Practice Point
Lower extremity ulcers in young black patients, especially males, could be due to undiagnosed sickle cell disease.
Ulcer Pathogenesis
The abnormal hemoglobin molecule in the red blood cell in the patient with sickle cell disease does not affect the amount of oxygen the red blood cell can carry. After the red blood cell and its hemoglobin give up the oxygen to the tissues, the abnormal hemoglobin causes the red blood cell to lose its water content and polymerize resulting in the red blood cell distorting, becoming rigid, and deforming into the sickle shape (Fig. 17-1). Unfortunately, while the cells are in the sickled shape, they tend to increase blood viscosity and become “sticky.” This results in slowing of the blood flow in small vessels and subsequent clotting of the vessels especially in the postcapillary venules,5 causing ischemia of tissues and organs. Over time, the patient suffers repeated episodes of pain, tissue damage, and, eventually, organ failure. Because the cells become damaged while they are in the sickled shape, they are removed from the circulation faster than normal, resulting in anemia (Table 17-1). When the red blood cell is reoxygenated and regains its water content, it can regain its normal shape, but a significant percentage of sickle cells do not regain their normal shape and remain irreversibly sickled despite being fully oxygenated.5 This leads to an even more rapid removal of these irreversibly damaged cells from the circulation.
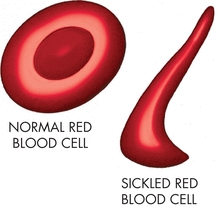
Figure 17-1. Sickled cell. The diagrams show a normal red blood cell and a sickled cell.
Table 17-1 Conditions Associated with Sickle Cell Anemia
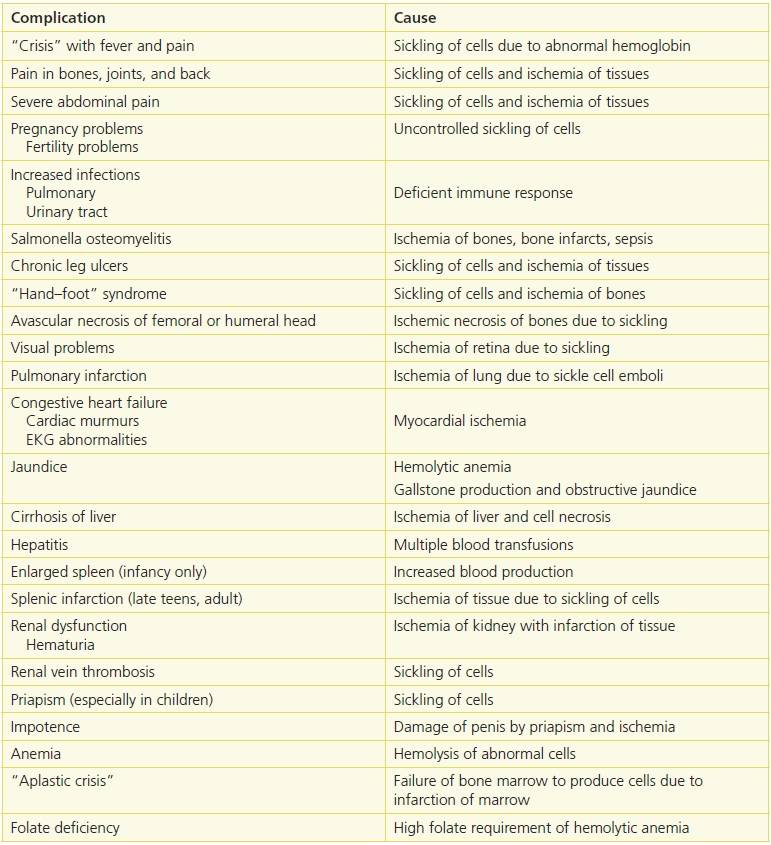
Adapted from Conley, C.L. “The hemoglobinopathies and Thalassemias,” in P.B. Beeson, W. McDermott, eds. Textbook of Medicine, 13th ed. Philadelphia, PA: W.B. Saunders Co., 1971:1501–503, with permission.
Although the underlying pathophysiology remains ischemia due to occlusion of the microcirculation, sickle cell ulcers have been associated with homozygous hemoglobin SS disease, trauma, infection, severe anemia, warm temperatures,9,10 venous insufficiency,11,12 and indications of severe sickle cell disease as reflected by increased cardiovascular risk, pulmonary hypertension, and lower body mass index.13
These ulcers are most likely to occur in the malleolar area of the lower extremities. Occlusion of the small vessels in the microcirculation results in chronic damage to the microcirculation of the skin around the ankle including injury to the capillary walls, thickening of the intimal lining of the capillary, and increase in permeability of the vessel wall, allowing macromolecules to escape into the tissues.14–16 These changes can also result in ischemia of the tissues making them more susceptible to minor trauma and less able to heal.14,17 As a result, these areas are more likely to be the sites of skin breakdown and ulceration. Recently, new information shows that the endothelial dysfunction in sickle cell disease may be due to impaired bioavailability of endogenous nitric oxide resulting in unrestrained vasoconstriction of the small vessels, ischemia of the skin, and skin necrosis.17,18
Diagnosis
Medical History
Evaluation of the patient with a suspected sickle cell ulcer is of utmost importance so that the correct diagnosis can be made and appropriate treatment planned. The patient’s medical history should be recorded, and the events surrounding the ulcer development should be investigated. Is this the first ulcer the patient has had? How long has the ulcer been present? How did the ulcer first develop? Was there trauma to the area? How did the area first look? Any history of lower extremity edema; unexplained swelling of the hands, feet, or knees; osteomyelitis; episodes of abdominal or joint pain; episodes of severe unexplained pain; recurrent urinary tract infections or pneumonia; or anemia should be noted. Sickle cell patients are prone to develop unexplained episodes of fever, which tend to resolve without therapy. These patients may carry a diagnosis of FUO (fever of unknown origin).
Physical Examination
A complete physical examination should be part of the evaluation of the patient. Vital signs, especially temperature, should be taken because, as mentioned above, unexplained fever may be a sign of sickle cell disease. Cardiac and pulmonary exam may reveal signs of cardiovascular disease and pulmonary hypertension. These conditions are more frequent and more severe in sickle cell patients with ulcers than in those without ulcers.13 Abdominal examination can detect enlargement of the liver. The spleen may be enlarged in early childhood as it is a primary blood forming organ but is usually small in later life due to ischemic infarction.
The presence of scars on the extremities indicates previous ulcer incidence. The location of the ulcer or ulcers is important as most sickle cell ulcers are found on the lower one-third of the leg and usually over the medial or lateral malleoli (or both) of the ankle10; however, less common sites include any area with little subcutaneous tissue such as the anterior tibial area, dorsum of the foot, and the Achilles tendon area.19 The size of each ulcer should be measured by determining the length and width or by using one of the more advanced measuring modalities described elsewhere in the book. (See Chapter 6, Wound Assessment.)
The presence of an ulcer in a patient with varicose veins, venous insufficiency, and sickle cell disease can be especially troublesome as it may lead to misdiagnosis (Fig. 17-2). In addition, venous incompetence in the patient with sickle cell disease may predispose him to develop an ulcer and can be correlated with the development of a recurrent sickle cell ulcer.12 Noninvasive venous studies have shown that venous insufficiency is not a primary contributing factor to the development of a sickle cell ulcer.19 These studies can be helpful in establishing the correct therapeutic approach in these patients.
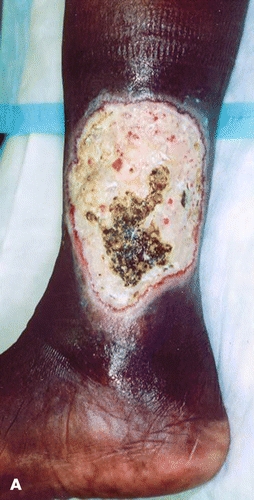
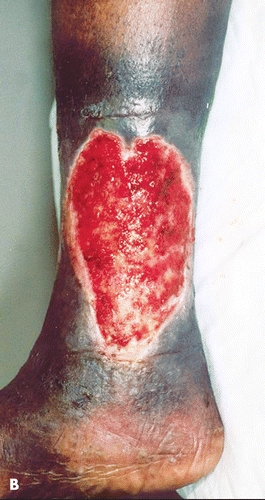
Figure 17-2. Undiagnosed sickle cell ulcer treated initially as a “venous ulcer.” (A) Predebridement. (B) Postdebridement. (Used with permission from T. Treadwell, MD.)
 Practice Point
Practice Point
Misdiagnosis—and thus, mistreatment—of sickle cell ulcer as a venous “stasis” ulcer makes it imperative to get the diagnosis correct.
Ulcer Assessment
Examination of the wound bed is essential to determine the presence of granulation tissue or fibrinous material (“slough”) (Figs. 17-2 and 17-3). In addition, the presence of periulcer erythema or cellulitis should also be documented. The presence and character of any drainage should be noted. Tenderness of the lower extremity to palpation or the presence of pain in the ulcer or surrounding area should be also recorded. A vascular examination should always be performed by checking for the dorsalis pedis and posterior tibial pulses to be sure the patient has adequate blood flow. Documentation of their presence or absence is important. If there is a question about the adequacy of the circulation, the patient will need to be referred for noninvasive vascular studies or arteriography. The microcirculation in the periwound area can be evaluated with pulse oximetry, laser Doppler, and TcPO2 measurements. Measurements of tissue oxygen levels by whatever method showed that readings are lower in patients with ulcers than sickle cell patients without ulcers.13
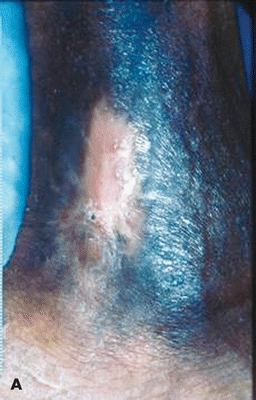
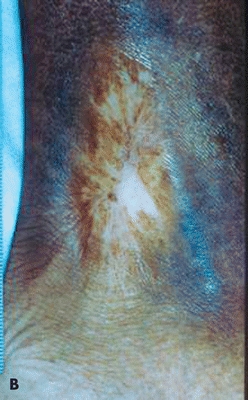
Figure 17-3. Recurrent sickle cell ulcer treated with tissue-engineered skin. (A) Healed 8 weeks postapplication. (B) Ulcer remains healed 8 months later—note improved scar. (Used with permission from T. Treadwell, MD.)
 Practice Point
Practice Point
A complete history and physical examination are vital when evaluating a patient with a sickle cell ulcer.
Laboratory Assessment
Laboratory evaluation can help make the diagnosis if a patient is seen with a nonhealing ulceration of the ankle area. It, also, will help identify the severity of the patient’s sickle cell disease. If the patient does not have a diagnosis of sickle cell disease, but it is suspected, blood tests that check for anemia, sickle cells, and abnormal hemoglobin should be ordered. This usually involves a complete blood count (CBC) with platelet count, sickle “prep,” and a hemoglobin electrophoresis. Although hemoglobin electrophoresis is considered the diagnostic tool of choice, it has its limitations especially if the patient has had recent blood transfusions.9 In such a case, referral to a hematologist may be necessary. If the patient with known sickle cell disease has an ulcer, the laboratory workup should consist of a CBC with differential white blood cell count, platelet count, and reticulocyte count.
Laboratory evaluation has shown that sickle cell ulcer patients, in addition to lower hemoglobin levels, have higher markers of hemolysis such as elevated levels of lactate dehydrogenase (LDH), bilirubin, aspartate transaminase (AST), alkaline phosphatase, and uric acid, serum amino-terminal probrain-type natriuretic peptide, and higher reticulocyte counts than do patients with sickle cell disease with no ulcers.13,14 High ferritin levels are noted and are felt to be due to the chronic inflammatory state of these patients with ulcers.13 High levels of uric acid are seen in patients with sickle cell ulcers. It has been determined that high uric acid levels may be an indicator of microvascular problems even if there is no current sickle cell ulcer.13
Patients with sickle cell disease are especially prone to develop infections; therefore, it is important to obtain a wound culture using appropriate technique if the wound bioburden appears elevated. We have found that a significant number of patients with sickle cell ulcers have wounds covered by biofilm, which must be removed before the ulcer can heal (see Fig. 17-2). The most frequent way to remove a biofilm is with sharp debridement, but recently, we have turned to ultrasonic debridement techniques, which are less painful for the patient. Dressings that enhance autolytic debridement can, also, be helpful. Following debridement, the use of the enzymatic debriding agent, collagenase, will help maintain the debrided state.20 Other methods for removing biofilms will be available in the near future. (See Chapter 7, Wound Bioburden and Infection.)
Infection and Osteomyelitis
Patients with sickle cell disease and a deep, painful ulcer should be evaluated radiologically for the presence of osteomyelitis especially if the patient has fever or leukocytosis. Patients with sickle cell disease are prone to developing Salmonella osteomyelitis.9 Radiologic evaluation can be done by several methods. Plain film x-rays are the least sensitive method and usually don’t show any evidence of osteomyelitis until late in the course of the infection. In addition, they can be confusing in the patient with sickle cell disease as the disease can cause periosteal elevation and other bone changes mimicking osteomyelitis.9 Nuclear medicine bone scans are slightly more helpful, but it must be remembered that routine bone scans only detect areas of inflammation. For this reason, if the patient has an ulcer overlying the bone in question, the bone scan is not helpful without special imaging techniques. Magnetic resonance imaging appears to be the imaging modality of choice in terms of sensitivity and specificity.
It has been suggested that bone biopsy and culture may be the only definitive way to determine if osteomyelitis is present,10 but it should be done with great care so as not to cause an infection in the bone. Biopsy of the wound bed and wound margin may be advisable if the ulcer has been present for over 3 months, does not respond to therapy, or just doesn’t “look right.” This should be done to rule out the possibility of malignancy and can help with the diagnosis.21
Pain
It must be remembered that sickle cell ulcers are extremely painful. Pain assessment is an area that must not be overlooked as, often times, the patient is fearful of experiencing even more pain with your evaluation and treatment and may not want you to look at the ulcer much less touch it. This makes treatment of these ulcers very difficult. (A useful pain assessment tool for evaluating a patient’s pain is outlined elsewhere in this book [see Chapter 12, Pain Management and Wounds].) It has been the author’s experience that if the provider does not address the pain problem, many of the patients will not return for follow-up care. Indeed, most patients would rather keep their ulcer than deal with potentially being in more pain than they already are. For this reason, any procedure to the wound must be preceded by some type of anesthesia: topical anesthesia (Xylocaine ointment or EMLA cream), injectable local anesthesia, regional anesthesia, or general anesthesia. Some of these techniques will require hospitalization of the patient.
Pain control can be managed with topical anesthetic agents. Topical Xylocaine ointment or EMLA cream can be used on a regular basis for pain control. Applied every 4 to 6 hours, these agents can make the patient’s daily activities much more manageable. It also makes dressing changes more comfortable. Other therapies, including opioid analgesics and regional medications (Xylocaine patches), are useful but many times have to be managed by a pain specialist. (See Chapter 12, Pain Management and Wounds.)
Because of the painful nature of sickle cell ulcers, debridement, biopsy cultures, or even dressing changes can be a challenge. If biopsy cultures are indicated, pain management will be required, but the practitioner should be aware of recent studies about the effects of anesthetics on bacteria. Berg et al.22 have shown that EMLA cream, a topical anesthetic agent commonly used before doing wound biopsies, is highly antibacterial. Within 1 hour of exposure to EMLA cream, most common bacteria are killed, including strains of Staphylococcus aureus (both methicillin-resistant and methicillin-sensitive strains), Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa. Injected solutions of 1% lidocaine are, also, antibacterial for the same organisms but at greater than 2 hours after local injection. It is the recommendation of the author that EMLA cream not be used for anesthesia when biopsy cultures are being done. Local injection of preservative-free 1% lidocaine would be satisfactory to use if the biopsy culture is done within 2 hours of the injection and the specimens are promptly plated.22
 Practice Point
Practice Point
Sickle cell ulcers are extremely painful. Evaluation and treatment of the patient’s pain should be a top priority and should be the first steps in instituting therapy for the ulcer.
Treatment
Treatment of sickle cell ulcers can be challenging and frustrating. Even in this day of evidence-based therapies, it is noteworthy that there are no published trials of treatments of sickle cell ulcers.10 One of the more interesting findings about the treatment of sickle cell ulcers is that most ulcers will heal with prolonged bed rest.10 Obviously, this is not a practical therapy as extended hospitalization is no longer possible, and complete bed rest at home is not realistic. However, any therapy that results in a long period of immobilization, such as from operative intervention, must take into account that the bed rest is healing the ulcer, not necessarily the treatment. With this in mind, one must begin with the basics of what we know constitutes good wound care.
The basics of good wound care include debridement of devitalized tissue, control of infection, assurance of adequate circulation, maintenance of a moist wound environment, and control of the edema in the extremity. The major addition in the treatment of sickle cell ulcers is control of wound pain. Many times, the ulcers are so painful that manipulation of the wound is impossible. Treatment of the wound with topical anesthetics has been previously addressed and must not be overlooked. If the therapy for the wound is painful, most people will forgo your advice and treat the ulcers themselves in ways that do not cause more pain. It is unfortunate that many of these patients are labeled as “noncompliant” when it is a poor choice of therapy by the clinician and not the compliance of the patient that should be in question. Once the pain is controlled, the wound can be debrided and treated, as indicated. Use of dressings that enhance autolytic debridement or enzymatic debridement should be strongly considered. Debridement with sterile maggots can be a consideration and is less painful, but convincing the patient may be difficult!
The evaluation of sickle cell ulcers for infection and the evaluation of the patient for circulation problems have been covered previously. The importance of moist wound care has been known since Winter’s publication in 1963.23 It is now known that wounds treated with wet-to-dry dressings do not heal well and removing a dry dressing that adheres to the wound causes pain and reinjures the wound.24 Wounds treated by moist wound healing techniques and dressings heal faster, are less painful, and have less scarring than those allowed to dry out. There are numerous wound dressings currently available, which will maintain a moist wound environment. (See Chapter 9, Wound Treatment Options.)
If the wound is felt to be infected, topical antimicrobials should be considered. Oral or IV antibiotics are only indicated if the patient has a leukocytosis, cellulitis, or fever.25,26 Silver dressings have become popular in the treatment of the wound with a clinically significant bacterial burden (critical colonization) or with frank infection. There are numerous silver dressings available for use on these wounds. It is suggested that once the bacterial burden is under control, the silver dressing should be discontinued and other moisture control dressings used. This is because of the potential for toxicity of the silver to the growing tissues.27,28 Dressings containing Cadexomer iodine are useful in treating wounds critically colonized with bacteria or wounds, which are infected.29,30
Since a number of patients with sickle cell ulcers have a low body mass index and are underweight, attention to nutritional factors should be remembered.13 Restoration of normal protein, vitamin, and electrolyte levels should be considered critical in the treatment of these patients.31 It has been shown that many patients with sickle cell ulcers are zinc deficient. Some early studies showed that oral zinc sulfate (200 mg three times/day)32 is helpful in the treatment of patients with sickle cell ulcers.
Another major issue is to manage the edema of the extremity associated with the ulcer. It is known that edema fluid inhibits mitogenic activity and DNA synthesis, the cytokine environment in edema fluid is more proinflammatory, the protease activity is higher in edema fluid, and growth factor levels are decreased in edema fluid. All of these factors contribute to impaired healing of any wound including sickle cell ulcers.33 Compression bandaging should be considered in the treatment of any patient with a lower extremity ulcer and edema.
The use of the tissue-engineered skin in the treatment of sickle cell ulcers has met with some degree of success.34 Prior to applying any advanced therapy product, the wound bed must be well prepared, which means assuring that the wound bed has been debrided to remove all necrotic tissue, that infection has been controlled, and that the wound environment has been optimized.35 To achieve the goal of wound environment optimization, Treadwell et al.36 recommend pretreating the wound with a protease modulating agent for 2 to 3 weeks to reduce the abnormal protease levels. These agents include oral or topical doxycycline or an oxidized, regenerated cellulose (ORC) collagen product.37,38 His early data show that pretreatment of any chronic wound with these protease modulating agents prior to application of human skin equivalent improves the healing rate.36 Once treated with the tissue-engineered skin product, the patients can experience significant relief of their pain as well as healing of their ulcer (see Fig. 17-3A). It is also of note that sickle cell ulcers treated with tissue-engineered skin seem to have a more “normal” appearing and stable scar (see Fig. 17-3B).
The use of the split-thickness skin graft or pinch grafts to treat patients with sickle cell ulcers may be a reasonable therapeutic alternative. However, the procedures require hospitalization and anesthesia, thus making them less cost-effective. It has also been reported that split-thickness skin grafting has a very low success rate in healing sickle cell ulcers, and in those that do heal, the recurrence rate is very high.39 Success has been noted with the use of muscle flaps, myocutaneous flaps, and free flaps to cover large lower extremity sickle cell ulcers40,41 but not uniformly.42
A mainstay in the treatment of sickle cell disease is hydroxyurea, which is known to improve symptoms associated with the disease by increasing the levels of fetal hemoglobin (HbF). This results in a significant decrease in the polymerization of hemoglobin S and sickling of the red blood cells.5 Unfortunately, hydroxyurea is known to cause leg ulcers in patients taking this medication.43,44 The medication must be stopped before the ulcer will heal.44 Fortunately, it has been found that ulcers related to hydroxyurea therapy respond promptly to treatment with tissue-engineered skin.45
Transfusion therapy has been tried in the treatment of patients with sickle cell ulcers who have been resistant to all other therapies. The goal of the therapy is to keep the hematocrit between 30 and 35 volume percent and the percentage of normal hemoglobin (hemoglobin A) greater than 70% of the total.39 The transfusions are continued until the ulcer heals or for 6 months at which time they are discontinued. Unfortunately, 20% to 30% of patients treated with multiple blood transfusions can develop antibodies to blood products minimizing their use when anemia is profound.39 The possibility of iron overload with this therapy must be considered.15
Another interesting approach to treating sickle cell ulcers has been the use of IV arginine butyrate. The concept is that the arginine butyrate will change the concentration of abnormal hemoglobin, thus allowing the wounds to heal. Studies have shown reasonable success with this method,46–48 but no randomized controlled trials have been done as of this time.
Other therapies for sickle cell ulcers include medications, such as pentoxifylline (Trental) and the topical growth factor, molgramostim (GM-CSF)49; negative pressure wound therapy; hyperbaric oxygen therapy; and electromagnetic stimulation. Other therapies are still considered experimental, and their utility in treating these patients with difficult wound problems will be determined by future studies.
Recently, it has been suggested that strategies for the treatment of sickle cell ulcers should fall into one of the following categories: (1) agents with reduce or prevent sickle cell dehydration, (2) agents with reduce sickle cell–endothelial adhesive events, (3) nitric oxide or nitric oxide–related compounds, or (4) antioxidant agents.50 These strategies deal with the unhealed ulcer based on the pathophysiology unique to the sickle cell ulcer. Currently, most of the work on these strategies is still in the research phase but hold promise for future treatments.5,50 One exception is the role of nitric oxide in the treatment of the sickle cell ulcer.
Nitric oxide is a strong vasodilator, which also effects activation of white blood cells, platelets, and endothelial cells. Nitric oxide is produced by endothelial cells in the vasculature from the amino acid, l-arginine. It has been noted that nitric oxide levels are decreased in patients with sickle cell ulcers especially during episodes of crisis.50–52
Based on this information, Aslan17 suggested that sickle cell ulcers may be due to a decrease in microcirculatory smooth muscle nitric oxide with resultant vasoconstriction, tissue ischemia, and necrosis and that nitric oxide supplementation may improve healing of the ulcers or even help prevent them. Because of this information, we have been treating patients with sickle cell ulcers with a combination of l-methylfolate, pyridoxal 5′-phosphate, and methylcobalamin, the combination of which has been shown to reduce vascular homocysteine levels and raise nitric oxide levels in the microcirculation of wound bed and periwound skin compared to the skin away from the ulcer.52 An increase in nitric oxide in the microcirculation is reflected by vasodilatation and an increase in tissue TcPO2. An example is the patient represented in Figure 17-4. The “pretreatment” evaluation shows the periwound TcPO2 (#1) and the reference point (#2) levels of 16.0 and 69.1, respectively. The periwound level is below what would be expected for healing to occur. After 1 week of oral therapy with the medication (“posttreatment”), the periwound TcPO2 has risen to 46.1 (#2), while the reference point (#1) has stayed essentially the same at 67.2.53 With this improvement, the wound began to heal. For these reasons, we recommend all of our sickle cell ulcer patients be treated with this therapy. Recently, we have had the opportunity to utilize a device that delivers topical nitric oxide directly to the wound bed to assist in healing. This is the preferable way to deliver the nitric oxide so that systemic effects can be avoided in these patients.54 It is our impression that the sickle cell ulcers heal faster with these therapies, but the actual healing rates and whether this treatment will reduce the incidence of sickle cells ulcers or their recurrence are yet to be determined.
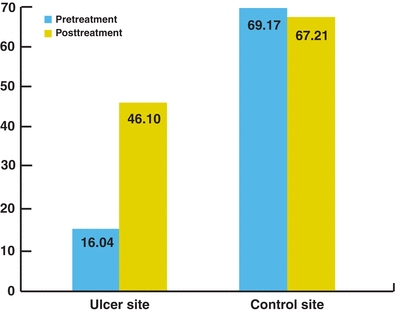
Figure 17-4. Treatment of sickle cell ulcer with nitric oxide–producing medication. Before treatment, TcPO2 levels at the ulcer site and control site were 16.04 and 69.17, respectively. The periwound TcPO2 level is below what would be expected for healing to occur. After 1 week of oral therapy with the nitric oxide–producing medication Metanx, there was marked improvement in the periwound TcPO2 while the control site stayed essentially the same.25 With this improvement, the wound began to heal. Based on this, we treat all of our sickle cell ulcer patients with this medication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


