Ronald A. Kline, MD, FACS, FAHA
“Peripheral vascular disease” is a term commonly used in reference to an arterial problem, even though it includes diseases and conditions of the venous and lymphatic systems as well as the arterial system. Patients with leg ulcers may present with a combination of arterial, venous, and lymphatic disease. This chapter presents information in two separate sections: (1) the pathogenesis and management of wounds resulting from venous insufficiency and (2) lymphatic disease.
Scope of the Problem
Up to 50% of individuals may have some sort of vein abnormality.1 It is estimated that between 1% and 22% of the population over age 60 suffers from lower-extremity skin ulcers.2–5 One study found the problem to be underestimated when a self-report survey indicated that high numbers of patients cared for their own ulcers without consulting a healthcare provider.6 The principal leg ulcer etiology in most patients is some type of peripheral vascular disease. Chronic venous disease is the seventh most common chronic disease and is the underlying cause in 95% of leg ulcers.1,7–9 In a US community health survey, 5% of adults had skin changes in the leg and more than 500,000 suffered from venous ulcers. Over 2,000,000 workdays are lost in the United States per year due to the associated morbidity of postphlebitic syndrome.9 Although it’s understood that chronic wounds have physical, financial, and psychological effects, it’s difficult to measure these effects on a patient’s quality of life.10 It’s also difficult to obtain accurate etiological information about leg ulcers, and in about one-third of medical records, no ulcer etiology might be documented.
Venous Anatomy and Physiology
Venous System
The venous system begins at the postcapillary level. Venules begin to coalesce, forming small veins, which again coalesce into larger veins from the periphery to a more central location. The venous system mimics the arterial system in many respects but has greater anatomic variability than the arterial tree. In the leg, the veins that course with the tibial and peroneal arteries are usually paired with numerous cross-linking branches, resulting in a retia appearance in some patients. These branches ascend along the respective arteries to form the popliteal vein, which is the first vein of significant size in the lower leg. The popliteal vein proceeds toward the head and becomes the femoral vein, commonly called the superficial femoral vein—a name that causes confusion because the vein in question is actually a deep vein. The superficial femoral vein joins the deep femoral vein to form the common femoral vein. The deep femoral vein is the deep drainage system of the thigh (Fig. 14-1).
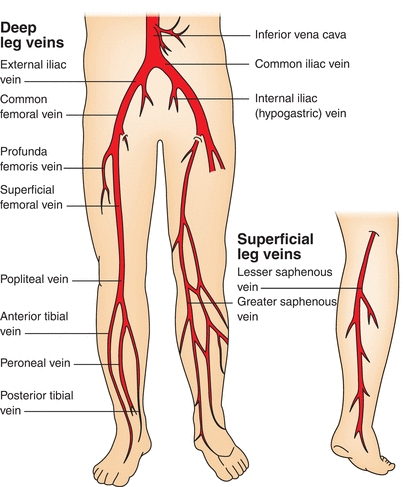
FIGURE 14-1. Deep and superficial venous systems. This illustration shows deep and superficial veins of the lower extremities.
Dual Venous System
The leg has a dual venous system—the deep system just described and the superficial system represented by the saphenous veins. The greater saphenous vein (GSV) courses along the medial aspect of the leg. The dorsal digital veins in the foot coalesce to form the GSV, which is found medial and anterior to the medial malleolus. It ascends in the leg through a variable course and may be bifurcated or even trifurcated. At knee level, its course becomes deeper in relation to the skin. As it ascends the leg, it joins the common femoral vein at the fossa ovale. The lesser saphenous vein drains the posterior aspect of the calf. It perforates into the deep compartment of the calf at the level of the popliteal fossa to join the popliteal vein. As the common femoral vein ascends behind the inguinal ligament, it becomes the external iliac vein and joins the internal iliac vein to become the common iliac vein. The common iliac veins join at the level of the umbilicus and to the right of the aorta to become the inferior vena cava. The renal veins drain into the vena cava. More cephalad, the hepatic veins join the vena cava, which then empties into the right heart chamber.
The saphenous system is connected to the deep venous system through numerous perforator veins. Perforator veins shunt blood from the subcutaneous tissue and the greater saphenous system into the deep veins of the leg. They cross through the superficial fascia of the leg, hence their name. The location of perforator veins is somewhat variable, and some are ascribed proper names. The lowest perforator connecting the saphenous system with the deep venous system is just above the medial malleolus.
Valve Anatomy
Unidirectional valves are present in the deep and superficial venous systems and in the perforator veins. These valves are located just before bifurcation points. The GSV contains approximately six to eight valves. With rare exception, a valve is always present just below the insertion of the GSV into the common femoral vein at the fossa ovale. The orientation of the valves allows venous blood to flow from distal to proximal. Perforator veins valves are oriented to shunt blood from the lesser saphenous vein and the greater saphenous system into the deep veins of the leg.
Valve anatomy is that of a bileaflet with valve sinuses present on the lateral bases of each valve leaflet. These sinuses represent a dilation in the normal contour of the vein wall. Their function is to assist in valve closure, a passive act caused by the retrograde flow of venous blood into the sinus, thereby coapting (fitting together) the two valve leaflets. The valve leaflets are oriented parallel to the surface of the skin. It’s the loss of valve function at various levels that results in varying degrees of venous insufficiency. Valve function is lost under a number of disease states. Inability of the valve to coapt can also occur with overdistention of the venous segment. This effectively stretches the valves apart so that they no longer come in direct contact, thereby allowing blood to reflux into the more dependent portion of the vein. Disease states that cause loss of valve function include:
- congenital valve absence
- deep vein thrombosis
- ectasia
- phlebitis
- valve atresia
- venous engorgement
- venous hypertension.
Venous Wall Architecture
Venous wall anatomy is similar to arterial wall anatomy except that the respective laminae are thinner. The outermost layer of a vein is the adventitia. The media varies most from the arterial media. The media within a vein contains both elastic and muscular fibers, but to a much lesser degree than the arterial media. Nonetheless, a vein can contract and adjust its size to correspond to the degree of venous blood flow. The intima layer is a delicate single layer of endothelial cells.
The relatively thin media accounts for the lack of venous compliance at increased pressures. At low pressures, the venous system is fairly compliant, but once arterial pressure is reached, the venous wall becomes distended and rigid. Venoconstriction occurs in both the superficial and deep veins; the more peripheral the vein, the more readily it contracts. This reactivity is under sympathetic adrenergic control. Peripheral veins are more sensitive than central veins to this sympathetic drive. The ability of veins to relax and dilate enables the venous system to hold 75% of the total blood volume.
 Practice Point
Practice Point
Loss of consciousness can occur from venous pooling in motionless lower extremities—a fate not unknown to young military recruits who must stand at attention for prolonged periods of time.
The upward flow of lower-extremity venous blood, although aided by unidirectional valves and arterial pressure, is mostly dependent upon the “muscular pump.” Pedal dorsal vein pressure in the supine position should approximate that of central venous pressure. Upon assuming an erect posture, this pressure can approach 100 mm Hg. With active muscle contraction, intracompartmental pressure markedly increases, thereby causing deep veins of the leg to compress and push venous blood upward. This pressure then approaches 200 mm Hg. This is possible because the muscular compartments of the leg are enclosed by relatively rigid fascial encasement. Backflush of blood is reduced when valves are competent and reflux into the saphenous system is prevented by the unidirectional perforator valves.
Ulcer Pathophysiology
Venous Ulcers
Venous ulcers are chronic skin and subcutaneous lesions usually found on the lower extremity at the pretibial and the medial supramalleolar areas of the ankle, where the perforator veins are located. Venous ulcers were formerly known as “venous stasis” ulcers because their development was thought to be caused by blood pooled in the veins. More recent literature indicates that venous hypertension rather than venous stasis is both the cause of these ulcers and the reason they don’t heal.11,12 It’s difficult to restore skin integrity in the presence of chronic venous hypertension because the underlying edema must be controlled in addition to healing the ulcer.
Venous ulceration may be precipitated by deep vein thrombosis (DVT), which can remain undiagnosed for years prior to the onset of the ulcer (Fig. 14-2). It has long been thought that the natural course of lower-extremity DVT is the eventual development of leg ulcers.13
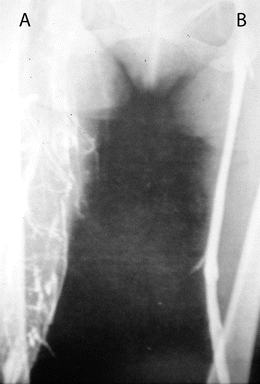
FIGURE 14-2. Venogram. In this venogram, the patient’s left venous valve (B) is intact. On the patient’s right (A), collateral veins are present due to venous occlusion, possibly from an undiagnosed deep vein thrombosis.
Symptomatic and asymptomatic thrombi may cause long-term complications by scarring the intima and creating valvular incompetence. When the valves are rendered incompetent, blood backs into the distal veins during diastole. With loss of perforator valve function, the high intracompartmental venous pressure, which can approach 200 mm Hg during active muscle contraction, results in distention of the saphenous system. This in turn causes a cascading effect with dilation of the GSV and worsening of already compromised valvular function. The weight of the column of blood increases the pressure inside the capillaries.
Characteristics of Venous Ulcers
Venous hypertension distends the superficial veins, resulting in vein wall damage and exudation of fluid into the interstitial space, thereby causing edema of venous insufficiency. Over time, an actual leakage of red blood cells occurs through these compromised veins. As they break down, the red blood cells deposit hemosiderin into the tissues, causing a form of “internal tattooing” of the skin; the coloration is that of a brownish hue noticeable even in black skin (Fig. 14-3). The skin loses its normal texture and becomes somewhat shiny and subsequently sclerotic, giving a taut skin appearance in these areas.
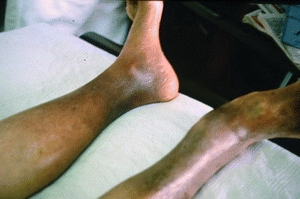
FIGURE 14-3. Hemosiderin staining. Hemosiderin staining caused the discoloration seen here in the patient’s right leg from chronic venous hypertension.
Edema and loss of red cells into the subcutaneous tissue occur at the point of greatest gravitational pressure, the ankle. This gives rise to the pathognomonic features of chronic venous stasis, hyperpigmentation, and stocking distribution induration of the subcutaneous tissues,14,15 the characteristics of long-standing venous insufficiency called lipodermatosclerosis. These areas are prone to subsequent ulceration or infection; extreme pruritus and excoriation are usually present, potentially aggravating the injured skin. Dermatitis due to endogenous or exogenous sources and severe allergic reactions may complicate the situation. The skin may present as itchy, erythematous, and weeping or dry and scaly (Fig. 14-4). Chemical or mechanical factors may be responsible for contact dermatitis surrounding a leg ulcer.16
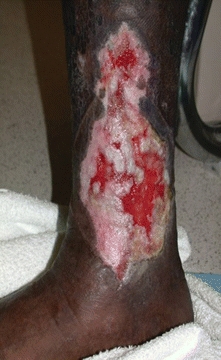
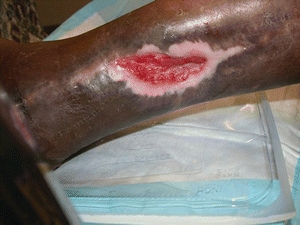
FIGURE 14-4. Venous ulcer with granulating base. Venous ulcer with granulation tissue and epithelial islands and epithelial growth from the borders.
Another sequela of venous hypertension is irritability of the musculature. Many patients with venous insufficiency—even those in whom the condition is mild—report nocturnal leg cramps. Depolarization may occur due to fluid distention of the muscular cells, causing tetanic-like contractions of various muscle groups. Distention of veins in the subdermal plexus results in the varicosities typically seen with venous insufficiency (Fig. 14-5). The appearance of telangiectasias, more commonly called “spider veins,” is the result of distention of the smaller subdermal capillary network (Fig. 14-6).
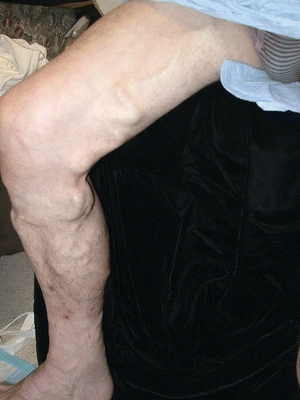
Figure 14-5.Note the presence of varicose veins in the patient’s right leg.
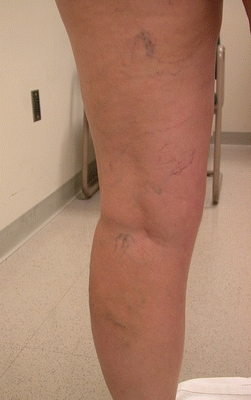
FIGURE 14-6. Telangiectasias, also known as “spider veins,” are shown.
In some circumstances, venous aneurysms can occur due to massive dilation of the GSV and its tributaries. Further stagnation of flow in these areas in the presence of an abnormal vessel wall can result in thrombophlebitis, which worsens the venous outflow of the leg and aggravates an already deleterious condition. Thrombosis adheres to the wall of the vein and although recanalization occurs eventually, the valves remain incompetent. In an attempt to compensate for the reduced venous return, the surrounding collateral veins dilate. Chronic edema occurs in the ankle. Increased venous pressure impedes capillary flow, decreasing oxygen available for transport from the capillaries to the tissues, and protein and red blood cells leak into the interstitial tissues. The effect is cumulative, eventually leading to tissue damage, scar formation, and, ultimately, ulceration.
Endothelium in the normal saphenous vein facilitates contraction in response to noradrenaline. In varicose veins, the endothelial-enhanced noradrenaline vasoconstriction is decreased. Endothelial damage is thought to be a possible cause of venous dilatation and subsequent varicose veins.17
Venous leg ulcers are also correlated with increased ambulatory venous pressures (AVP). Nicolaides18 obtained AVP from 220 patients admitted with venous problems. He found that no patients with an AVP less than 30 mm Hg had leg ulcers, while 100% of those with AVP greater than 90 mm Hg had ulcers. The incidence of ulceration wasn’t preferentially associated with either superficial or deep venous disease.
 Evidence-Based Practice
Evidence-Based Practice
Nicolaides’ study18 suggests that AVP should be measured in patients with nonhealing venous ulcers to determine whether they may benefit from a procedure such as a venous valve transplant, which reduces the AVP to less than 30 mm Hg.
Pathogenesis of Venous Ulcers
Several theories have been proposed to explain the mechanism of venous hypertension leading to ulceration. In 1917, Homans suggested that stasis of blood in dilated veins in the skin may cause anoxic cell death, leading to ulcers. In 1929, Blalock19 found blood oxygen content to be higher than normal in varicose veins, suggesting that arteriovenous communications may be responsible for venous hypertension. In 1972, however, a study using radioactive macroaggregates refuted the arteriovenous shunting hypothesis.20
Two hypotheses—the “fibrin cuff” and “white cell trapping” theories—are more recent attempts to explain venous ulcer formation. The fibrin cuff theory states that sustained venous hypertension causes distention of dermal capillary beds, which allows plasma exudate to leak into the surrounding tissue. Fibrin precipitation in the peripapillary space forms fibrin cuffs, which impair oxygen, nutrient, and growth factor transport. The tissues undergo inflammation and fibrosis.21 A subsequent study suggests that peripapillary fibrin is present but doesn’t influence healing of lower-extremity ulcers.22
The white cell trapping theory states that the neutrophil aggregation in the capillaries causes lipodermatosclerosis. Increasing venous pressure is thought to reduce capillary perfusion pressure and flow rate. Low capillary flow rate initiates white blood cell adherence to the cell wall. Endothelial cells and leukocytes interact and release proteolytic enzymes, oxygen-free radicals, and lipid products. The white cells are then activated, damaging the vessel walls, increasing capillary permeability, and allowing larger molecules such as fibrinogen to exit the capillaries.23,24
The trap hypothesis of venous ulceration was proposed by Falanga and Eaglstein.25 This hypothesis proposes that fibrin and other macromolecules that leak out bind or trap growth factors and other substances necessary for maintaining normal tissues and healing. There has not been any new research to support either of these theories.
Classifying Venous Disease
Chronic venous insufficiency (CVI) has been defined as an abnormally functioning venous system caused by venous valvular incompetence with or without associated venous outflow obstruction, which may affect the superficial venous system, the deep venous system, or both.26 CVI can result in postphlebitic syndrome, which manifests as varicose veins and venous ulcers.
In 1994, the American Venous Forum developed a descriptive system based on clinical, etiologic, anatomic, and pathophysiologic (CEAP) data to categorize the key elements in chronic venous disease.27 The CEAP system provides an objective classification method that clarifies relationships among contributing factors and improves communication regarding venous disease (Table 14-1). The system is subdivided into seven categories based on objective signs of chronic venous disease.28 In 2004,29 this system was refined to add C0 to the clinical component to represent no visible or palpable signs of venous disease, and subclasses were also added.
Table 14-1 CEAP Classification System
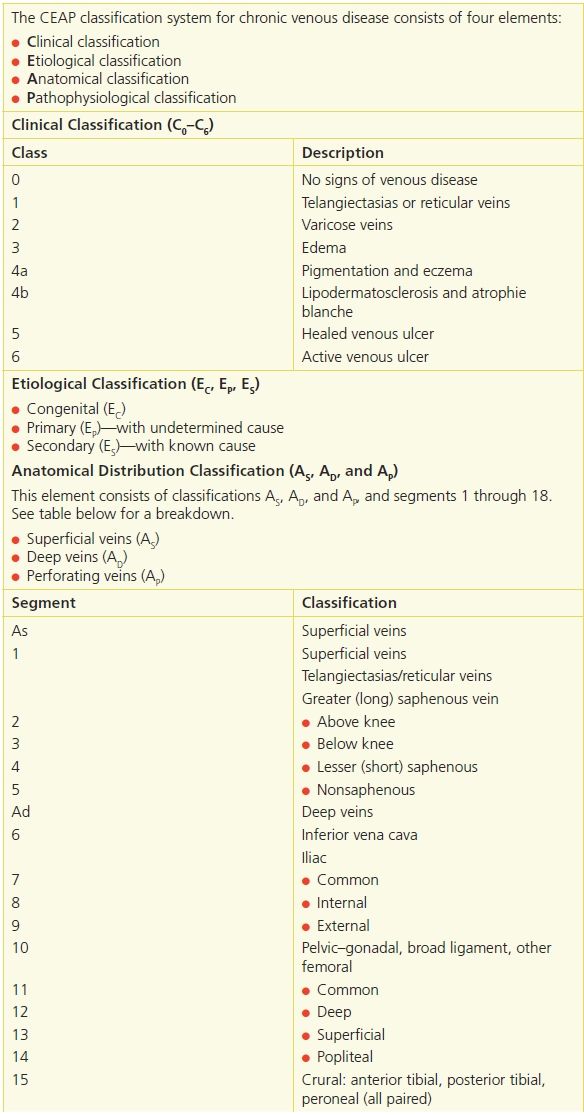
Adapted from: (1) Porter, J.M., Moneta, G.L. “Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease,” Journal of Vascular Surgery 21:635–45, 1995, (2) Eklof, B., Rutherford, R.B., Bergan, J.J., et al. “Revision of the CEAP Classification for Chronic Venous Disorders: Consensus Statement,”. Journal of Vascular Surgery 40:1248–52, 2004, (3) Meissner, et al. “Primary Chronic Venous Disorders,” Journal of Vascular Surgery 2007;46:54S–67S.
C4 was added to better define the differing severity of venous disease:
C4a: Pigmentation or eczema
C4b: Lipodermatosclerosis or atrophie blanche
In 2000, The American Venous Forum developed an assessment tool called the Venous Clinical Severity Score (VCSS) to supplement the CEAP tool.30 This assessment tool was revised and updated in 2010.31 Designed to provide more information about severe chronic venous disease, the VCSS tool consists of six descriptors—pain, varicose veins, venous edema, skin pigmentation, inflammation, and induration—that are rated 0 (absent), 1 (mild), 2 (moderate), or 3 (severe). In addition, there are four descriptors for ulcers: number of active ulcers (0, 1, 2, or 3 or more), active ulcer duration (N/A, <3 months, >3 months but <1 year, not healed for 1 year), active ulcer size (N/A, <2 cm diameter, 2 to 6 cm diameter, >6 cm diameter), and compressive therapy (not used, intermittent use, wears most days, full compliance). This scoring system is an elaboration of the C or clinical component of the CEAP classification system. It is dynamic in that the numbers can change as the ulcers heal or the venous disease process is treated. Combining the two tools provides a method to communicate information about the ulcer in both objective and subjective terms. The VCSS and CEAP tools are expected to improve clinicians’ ability to follow venous treatment outcomes.
Diagnosing Venous Ulcers
Venous disease and ulcer etiology can be determined by obtaining a thorough patient history and performing a physical examination. A focused vascular history includes a clear description of the presenting complaint, past medical history for vascular and related conditions, current and previously taken medications, and risk factors. Signs and symptoms of lower-extremity venous disease may include pain, tissue loss, or change in appearance or sensation. It is important to include assessment of the arterial system when evaluating venous and lymphatic disease. Adequate arterial perfusion is essential when using compression therapy for venous and lymphatic ulcers. An ankle–brachial index (ABI) is a reliable test that can be performed in any office (Fig. 14-7). An ABI should be conducted before applying any compression therapy. It may be necessary to correct the arterial problem before the ulcer can be treated. Noninvasive vascular laboratory testing is used to identify the location of vascular pathology.
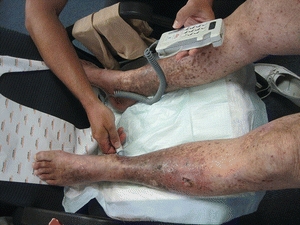
FIGURE 14-7. Ankle–brachial index (ABI) evaluation.
Physical Examination
Physical examination provides direction for intervention, starting with skin inspection. Skin changes in venous disease include hyperpigmentation, dermatitis, lipodermatosclerosis, or atrophie blanche, a characteristic white patchy scarring at the site of previous ulcers. Because skin color may indicate venous congestion, the color of each toe should be noted and compared with the color of other foot and toes. In venous insufficiency, the skin appears a dusky ruddy color. In CVI, the skin may become atrophied with scarring from a previous ulcer, or it may have weeping blisters or dry, scaly crusts.
Skin should be palpated for temperature changes. The skin over varicose veins is often warmer than the surrounding skin. Patients presenting with foot or leg ulcers should be tested for neuropathy, which is a common finding in the diabetic patient.
Edema is commonly found in lower-extremity venous disease. Early edema may be observed as a difference in calf circumference between legs and should be confirmed by measurement. After edema has been long-standing, tissue fibrosis occurs. This makes the skin and subcutaneous tissues less resilient to palpation.
Venous Signs and Symptoms
Patients may report a gradual onset of discomfort associated with venous disease; however, often, no symptoms are present initially. Most patients describe general nondescript aching rather than specific pain. Some terms used to describe sensations in the legs include fullness, swelling, tightness, aching, or heaviness. These symptoms can be reduced with leg elevation. Venous insufficiency accompanied by acute DVT may be described as sharp, severe, deep, aching pain.32 Varicose veins occasionally produce a pulling, prickling, or tingling discomfort localized to the area of the varicose vein.7 In severe cases of venous insufficiency, a form of claudication can occur. The patient may complain of foot edema that makes it difficult to wear shoes.
A venous ulcer is moist and may have a yellow fibrous film covering its surface. This fibrous tissue isn’t a sign of infection and doesn’t interfere with healing.33 The ulcer edges may be irregular with firm fibrotic and indurated surrounding skin. The surrounding tissue may have a brownish rust color due to erythrocyte breakdown and deposition of hemosiderin. Scar tissue may indicate the site of a previous ulcer. Because of the subcutaneous scarring, there is no allowance for the tissue expansion that occurs with edema in skin of normal elasticity. Scar tissue also prevents blood vessels from transporting oxygen to the skin, further compromising healing.34 Lipodermatosclerosis is the term used for chronic inflammation and fibrosis of skin and subcutaneous tissues. It may be associated with diffuse inflammatory painful skin edema.
Vascular Testing
Although an experienced vascular clinician can make a vascular diagnosis based on history and physical examination alone, vascular laboratory studies contribute to the accuracy of the diagnosis. The presence, location, and severity of arterial and venous disease are confirmed by vascular laboratory procedures. Information obtained by vascular studies can predict ulcer healing when the cause is arterial insufficiency.7 Laboratory tests differentiate among conditions that contribute to a nonhealing ulcer.
Noninvasive vascular testing is divided into direct tests that image the vessel itself and indirect tests that demonstrate changes distal to the diseased vessel. These tests include Doppler ultrasound, venous duplex ultrasound, ABI, transcutaneous pressure of oxygen (TCPO2), segmental systolic pressures, and plethysmography.
Doppler Ultrasound
In a Doppler ultrasound, a transmitting probe sends a signal that is reflected from an object to the receiving probe. If the signal strikes a moving object such as blood cells, a frequency shift is detected and reflected as sound. The audible signals of venous and arterial flow patterns can be distinguished.
Duplex Ultrasound
The venous duplex allows one to evaluate various segments of the venous tree looking for more than 0.5 seconds reflux after either a muscle contraction or manual augmentation of cephalad flow.35 An advantage of venous duplex imaging is that it can identify sites of thrombosis with high levels of accuracy. The findings of venous reflex may influence the treatment choice. The disadvantage of this test is that it’s fairly time-consuming, taking one to two hours per evaluation for a full leg imaging.
Venous Testing
It’s possible to perform a crude venous assessment by physical exam using a Doppler ultrasound. By compressing the limb manually, the flow in the veins can be augmented and noted by the audible Doppler signal heard distal to the site of compression. This is a subjective test, and reliability is clinician dependent. The introduction of noninvasive vascular testing has provided much anatomical and physiological information to increase the accuracy of diagnosing venous diseases. Two tests have been commonly used to assess the severity of venous insufficiency. One is venous photoplethysmography (PPG), and the other is venous duplex imaging.
Plethysmography
Plethysmography records volume changes in the limb. Several types of plethysmography are available:
- air plethysmography, which uses a pneumatic cuff as a segmental volume sensor
- strain-gauge plethysmography, which uses a fine-bore silicone rubber tube filled with mercury wrapped around the limb to be studied
- impedance plethysmography, which measures the relative change in resistive impedance of the passage of an electrical current through a segment of the body
- PPG, which measures the degree of light attenuation, which is proportional to the quantity of blood present and not actual volume change.7,36
Air plethysmography and PPG are the more common tests for chronic venous disease. A photoplethysmograph consists of an infrared light-emitting diode and a photo sensor mounted on a probe. The probe is applied to the skin over the area to be tested.7 The advantage of venous PPG is that it’s quick and gives assessment of overall venous refill time. On the other hand, it only evaluates the most dependent portion of the leg in the gator area.
Treating Venous Ulcers
Treatment of venous leg ulcers necessarily involves treatment of the underlying venous disorder. Edema reduction using compression, leg elevation, and exercise are part of the ongoing intervention for venous disease.
Treatment goals for all ulcers are to:
- provide an environment conducive to new tissue growth
- protect the wound
- prevent further tissue destruction.
Topical and systemic treatments are addressed simultaneously. It’s imperative to consider the cause when deciding treatment because ulcers aren’t all alike and treatment for one type may be inappropriate or harmful for another type. A vascular specialist consultation is appropriate for ulcers of mixed etiology.
Wound Infection
Infected leg ulcers, soft tissue cellulitis, and osteomyelitis are treated by administering systemic IV or oral antibiotics. Topical antibiotics are not indicated for all leg ulcers.37 Chronic wounds are colonized with normal skin flora and should not be treated with antibiotics. Rigorous and frequent ulcer cleansing assists in removing surface bacteria. Silver-impregnated dressings have not been shown to have a significant difference in ulcer healing and routine use of these dressings is not recommended.38
Biopsy for a quantitative culture of the inflamed tissue surrounding nonhealing ulcers should be considered if true infection isn’t responding to antibiotics. If carcinoma is suspected, biopsy of the lesion should be obtained (see Chapter 7, Wound Bioburden and Infection).
 Practice Point
Practice Point
Bone scans are expensive and may give false-positive results in the presence of inflammation; therefore, their use is indicated only when bone infection, abscess, or fluid collection is suspected.
Skin and Wound Care
A clean wound, free from dead tissue and wound debris, is necessary for healing to occur. Wound cleaning and debridement are the initial steps in wound care. Many commercial wound cleaners and disinfectants are cytotoxic. Povidone–iodine, hydrogen peroxide, and 0.25% acetic acid have shown evidence of interfering with fibroblast formation and epithelial growth.39–42 There may be indications for using cidal agents in a wound; however, their use should be time limited, and each caregiver should have a clear understanding of what the goals are and how to recognize when the goals have been reached.
 Evidence-Based Practice
Evidence-Based Practice
The advantage of cleaning the wound should be weighed against the risk of damaging new tissue growth.43
The safest wound cleanser is 0.9% saline solution. Wounds should be cleaned with a force strong enough to dislodge debris but gentle enough to prevent damage to newly growing tissue. The pressure to accomplish this goal ranges from 4 to 15 psi.44 A 19-gauge needle or 19-gauge angiocatheter distributes approximately 8 psi when used with a 35-mL syringe. Commercial irrigation products with caps designed to deliver adequate pressure are available. Leg ulcers treated in the home are commonly irrigated with running tap water.
Hydrotherapy or whirlpool has been used to aid in cleaning and debridement of both arterial and venous leg ulcers.45 A clinical pilot study found that whirlpool followed by vigorous rinsing reduced the bacterial load in venous ulcers more than the whirlpool alone.46 This may suggest that the vigorous irrigation is the significant factor in cleaning the wound. Whirlpool may be contraindicated if the water and dependent position increase edema in the leg. There are no current studies to support or refute whirlpool as a standard recommendation for cleansing venous ulcers.
Dressings chosen for specific wounds depend on the condition of the wound bed and the goal for the wound. Many new dressings are designed to support moist wound healing (see Chapter 9, Wound Treatment Options). Because the skin is fragile in patients with either arterial or venous disease and can be easily injured, tape and adhesive products should be avoided or used with extreme caution. Use other methods of securing dressings that won’t injure the skin. A Cochrane review on leg ulcer dressings found that the type of dressing material placed under compression had no influence on healing rates.47,48 Other studies found no advantages with the use of either silver-impregnated49 or honey-impregnated50 dressings.
 Practice Point
Practice Point
The preferred attachment device for dressings on vascular leg ulcers is roller gauze or commercial devices (such as netting or tube gauze) that hold dressings in place without adhesive, which can damage fragile skin.
Compression
Venous hypertension and venous wounds are treated simultaneously. Wound care and edema management depend on whether or not the patient can be immobilized. Edema is controlled by conservative means: intermittent elevation, compression bandages, and intermittent pneumatic compression.51 Studies have demonstrated that moist wound healing combined with compression improves wound healing rate of venous ulcers.52 Compression therapy is the mainstay of venous ulcer therapy.53 Elevating the legs above the heart is recommended whenever the patient can be placed in this position. A compression dressing isn’t required when the patient is immobilized with the leg elevated, such as during sleeping hours. Moist or dry (depending on the amount of exudate from the wound) gauze dressings with frequent changes can be used instead.
Compression is the application of pressure to the limb. It is measured in millimeters of mercury (mm Hg) and is applied by bandages, elastic stockings, and/or intermittent pneumatic compression pumps. The amount of compression prescribed is determined by the diagnosis, comorbid conditions, and the patient’s ability or willingness to accept the treatment. The following standard pressure classification54 is suggested: mild (<20 mm Hg), moderate (21 to 40 mm Hg), strong (41 to 60 mm Hg), or very strong (≥61 mm Hg). Compression strength of 30 to 40 mm Hg is recommended to counteract the capillary filling pressures within the leg; however, elderly or frail individuals or those who have difficulty donning the stockings may have compression ordered at 20 to 30 mm Hg. Many factors affect bandage pressure, including the bandage itself, the calf muscle and foot pump function, the shape of the leg, and the skill of the person applying the bandage.
The ambulatory venous patient is best served by semirigid dressings, such as the Unna boot or by multicomponent system compression wraps. Multicomponent compression has been shown to be more effective than single-component compression; both four-layer and short-stretch bandages have higher healing rates than paste plus an outer support (Unna boot). With the discovery of moist wound healing55 and the advent of hydrocolloid and foam dressings, occlusive dressings may be used under compression wraps to promote growth of granulation tissue, reduce pain from the dressing rubbing against the ulcer, and promote autolytic debridement. One study found that ulcers treated with the foam dressing under the Unna boot healed twice as fast as ulcers treated without the foam.56
Bandages may be made of different materials, including elastic and inelastic materials or both. Stiff bandages are made of multiple layers of elastic or inelastic material. This type of bandage remains rigid and generates high pressure during exercise, which reduces venous hypertension.
Elastic bandages are considered long stretch if they are capable of stretching to double their size. Because these dressings can be stretched too tight, they are not recommended as a primary dressing for compression.57
Inelastic bandages are nonstretch bandages, short-stretch bandages, and paste bandages. The resting pressure under inelastic bandages decreases over a 24-hour period, but the working pressure (with muscle movement) decreases less.
Compression wraps should be applied starting just below the toes and ending just below (two finger breadths) the popliteal fossa. A gauze roll or padded gauze dressing is typically placed over the wound area, and the dressing is covered with an elastic bandage. If the leg is misshapen, padding the leg to create symmetry facilitates a better bandage fit. This will reduce bandage slippage and avoids a tourniquet effect that increases the distal edema. Extra padding around bony prominences reduces the possibility of the compression garment creating a device-related pressure ulcer. The concave area around the lateral and medial malleoli may need extra padding to ensure sufficient pressure is applied to those areas. Training should be ongoing to avoid complications associated with the application of compression wraps, such as ineffective pressure, pressure ulcers, bandage slippage, and limb distortion57 (Fig. 14-8).
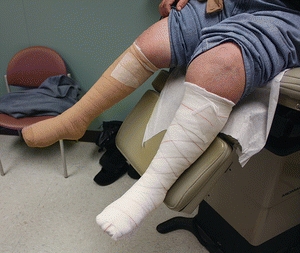
FIGURE 14-8. Compression wrapping. This photograph shows a leg being wrapped with a four-layer compression therapy dressing. (Photo courtesy of J.A. Niezgoda.)
Complications from compression include pain, damage to skin and subcutaneous tissue from pressure, reduction of calf muscle from nonuse, and skin problems. Interventions include carefully assessing to determine the cause of pain; avoiding excessive pressure under the dressing; encouraging exercise, including range of motion; and avoiding topical products that might cause allergies or irritations.57
Stockings reduce AVP by decreasing venous reflux and improving calf muscle ejection capacity during use.58 The benefit derived from stockings is in direct proportion to the fit.
In many cases, patients fail to wear the prescribed compression dressing or stocking because of difficulty donning the stockings or complaints of tightness. The importance of long-term external compression can’t be overemphasized. Patients should be taught that the stockings must be replaced every 3 to 6 months. Two pairs should be purchased so that one can be worn, while the other is laundered.
 Practice Point
Practice Point
Long-term compression therapy is an essential part of the treatment of venous leg ulcers.
A pneumatic compression pump may be used to reduce lower-extremity edema. One study found improved venous ulcer healing when compression pumps were used; however, not all third-party payers agree with the use of these pumps for treatment.59–62 Another study63 found 4 hours of pump compression per day improved ulcer healing when used in conjunction with compression stockings. In yet another study,64 an intermittent pneumatic compression device (PCD) provided improved healing when used for 1 hour twice weekly in conjunction with conventional dressings. Patients who are immobile, are unable to tolerate bandages, have arterial insufficiency, or have problems with edema may benefit from intermittent pneumatic compression.64
Assessment of Treatment
If a venous ulcer does not show signs of healing, the patient should be assessed for other comorbidities. Diabetes, heart failure, arterial insufficiency, venous reflux or history of venous thrombosis, fibrin on the wound surface, superficial colonization of bacteria, or infection all can slow healing.65,66
Exercise
A graded exercise program may be used to improve the calf muscle pump in those patients with abnormalities in pump function. One author64 determined that a structured exercise program to improve muscle function may have a significant positive outcome in patients with venous disease.67
Effective edema reduction from the leg reverses much of the associated comorbidity, particularly the skin changes. The achieved limb reduction must be maintained through the use of appropriately fitted compression hosiery or an alternative compression product. The application and removal of such hosiery can be problematic for individuals with limited hand function or who are unable to see or reach their toes (obese, blind, arthritic, etc.). However, application aids are available and instruction in technique does help. Other strap-type leggings and boot-like devices40 are available to decrease the functional burden. Therapists can be very creative in combining or layering different compressive products to achieve adequate levels of compression. Compliance is crucial to prevent recurrence of edema.
Surgical Treatment for Venous Ulcers
Surgical treatment for venous ulcers is aimed at correcting the cause of the venous hypertension. Patients can have venous reflux without the symptoms of insufficiency. It is when reflux is severe enough that the insufficiency results in dermal venous hypertension and the eventual skin changes with which patients present. Procedures aimed at correcting insufficiency of the deep venous system include vein valve transplantation, direct valve repair, and venovenous bypass. Outflow obstruction of a limb is addressed with venovenous bypass, endovascular intervention, or a combination of the two. Varicose veins, the manifestation of superficial venous insufficiency, generally require ablation. Their treatment is usually by excision, ligation, injection, or the more recent method of endovenous ablation, depending on the size of the vein.
Surgical treatment for venous insufficiency remains far behind the more established treatment of arterial occlusive disease. Venous insufficiency can be grouped under two broad categories:
- venous reflux
- venous outflow obstruction.
The net result of both of these disease entities is venous hypertension and the sequelae resulting in dermal injury that ultimately ends with venous ulcerations. The mainstay for the treatment of venous insufficiency continues to be good external compression. In many patients, this is all that is required. Compression acts both as treatment for various states of venous insufficiency as well as prophylaxis for the development of the adverse sequelae. In some patients, the use of compression alone is inadequate; for these patients, surgical intervention is usually necessary.
Venous outflow obstruction is usually the result of DVT; however, other causes include obstruction from surgical excision of outflow vessels, tumor encasement of venous return, radiation-induced fibrosis, congenital atresia, injection-induced venous destruction (illicit or otherwise), obesity with immobility, and infection. When it involves isolated segments with normal segments proximal and distal, the obstruction can result in a cascading event resulting in venous insufficiency. In some patients, the outflow obstruction is the cause of the symptoms. In these patients, it may be necessary to relieve the venous outflow obstruction and the corresponding venous hypertension by bypassing the obstructed segment, by balloon angioplasty of a sclerotic or stenotic segment with or without stent placement or, if the DVT is not chronic, by either open venous thrombectomy or mechanical thrombectomy.
Endovascular treatment of iliac vein obstruction is now possible on a routine basis. The morphology of this outflow obstruction and the measures to best treat it are ideally evaluated with the use of intravascular ultrasound (IVUS).68 Although there is an early reocclusion rate of around 35% at 3 months, subsequent ulcer recurrence-free rates of almost 60% exist out to 5 years.69 Early thrombosis and in-stent stenosis remain the most common causes of these early failures.
For years, obstruction from DVT has been approached in Europe with open venous thrombectomy, but this technique has never caught on in the United States. Mechanical thrombectomy via percutaneous routes is a focus of current interest. Two devices currently popular are Trellis drug dispersion and thrombectomy catheter (Bacchus, Santa Clara, CA),70,71 and the AngioJet (Possis Medical, Minneapolis, MN).72,73 Each uses a unique method of clot destruction and aspiration combined with lytic therapy. Trellis traps the thrombus between two balloons and emulsifies it with a rheolytic agent and oscillating wire. Once the clot is emulsified, it is aspirated from between the two balloons. The AngioJet uses lytics and a high-velocity jet of liquid to fragment and then rapidly aspirate the thrombus fragments back into the catheter. Both methods are effective, with Trellis being quicker and AngioJet gentler on the vessel’s intima. Whether these devices will have good long-term results is still in debate. Posttreatment patients need to be on warfarin.
Patients who require bypass are best served using an autogenous venous conduit. The proximal and distal anastomoses of the venous bypass are dictated by the obstruction site. For example, if a patient has an isolated iliofemoral thrombosis that has failed to recannulize or has only partially recannulized and the affected leg is symptomatic, a femoral–femoral bypass from the proximal portion of the symptomatic leg to the more distal portion of the contralateral leg can be performed. The saphenous vein is usually used for this, but in contrast to arterial bypasses, the direction of the valve isn’t reversed; rather the valve leaflets are oriented to prevent reflux. Similarly, a bypass from a more proximal vein within a symptomatic leg to the more cephalad iliac vein may be indicated.74
In patients with an outflow obstruction, but in whom insufficiency or hypertension is caused by occlusion of the GSV, the venous hypertension may be alleviated by isolated partial saphenous vein ligation and stripping. This is usually done at the knee level with stripping of the affected saphenous segment. If, however, the reflux or hypertension is the result of the deep venous system, then stripping the GSV wouldn’t help and actually may be detrimental due to elimination of one of the venous outflow tracts of the extremity. This information must be known before a surgical procedure is performed to correct venous insufficiency. Three tests are available to evaluate reflux:
- ascending/descending venography
- duplex imaging
- venous PPG with sequential tourniquet placement.
These tests are all readily available through either an accredited vascular laboratory or a radiology department with experience in venous testing. Venous PPG can determine whether the deep or superficial system is involved with venous reflux. It is an excellent assessment of overall limb reflux and helps assess for perforator incompetence. Venous duplex, which interrogates specific segments of both the superficial and deep veins for reflux, gives a more detailed anatomic evaluation of the affected extremity. Both are needed to properly evaluate a patient with venous disease. Isolated valve segments of the more proximal venous system can be evaluated using descending venography looking for contrast reflux past the incompetent valve. Venography and venous duplex imaging can also determine sites of stenoses within the venous system. One of the problems associated with accurate duplex imaging is that it’s dependent upon the competence of the technician performing the test. Certain laboratories have more expertise in these areas than do others. This notwithstanding, duplex imaging has largely replaced venography in evaluating patients with venous disease. Venography is still used if surgical bypass and/or valvular surgery is planned.
When deep venous insufficiency is due to valvular incompetence, it isn’t known how many competent valves are required and in what locations for the deep venous system to become competent again. Research in these areas is still ongoing as more attention is given to venous insufficiency.75
Three techniques are available for surgical correction of venous insufficiency due to valvular incompetence:
- artificial venous valve insertion
- autogenous vein valve transplantation using a segment of vein, usually from the upper extremities or axilla
- direct valvuloplasty.
Autogenous valve transplantation is a procedure in which a segment of vein with a competent valve, usually from the upper extremity or axilla, is identified. This section of vein is resected and an interposition graft placed at the harvest site. The vein is then transposed into the venous system of the affected extremity, maintaining the orientation of the valve to keep the leaflets open in a cephalic direction. Postoperative anticoagulation with heparin and subsequently Warfarin is commonly used.
Approximately 75% of the stasis ulcers remain healed 12 months after valve transplantation.76 However, considerable degradation occurred over the course of the 2nd year in these patients, such that only 40% of limbs remain healed.76 After the second year, results appear to stabilize without further deterioration although the reports on this are limited in both scope and number.76
Variations of this procedure using valve segments from other areas or even transposing a deep vein with a competent valve with another deep vein with an incompetent valve can be done. In Ko et al.’s study, the overall results with this method appeared to be similar to that of transposition of competent vein valve segments.76
Direct valvuloplasty is another technique for correcting valvular insufficiency. This is performed by suture approximation of two valve leaflets that don’t fully close. It’s done either with direct suturing within the areas of the cusps to obtain good apposition or by external buttressing of an incompetent vein valve sinus. Valve leaflets themselves are brought into apposition by placing the equivalent of a “girdle” around the dilated valve to reduce dilation and allow the valves to come in apposition in a more normal fashion. This sleeve technique is usually done with prosthetic material. Similarly, a transplanted valve that may deteriorate due to dilation can be made competent again by using this technique.77
An external valve repair known as the Psathakis Silastic sling procedure has been developed.78 This procedure involves placing a Silastic sling around the popliteal vein and then attaching it to the two heads of the biceps femoris muscle. When these muscles contract, the sling is intended to occlude the popliteal vein during ambulation. The problem with this procedure is that the sling becomes intimately adherent to the vein and surrounding tissue and over time no longer functions in this fashion.
Two publications on neovalve construction and valvular repair highlight the various techniques employed to restore venous competency of the deep system and their outcomes.79,80 These are technically challenging operations that are not widely available. When successful, ulcer healing rates exceed 88%.
In patients in whom no suitable vein valve segment can be found or it’s deemed an inadequate operation, the development and implantation of a prosthetic valve holds some promise. Currently, a prosthetic venous valve comprised of a complex titanium double-leaflet system is being developed and may hold promise.
The appropriate use of adequate compression is necessary in conjunction with all the surgical treatments. The application of compression hose and management of these patients are addressed elsewhere within this chapter.
Patients with recurrent leg ulcers due to incompetent perforators in the affected area were thought to benefit from a Linton flap. This procedure requires elevation of the skin and fascia at the site of ulceration and transection with ligation of the incompetent perforator veins feeding the area. Proper application of compression is required afterward to reduce local venous hypertension. The morbidity associated with this procedure includes tissue slough along the area of incision and the overlying tissue, resulting in a prolonged healing period. This is thought to be due to the chronic disease state of the tissue at the ankle.
A subfascial ligation of incompetent perforator veins with an endoscope (SEPS) is a significant advancement in the Linton technique. Using equipment developed for laparoscopic cholecystectomies, the scope is passed from the medial infrageniculate area into the subfascial space. The fascia is raised, often with CO2 insufflation. The perforator veins are ligated and transected through the endoscope. Advances in technology, such as smaller-diameter scopes (5 to 10 mm), high-resolution camera and LCD screens, and single-step electrocautery/transection, have made this procedure much easier to perform. The smaller-diameter endoscopes allow easy perimalleolar inspection for perforators. This entire procedure routinely takes less than an hour to perform and in select patients can be done on an outpatient basis. The reason for ligating incompetent perforators is to eliminate the venous hypertension associated with the reflux of venous blood.79 In a meta-analysis by Tenbrook et al., ulcers treated by SEPS with or without additional venous ablation healed in 88% of patients.81,82
There has been some interest in duplex ultrasound–guided foam sclerotherapy, which scleroses the perforator veins to achieve the same effect.83,84 While this treatment is used extensively in Europe, it’s not yet approved in the United States.85 Further studies are needed to establish the overall effectiveness of this procedure for wound healing.
The use of endovenous ablation has really become widespread in the United States, supplanting traditional vein stripping. In most places, endovenous ablation is an outpatient office-based procedure. It involves ultrasound-guided cannulation of the distal saphenous vein, either lesser or greater, with a catheter whose tip is positioned 2 cm distal to the saphenofemoral junction. The length of the vein is surrounded with tumescent anesthesia usually consisting of a dilute buffered lidocaine solution (usually 300 to 500 mL). This acts as a heat trap for the delivered energy, which destroys the vein wall and coagulates the blood within. The energy delivered is either in the form of a laser (endovenous laser therapy, EVLT) or radiofrequency (RF). Proponents of both forms claim superiority. The end result, if successful, is controlled thrombosis and destruction of the vein and thereby prevention of reflux through it. US data show 99.6% successful occlusion initially,86 falling to 86% to 89% at 4 years.87 Devices of both types exist that can be used to directly ablate incompetent perforators under ultrasound guidance. However, neither of these devices is as easy to use as the main units.
RF and EVLT are quick procedures, routinely taking far less than 1 hour to perform. The patients generally do very well, and the procedures are well tolerated. The patient avoids a hospital stay and the need for a major anesthetic. Endovenous ablation has been widely accepted as a tool for the patient seeking removal of mostly asymptomatic varicosities, but when appropriately applied, the procedure can correct isolated saphenous reflux disease. While the procedure has a low complication rate, it has two major drawbacks that open vein stripping does not: It usually leaves behind a distal segment of vein, and recannulation can occur. Some centers have combined endovenous ablation with a subsequent limited distal stripping or microphlebectomy, if needed. Depending on how extensive the condition, either of these procedures could be office based.
In some patients, the application of a split-thickness skin graft to an otherwise healthy stasis ulcer may be appropriate. This technique shouldn’t be used in patients whose underlying venous problems haven’t been addressed. The application of a split-thickness skin graft to an ulcer with persistent venous hypertension will fail, even if the patient is compliant with the use of a compression garment.
Options other than split-thickness grafting involve the use of bioengineered human dermal substitutes. These are intended to be used as carriers of human growth stimulants and to provide a scaffold upon which the patient’s own dermis can regenerate, either spontaneously or with the use of a delayed thin skin graft. Dermal substitutes are not inexpensive, however, and the underlying venous issue as well as the wound bed must still be addressed. Although skin substitutes appear to hasten wound closure, recurrence rates are high.88
Modern venous surgery now has in its armamentarium a wider array of treatment options for those patients with venous disease. As this area of vascular surgery matures, better paradigms for patient treatment are emerging.89 No longer is it just an Unna boot and vein stripping.
Summary
CVI is a permanent condition. Because of this, patients are given information about the disease process and rationale for intervention. The more information they have, the more likely they are to manage the condition effectively. Activities that promote venous return are encouraged. Extremity elevation should become a daily routine, and external compression is needed for life. Patients must understand the importance of this fact. Protection from skin trauma is essential. A small lesion may progress quickly to a large ulcer because of the edema. It may take years to heal, if at all. Small cuts or bruises should receive immediate medical attention. Leg exercises to increase muscle pump activity are taught to the patient. Patients are encouraged to use these exercises during long periods of standing or sitting. When sitting, the legs should be elevated.
Success in managing venous ulcers requires a total patient commitment. Risk factors and ulcer management are dependent upon the patient’s activities; therefore, the patient must have as much information as possible to participate in therapy. An understanding of venous pathophysiology and its contribution to leg ulcers is critical in managing the ulcers. Venous reconstruction is in its infancy but shows promise to reduce the sequelae of postphlebitic syndrome. Venous ulcers always require external compression, ultimately in the form of compression stockings or layered bandaging systems.
Venous ulcers always require external compression, ultimately in the form of compression stockings or layered bandaging systems. While nurses know the importance of compression as part of the treatment of venous ulcers, in a recent study, they may not feel confident in how to apply these 3- or 4-layer bandaging systems.90 In another recent study, nurses may overestimate their ability to apply these bandaging systems.91 Both of these studies underscore the importance of making use that nurses can correctly apply compression.90,91
Ulcers associated with lymphedema usually respond when the edema is reduced. A variety of wound care products are available for leg ulcers, but no research exists showing one product to be more effective than another. Economic concerns make it imperative to choose the appropriate dressings and treatment, but research demonstrates little increased benefit of the newer treatments over the old. Although surgical treatment for venous ulcer lags behind that for arterial occlusive disease, there are procedures that correct the insufficiency of the deep venous system. These are vein valve transplantation, direct valve repair, and venovenous bypass.
 PATIENT SCENARIO
PATIENT SCENARIO
Clinical Data
Mrs. MC is an 82-year-old woman who has had multiple recurrent superficial ulcers in the medial aspect of the left ankle region, presumably resulting from venous insufficiency. Some ulcers healed spontaneously, but more recently, they required compression therapy at a wound center. The current episode was indolent for more than 8 months and has left Mrs. MC with macerated skin that cracks and weeps serous fluid (Fig. 14-9A). She recently moved in with her daughter but is quite independent. Although Mrs. MC is thin, her nutrition is adequate and she is compliant with leg elevation. She has worn proper compression hose in the past. She was referred to the Vascular Surgery Service for evaluation.
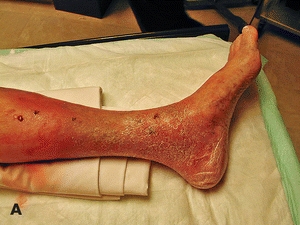
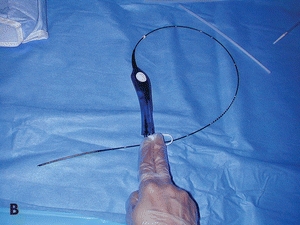
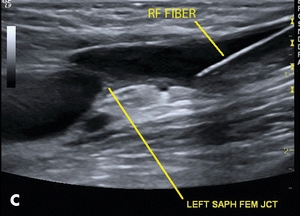
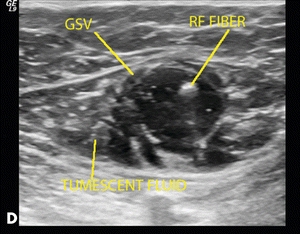
Figure 14-9. (A) Macerated skin due to multiple recurrent superficial ulcers in the medial aspect of the left ankle region. (B) Radiofrequency fiber. (C) Placement of the radiofrequency fiber approximately 2 cm distal to the junction of the common femoral vein. (D) Tumescent fluid infused around the GSV.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


