Macrolides, Tetracyclines, Aminoglycosides, and Fluoroquinolones
Objectives
• Describe the pharmacokinetics and pharmacodynamics of erythromycin.
• Apply the nursing process for tetracyclines, including patient teaching.
• Explain the importance for ordering peak and trough concentration levels for aminoglycosides.
• Develop a teaching plan for a patient prescribed a fluoroquinolone (quinolone).
Key Terms
bactericidal, p. 414
bacteriostatic, p. 414
hepatotoxicity, p. 415
nephrotoxicity, p. 419
ototoxicity, p. 418
pathogen, p. 414
photosensitivity, p. 419
superinfection, p. 419
The groups of antibacterials discussed in this chapter include macrolides (erythromycin, clarithromycin, azithromycin, and dirithromycin), lincosamides, glycopeptides, ketolides, tetracyclines, glycylcyclines, aminoglycosides, fluoroquinolones (quinolones), and lipopeptides. The macrolides, lincosamides, and tetracyclines are primarily bacteriostatic (inhibit bacterial growth) drugs and may be bactericidal (kill bacteria), depending on the drug dose or pathogen (microorganism capable of producing disease). Glycopeptides, aminoglycosides, and fluoroquinolones are bactericidal drugs.
Macrolides, Lincosamides, Glycopeptides, and Ketolides
These four groups of drugs are discussed together, because they have spectrums of antibiotic effectiveness similar to penicillin, although they differ in structure. Drugs from these groups are used as penicillin substitutes, especially in individuals who are allergic to penicillin. Erythromycin is the drug frequently prescribed if the patient has a hypersensitivity to penicillin.
Macrolides
Macrolides, including azithromycin (Zithromax), clarithromycin (Biaxin), and erythromycin (E-Mycin), are called broad-spectrum antibiotics. Erythromycin, the first macrolide, was derived from the funguslike bacteria Streptomyces erythreus and was first introduced in the early 1950s. Macrolides bind to the 50S ribosomal subunits and inhibit protein synthesis. At low to moderate drug doses, macrolides have a bacteriostatic effect, and with high drug doses, their effect is bactericidal. Macrolides can be administered orally or intravenously (IV) but not intramuscularly (IM), because it is too painful. Administration of IV macrolides should be infused slowly to avoid unnecessary pain (phlebitis).
Gastric acid destroys erythromycin in the stomach; therefore, acid-resistant salts are added to erythromycin (e.g., ethylsuccinate, stearate, estolate) to decrease dissolution (breakdown in small particles) in the stomach. This allows the drug to be absorbed in the intestine. Normally, food does not hamper the absorption of acid-resistant macrolides. Table 30-1 lists the dosages, uses, and considerations of macrolides.
TABLE 30-1
ANTIBACTERIALS: MACROLIDES, LINCOSAMIDES, GLYCOPEPTIDES, KETOLIDES, AND LIPOPEPTIDES
| GENERIC (BRAND) | ROUTE AND DOSAGE | USES AND CONSIDERATIONS |
| Macrolides | ||
| azithromycin (Zithromax) | See Prototype Drug Chart 30-1. | |
| clarithromycin (Biaxin) | A: PO: 250-500 mg q12h × 7-14 d or XL 1 g/d; max: 1 g/d C: PO: 7.5 mg/kg b.i.d.; max: 15 mg/kg/d | For upper and lower respiratory tract infections, skin and soft-tissue infections, Helicobacter pylori and mycobacterial species, and gram-positive and gram-negative organisms. Report persistent diarrhea. Pregnancy category: C; PB: 65%-75%; 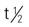 : 3-6 h : 3-6 h |
| erythromycin base (E-Mycin); erythromycin ethylsuccinate (E.E.S., Pediamycin) erythromycin stearate (Erythrocin Stearate) | A: PO: 250-500 mg q6h; max: 4 g/d C: PO: 20-50 mg/kg q6h; max: 2 g/d | For moderate to severe infections (e.g., pneumococcal pneumonia, acute pelvic inflammatory disease, intestinal amebiasis, Legionnaires’ disease, chlamydial infections). Report persistent diarrhea. Pregnancy category: B; PB: 73%-81%; 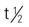 : PO: 1-2 h; IV: 3-5 h : PO: 1-2 h; IV: 3-5 h |
| erythromycin IV (Erythrocin lactobionate) | A/C/older adults: IV: 15-20 mg/kg/d in 4 divided doses; max: 4 g/d | For IV administration. Drug should not be dissolved in solution that contains preservative. Same as erythromycin base. |
| Fidaxomicin (Dificid) | A: PO: 200 mg b.i.d. for 10 d; max: 400 mg/d | For treatment of Clostridium difficile–associated diarrhea. May cause GI bleeding, GI obstruction, and elevated liver enzymes. Pregnancy category: B; PB: UK; 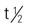 : UK : UK |
| Lincosamides | ||
| clindamycin HCl (Cleocin) clindamycin palmitate (Cleocin Pediatric) clindamycin phosphate (Cleocin Phosphate) | A: PO: 150-600 mg q6-8h; max: 2700 mg/d C: PO: 8-20 mg/kg/d in 3-4 divided doses; max: 20 mg/kg/d A: IM/IV: 600-1200 mg/d in divided doses; max: 2700 mg/d po, 4800 mg/d IV for severe infections C: IM/IV: 20-40 mg/kg/d in 3-4 divided doses; max: 40 mg/kg/d | For serious infections. Available in capsule form. Should be taken with full glass of water. Not affected by food. May cause fatal colitis. Pregnancy category: B; PB: 92%-94%; 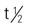 : 2-3 h : 2-3 h |
| lincomycin (Lincocin) | A: IM: 600 mg q12-24h IV: 600 mg-1 g q8-12h; max: 8 g/d C: IM: 10 mg/kg q12-24h IV: 10-20 mg/kg/d q8-12h | In most situations, this drug has been replaced by clindamycin. May cause fatal colitis. When IV, dilute in 100 mL of IV fluids. Pregnancy category: B; PB: 72%; 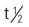 : 4-6 h : 4-6 h |
| Glycopeptides | ||
| vancomycin HCl (Vancocin) | A: IV: 500 mg q6h or 1 g q12h C: IV: 40 mg/kg/d in 4 divided doses; dilute in IV fluids; infuse over 60-90 min | For S. aureus–resistant infections and cardiac surgical prophylaxis in patients with penicillin allergy. Adverse reactions include vascular collapse, ototoxicity, nephrotoxicity, and red neck syndrome. Pregnancy category: B (oral)/C (IV); PB: 55%; 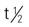 : 4-8 h : 4-8 h |
| telavancin (Vibativ) | A: IV: 10 mg/kg/d over 60 min for 7-14 d; max: 10 mg/kg/d | For treating complicated skin and skin structure infections, as well as MRSA. Adverse reactions include hearing loss, red neck syndrome, and blood dyscrasias. Pregnancy category: C; PB: UK; 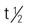 : 4-8 h : 4-8 h |
| Ketolides | ||
| telithromycin (Ketek) | A: PO: 800 mg/d for 7-10 d | For treating community-acquired pneumonia. May cause serious hepatotoxicity and worsen myasthenia gravis. Pregnancy category: C; PB: 60%-70%; 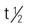 : 10 h : 10 h |
| Lipopeptides | ||
| daptomycin (Cubicin) | A: IV: 4-6 mg/kg/d | To treat complicated skin and skin structure infections. Effective against a broad spectrum of gram-positive bacteria and S. aureus–resistant infections. Pregnancy category: B; PB: 92%; 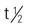 : 8 h : 8 h |
Macrolides are active against most gram-positive bacteria and moderately active against some gram-negative bacteria. Resistant organisms may emerge during treatment. Macrolides are used to treat mild to moderate infections of the respiratory tract, sinuses, gastrointestinal (GI) tract, skin and soft tissue, and diphtheria, impetigo contagiosa, and sexually transmitted infections (STIs).
Erythromycin is the drug of choice for the treatment of mycoplasmal pneumonia and Legionnaire’s disease. Biaxin XL is clarithromycin in a once-a-day extended-release tablet to be taken for 7 days. Azithromycin is frequently prescribed for upper and lower respiratory infections, STIs, and uncomplicated skin infections. Table 30-1 lists the drugs developed from the derivatives of erythromycin. Prototype Drug Chart 30-1 details the pharmacologic behavior of azithromycin.
Pharmacokinetics
Clarithromycin and erythromycin are readily absorbed from the GI tract, mainly by the duodenum. Azithromycin is incompletely absorbed from the GI tract, and only 37% reaches systemic circulation. Azithromycin and erythromycin are available IV, but intermittent infusions should be diluted in normal saline (NS) or in 5% dextrose in water (D5W) to prevent phlebitis or burning sensations at the injection site. Azithromycin 500 mg should be diluted in 250 to 500 mL of fluid, and erythromycin lactobionate 1 g should be diluted in 200 to 1000 mL. Macrolides are excreted in bile, feces, and urine. Because only a small amount is excreted in urine, renal insufficiency is not a contraindication for macrolide use.
Pharmacodynamics
Macrolides suppress bacterial protein synthesis. The onset of action of oral preparations of erythromycin is 1 hour, peak concentration time is 4 hours, and duration of action is 6 hours. Newer macrolides have a longer half-life and are administered less frequently. Clarithromycin (Biaxin) is administered twice a day, and Biaxin XL is administered once a day. Azithromycin (Zithromax) has up to a 40- to 68-hour half-life and is prescribed to be taken only once a day for 5 days.
Side Effects and Adverse Reactions
Side effects and adverse reactions to macrolides include GI disturbances such as nausea, vomiting, diarrhea, and abdominal cramping. Conjunctivitis may develop as a side effect of azithromycin. The patient should avoid wearing contact lenses if this occurs. Allergic reactions to erythromycin are rare. Hepatotoxicity (liver toxicity) can occur when erythromycin and azithromycin are taken in high doses with other hepatotoxic drugs, such as acetaminophen (high doses), phenothiazines, and sulfonamides. Liver damage is usually reversible when the drug is discontinued. Erythromycin should not be taken with clindamycin or lincomycin because they compete for receptor sites.
Drug Interactions
Macrolides can increase serum levels of theophylline (bronchodilator), carbamazepine (anticonvulsant), and warfarin (anticoagulant). If these drugs are given with macrolides, their drug serum levels should be closely monitored. To avoid severe toxic effects, erythromycin should not be used with other macrolides. Antacids may reduce zithromycin peak levels when taken at the same time.
Extended Macrolide Group
Derivatives of erythromycin have been effective in the treatment of numerous organisms. Like erythromycin, they inhibit protein synthesis. Many of these macrolides have a longer half-life and are administered once a day. The first extended macrolide group developed after the introduction of erythromycin was clarithromycin (Biaxin), which has been effective against many bacterial infections. Clarithromycin is administered twice a day and the XL formulation is given once a day. Another extended macrolide is azithromycin (Zithromax). This drug has a long half-life of up to 40 to 68 hours; therefore it is only prescribed once a day for 5 days.
Elimination of these drugs is via bile and feces. Azithromycin is frequently prescribed for upper and lower respiratory tract infections, STIs, and uncomplicated skin infections.
When erythromycin is given concurrently with verapamil (Calan), diltiazem (Cardizem), clarithromycin (Biaxin), fluconazole (Diflucan), ketoconazole (Nizoral), and itraconazole (Sporanox), erythromycin blood concentration and the risk of sudden cardiac death increase. Table 30-1 lists the drugs developed from the derivatives of erythromycin.
Common side effects of clarithromycin are nausea, diarrhea, and abdominal discomfort. With azithromycin, the side effects of nausea, diarrhea, and abdominal pain are common.
Lincosamides
Like erythromycin, lincosamides inhibit bacterial protein synthesis and have both bacteriostatic and bactericidal actions, depending on drug dosage. Clindamycin (Cleocin) and lincomycin (Lincocin) are examples of lincosamides. Clindamycin is more widely prescribed than lincomycin, because it is active against most gram-positive organisms, including Staphylococcus aureus and anaerobic organisms. It is not effective against gram-negative bacteria (e.g., Escherichia coli, Proteus, Pseudomonas). Clindamycin is absorbed better than lincomycin through the GI tract and maintains a higher serum drug concentration. Clindamycin is considered more effective than lincomycin and has fewer toxic effects. Table 30-1 lists the lincosamides.
Side Effects and Adverse Reactions
Side effects and adverse reactions to clindamycin and lincomycin include GI irritation, which may manifest as nausea, vomiting, and stomatitis. Rash may also occur. Severe adverse reactions include colitis and anaphylactic shock.
Drug Interactions
Clindamycin and lincomycin are incompatible with aminophylline, phenytoin (Dilantin), barbiturates, and ampicillin.
Glycopeptides
Vancomycin (Vancocin), a glycopeptide bactericidal antibiotic, was widely used in the 1950s to treat staphylococcal infections. Vancomycin is used against drug-resistant S. aureus and in cardiac surgical prophylaxis for individuals with penicillin allergies. Serum vancomycin levels should be monitored.
Vancomycin has become ineffective for treating enterococci. Quinupristin/dalfopristin is a combined antibacterial used to treat life-threatening vancomycin-resistant enterococci (VREF) infections. Antibiotic-resistant enterococci can cause staphylococcal endocarditis.
Telavancin (Vibativ), a glycopeptide, treats selected gram-positive bacteria and skin infections. This drug is a semisynthetic derivative of vancomycin, with bactericidal action against MRSA. Telavancin has an advantage of once-daily dosing.
Pharmacokinetics
Vancomycin is given orally for treatment of staphylococcal enterocolitis and antibiotic-associated pseudomembranous colitis due to Clostridium difficile. When vancomycin is given orally, it is not absorbed systemically and is excreted in the feces. Vancomycin is given IV for severe infections due to MRSA; septicemia; and bone, skin, and lower respiratory tract infections that do not respond or are resistant to other antibiotics. Intermittent vancomycin doses should be diluted in 100 mL for 500 mg and 200 mL for 1 g of D5W, NS, or LR and administered at a rate of 10 mg/min or a minimum of 60 minutes. Vancomycin is excreted in the urine when given IV. It is 30% protein-bound, and the half-life is 6 hours.
Pharmacodynamics
Vancomycin inhibits bacterial cell wall synthesis and is active against several gram-positive microorganisms. The peak action is 30 minutes after the end of the infusion.
Side Effects and Adverse Reactions
Vancomycin may cause nephrotoxicity and ototoxicity. Ototoxicity results in damage to the auditory or vestibular branch of cranial nerve VIII. Such damage can result in permanent hearing loss (auditory branch) or temporary or permanent loss of balance (vestibular branch). Side effects may include chills, dizziness, fever, rashes, nausea, vomiting, and thrombophlebitis at the injection site. Too rapid an IV injection of vancomycin can cause a condition known as “red man” syndrome or red neck syndrome. Characterized by red blotching of the face, neck, and chest, this is a toxic effect rather than an allergic reaction. Other adverse effects include eosinophilia, neutropenia, and Stevens-Johnson syndrome. Severe hypotension, tachycardia, generalized tingling, and, rarely, cardiac arrest are also adverse reactions.
Drug Interactions
Dimenhydrinate (Dramamine) can mask ototoxicity when taken with vancomycin. The risk of nephrotoxicity and ototoxicity may be potentiated when vancomycin is taken with furosemide, aminoglycosides, amphotericin B, colistin, cisplatin, and cyclosporine. Vancomycin may inhibit methotrexate excretion and increase methotrexate toxicity. The absorption of oral vancomycin may be decreased when given with cholestyramine and colestipol.
Ketolides
Ketolides are structurally related to macrolides. The first drug in this class is telithromycin (Ketek), which is used for adults 18 years of age and older to treat mild to moderate community-acquired pneumonia. This disorder is usually caused by Streptococcus pneumoniae and Haemophilus influenza.
Pharmacokinetics
Telithromycin is given orally and is well absorbed by the GI tract and not affected by food intake. Telithromycin is excreted in the feces and urine. It is 60% to 70% protein bound, and the half-life is 10 hours.
Pharmacodynamics
Telithromycin inhibits protein synthesis in microorganisms by binding to the bacterial ribosomal RNA site of the 50S subunit, resulting in bacterial cell death. The peak action is 1 hour.
Side Effects and Adverse Reactions
Side effects and adverse reactions to telithromycin include visual disturbances (blurred vision and diplopia), headache, dizziness, altered taste, nausea, vomiting, diarrhea, and liver failure. Telithromycin may also lead to an exacerbation of myasthenia gravis.
Drug Interactions
Telithromycin levels are increased when taken concurrently with antilipidemics (simvastatin, lovastatin, and atorvastatin), itraconazole, ketoconazole, and benzodiazepines. Class 1A or class III antidysrhythmics may lead to life-threatening dysrhythmias. Blood levels of telithromycin are decreased when taken with rifampin, phenytoin, carbamazepine, or phenobarbital, producing a subtherapeutic level. Telithromycin can increase levels of cisapride and pimozide, leading to toxicity; therefore, these two drugs are contraindicated for the patient taking telithromycin. Digoxin, metoprolol, midazolam, ritonavir, sirolimus, and tacrolimus levels are increased when taken concurrently with telithromycin. Concurrent use of telithromycin with ergot alkaloid derivatives leads to ergot toxicity (severe peripheral vasospasm and impaired sensation).
Tetracyclines
Tetracyclines, isolated from Streptomyces aureofaciens in 1948, were the first broad-spectrum antibiotics effective against gram-positive and gram-negative bacteria and many other organisms—mycobacteria, rickettsiae, spirochetes, and chlamydiae, to name a few. Tetracyclines act by inhibiting bacterial protein synthesis and have a bacteriostatic effect.
Tetracyclines are not effective against S. aureus (except for the newer tetracyclines), Pseudomonas, or Proteus. They can be used against Mycoplasma pneumoniae. Tetracycline in combination with metronidazole and bismuth subsalicylate is useful in treating Helicobacter pylori, a bacterium in the stomach that can cause a peptic ulcer. For years, oral and topical tetracycline has been used to treat severe acne vulgaris. Low doses are usually prescribed to minimize the toxic effect of the drug.
Continuous use of tetracyclines has resulted in bacterial resistance to the drugs. Tetracycline resistance has increased in the treatment of pneumococcal and gonococcal infections; therefore tetracyclines are not as useful in treating these infections.
The tetracyclines are frequently prescribed for oral use, although they are also available for IM and IV use (Prototype Drug Chart 30-2). Because IM administration of tetracycline causes pain on injection and tissue irritation, this route of administration is seldom used. The IV route is used to treat severe infections. The newer oral preparations of tetracyclines (i.e., doxycycline, minocycline) are more rapidly and completely absorbed. Tetracyclines should not be taken with magnesium and aluminum antacid preparations, milk products containing calcium, or iron-containing drugs, because these substances bind with tetracycline and prevent absorption of the drug. It is suggested that tetracyclines, except for doxycycline and minocycline, be taken on an empty stomach 1 hour before or 2 hours after mealtime. The absorption of doxycycline and minocycline is improved with food ingestion. Table 30-2 describes the tetracycline preparations and their dosages, uses, and considerations. The tetracyclines are listed according to short-acting, intermediate-acting, and long-acting.

 , half-life; UK, unknown; y, year.
, half-life; UK, unknown; y, year. : 68 h
: 68 h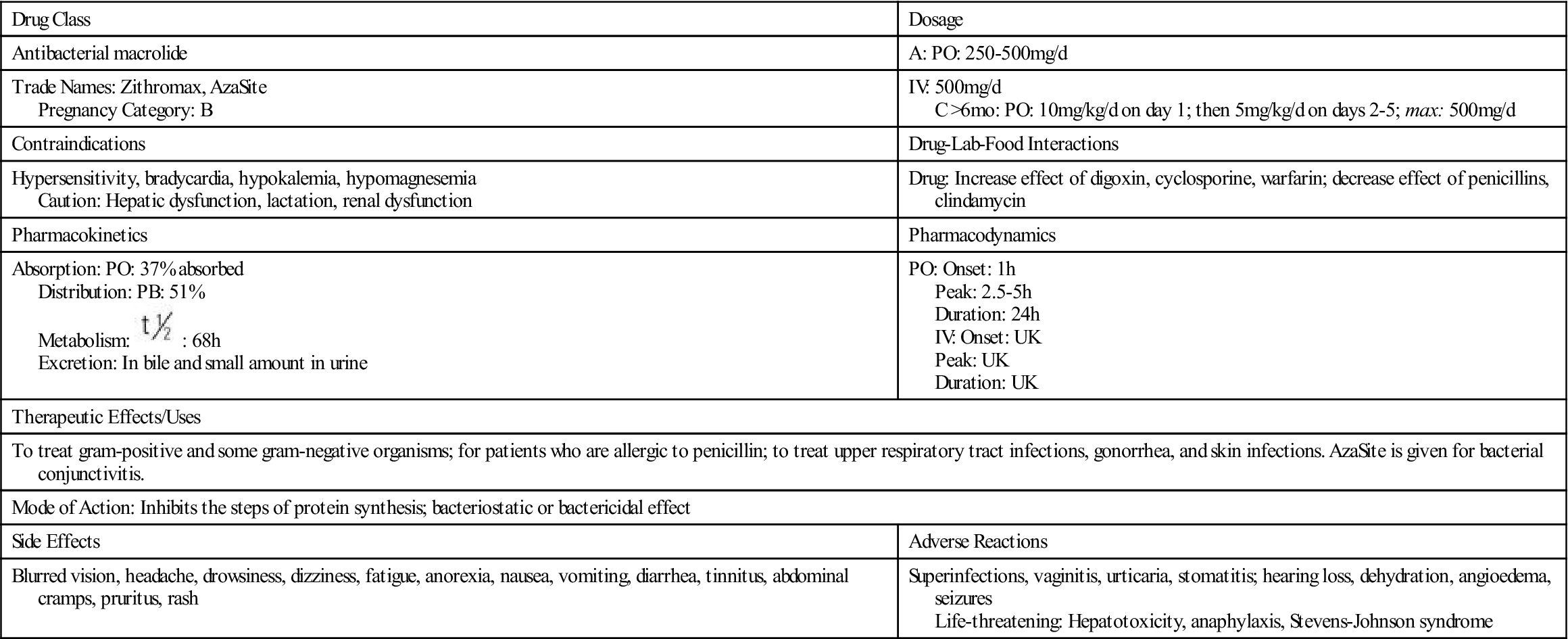
 , half-life; UK, unknown.
, half-life; UK, unknown.

















 : 14-24 h
: 14-24 h

