Drugs for Neuromuscular Disorders
Myasthenia Gravis, Multiple Sclerosis, and Muscle Spasms
Objectives
• Contrast the pathophysiology of myasthenia gravis and multiple sclerosis.
• Discuss the drug group used to treat myasthenia gravis.
• Compare the treatment strategies for the three phases of multiple sclerosis.
• Differentiate between the muscle relaxants used for spasticity and those used for muscle spasms.
• Apply the nursing process to drugs used to treat myasthenia gravis and muscle spasms.
Key Terms
acetylcholinesterase inhibitor, p. 327
cholinergic crisis, p. 327
fasciculations, p. 327
miosis, p. 327
multiple sclerosis, p. 326
muscle relaxants, p. 327
muscle spasms, p. 326
myasthenic crisis, p. 327
myasthenia gravis, p. 326
The authors gratefully acknowledge the work of Marilyn Herbert-Ashton, who updated this chapter for the eighth edition.
Myasthenia gravis (MG), a lack of nerve impulses and muscle responses at the myoneural (nerves in muscle endings) junction, causes fatigue and muscular weakness of the respiratory system, facial muscles, and extremities. Because of cranial nerve involvement, ptosis (drooping eyelid) and difficulty in chewing and swallowing occur. Respiratory arrest may result from respiratory muscle paralysis. The symptoms of MG are caused by autoimmune destruction of acetylcholine (ACh) receptor sites and a resultant decrease in neuromuscular transmission.
The neuromuscular disorder multiple sclerosis (MS) attacks the myelin sheath of nerve fibers, causing lesions known as plaques. While there are no definitive diagnostic tests, the sclerotic plaques are usually detected and measured by magnetic resonance imaging (MRI). Pharmacologic treatment is necessary to control the symptoms of this disorder.
Muscle spasms have various causes, including injury or motor neuron disorders that lead to conditions such as cerebral palsy, MS, spinal cord injuries (paraplegia [paralysis of the legs]), cerebral vascular accident (stroke), or hemiplegia (paralysis of one side of the body). Spasticity of muscles can be reduced with the use of skeletal muscle relaxants.
Myasthenia Gravis
MG is a chronic autoimmune neuromuscular disease that affects approximately 14 in 100,000 persons. It can occur at any age; however, MG occurs more commonly in women younger than 30 years and in men older than 50 years. It is not a genetic disorder, but there can be a familial tendency.
Pathophysiology
MG results from a lack of ACh receptor sites. This autoimmune disorder involves an antibody response against an alpha subunit of the acetylcholine receptor (AChR) site at the neuromuscular junction. Antibodies attack AChR sites, obstructing the binding of ACh and eventually destroying receptor sites. When AChR sites are reduced, ACh molecules are prevented from binding to receptors and stimulating normal neuromuscular transmission. The result is ineffective muscle contraction and muscle weakness. About 90% of patients with MG have anti-acetylcholine antibodies that can be detected through serum testing.
The thymus gland is involved in systemic immunity that is active during infancy and early childhood, but the gland normally shrinks during adulthood. Approximately 60% of MG patients have thymic hyperplasia. It has been suggested in some cases that if the thymus gland is removed during the early onset of MG, clinical symptoms are greatly decreased. Thymectomy has been an option for patients younger than 50 years.
MG is characterized primarily by weakness and fatigue of the skeletal (voluntary) muscles. Other characteristics of MG include dysphagia (difficulty chewing and swallowing), dysarthria (slurred speech), and respiratory muscle weakness. Early symptoms of MG are ptosis (drooping eyelids) and diplopia (double vision).
The group of drugs used to control MG is the AChE inhibitors, also called cholinesterase inhibitors and anticholinesterase; they inhibit the action of the enzyme. As a result of this action, more acetylcholine is available to activate the cholinergic receptors and promote muscle contraction. The AChE inhibitors are classified as parasympathomimetics.
When muscular weakness of the patient with MG becomes generalized, myasthenic crisis may occur. This complication is a severe generalized muscle weakness and may involve the muscles of respiration, such as the diaphragm and intercostal muscles. Triggers of myasthenic crisis include inadequate dosing of AChE inhibitors, infection, emotional stress, menses, pregnancy, surgery, trauma, hypokalemia, temperature extremes, and alcohol intake. Myasthenic crisis can also occur three to four hours after taking certain medications (e.g., aminoglycoside and fluoroquinolone antibiotics, calcium channel blockers, phenytoin [Dilantin], and psychotropics). If muscle weakness remains untreated, death could result from paralysis of the respiratory muscles. Neostigmine (Prostigmin), a fast-acting AChE inhibitor, can relieve myasthenia crisis.
Overdosing with AChE inhibitors may cause another complication of MG called cholinergic crisis, which is an acute exacerbation of symptoms. A cholinergic crisis usually occurs within 30 to 60 minutes after taking anticholinergic medications. This complication is due to continuous depolarization of postsynaptic membranes that create neuromuscular blockade. The patient with cholinergic crisis often has severe muscle weakness that can lead to respiratory paralysis and arrest. Accompanying symptoms include miosis (abnormal pupil constriction), pallor, sweating, vertigo, excess salivation, nausea, vomiting, abdominal cramping, diarrhea, bradycardia, and fasciculations (involuntary muscle twitching).
Acetylcholinesterase Inhibitors/Cholinesterase Inhibitors
The first drug used to manage MG was neostigmine. It is a short-acting acetylcholinesterase (AChE) inhibitor with a half-life of 0.5 to 1 hour. The drug is given every 2 to 4 hours and must be given on time to prevent muscle weakness. The AChE inhibitor pyridostigmine bromide (Mestinon) has an intermediate action and is given every 3 to 6 hours. Ambenonium chloride (Mytelase) is a long-acting AChE inhibitor and is usually prescribed when the patient does not respond to neostigmine or pyridostigmine. Prototype Drug Chart 24-1 presents drug data related to pyridostigmine. Table 24-1 lists the AChE inhibitors. Cholinesterase inhibitors are discussed in Chapter 23.
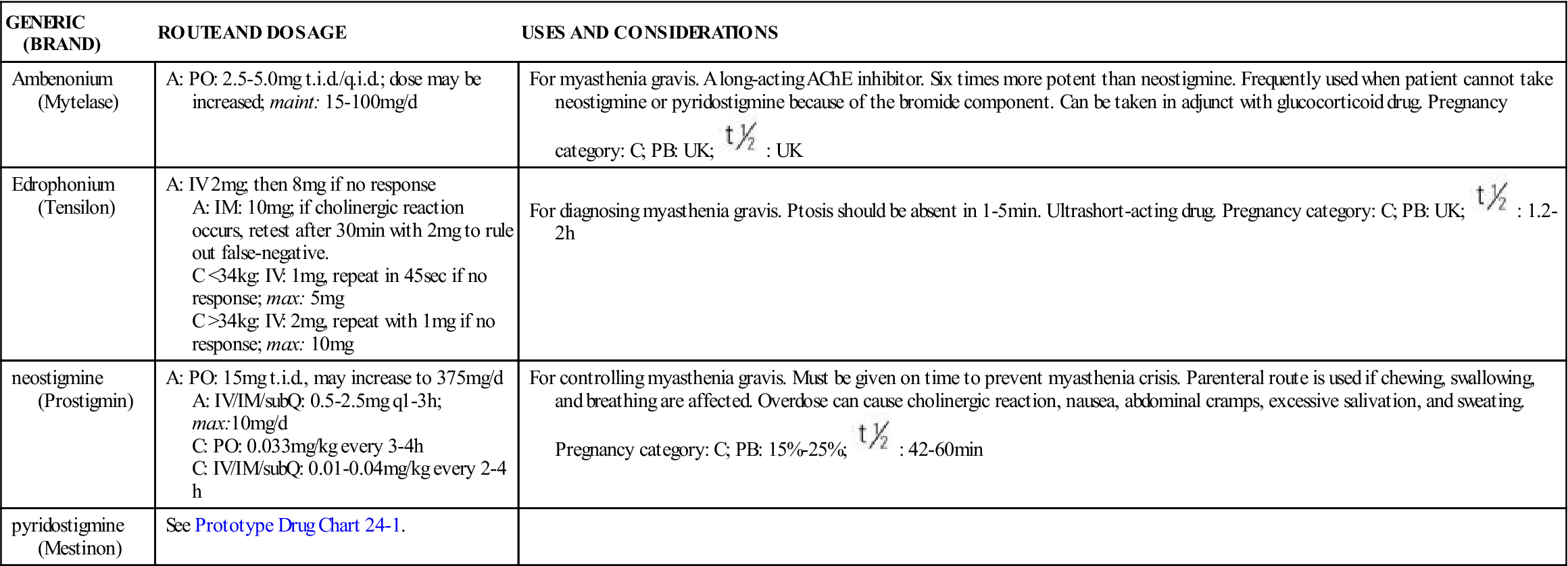
TABLE 24-1
ACETYLCHOLINESTERASE (AChE) INHIBITORS: MYASTHENIA GRAVIS
| GENERIC (BRAND) | ROUTE AND DOSAGE | USES AND CONSIDERATIONS |
| Ambenonium (Mytelase) | A: PO: 2.5-5.0 mg t.i.d./q.i.d.; dose may be increased; maint: 15-100 mg/d | For myasthenia gravis. A long-acting AChE inhibitor. Six times more potent than neostigmine. Frequently used when patient cannot take neostigmine or pyridostigmine because of the bromide component. Can be taken in adjunct with glucocorticoid drug. Pregnancy category: C; PB: UK; 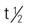 : UK : UK |
| Edrophonium (Tensilon) | A: IV 2 mg; then 8 mg if no response A: IM: 10 mg; if cholinergic reaction occurs, retest after 30 min with 2 mg to rule out false-negative. C <34 kg: IV: 1 mg, repeat in 45 sec if no response; max: 5 mg C >34 kg: IV: 2 mg, repeat with 1 mg if no response; max: 10 mg | For diagnosing myasthenia gravis. Ptosis should be absent in 1-5 min. Ultrashort-acting drug. Pregnancy category: C; PB: UK; 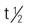 : 1.2-2 h : 1.2-2 h |
| neostigmine (Prostigmin) | A: PO: 15 mg t.i.d., may increase to 375 mg/d A: IV/IM/subQ: 0.5-2.5 mg q1-3h; max:10 mg/d C: PO: 0.033 mg/kg every 3-4 h C: IV/IM/subQ: 0.01-0.04 mg/kg every 2-4 h | For controlling myasthenia gravis. Must be given on time to prevent myasthenia crisis. Parenteral route is used if chewing, swallowing, and breathing are affected. Overdose can cause cholinergic reaction, nausea, abdominal cramps, excessive salivation, and sweating. Pregnancy category: C; PB: 15%-25%; 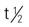 : 42-60 min : 42-60 min |
| pyridostigmine (Mestinon) | See Prototype Drug Chart 24-1. |
Pharmacokinetics
Pyridostigmine is poorly absorbed from the GI tract. Half of the sustained-release capsule is absorbed readily, but the balance is poorly absorbed. The duration of oral pyridostigmine is 3.5 to 4 hours; when given intravenously (IV), it is 2 hours. Because of its short half-life, pyridostigmine must be administered several times a day. The drug is metabolized by the liver and excreted in the urine.
Pharmacodynamics
Pyridostigmine increases muscle strength of patients with muscular weakness resulting from MG. The onset of action of oral preparations is 0.5 to 1 hour. The duration of action is longer with the sustained-release drug capsule. One thirtieth of the oral dose of pyridostigmine can be administered IV. Overdosing of pyridostigmine can result in signs and symptoms of cholinergic crisis (i.e., extreme muscle weakness; increased salivation, tears, sweating; miosis); the antidote atropine sulfate should be available. This crisis requires emergency medical intervention because of respiratory muscle weakness.
Patients who do not respond to AChE inhibitors may require prednisone, plasma exchange, intravenous immune globulin (IVIG), or immunosuppressive drugs. Prednisone decreases MG symptoms and promotes remission, but long-term use can cause adverse dermatologic effects.
The immunosuppressive agent azathioprine (Imuran) can be used in conjunction with a lower dose of prednisone. With azathioprine, the white blood cell (WBC) count and liver enzymes should be closely monitored to avoid leukopenia and hepatotoxicity.
Overdosing and underdosing of AChE inhibitors have similar symptoms: muscle weakness, dyspnea (difficulty breathing), and dysphagia (difficulty swallowing). Additional symptoms that may be present with overdosing are increased salivation (drooling), bradycardia, abdominal cramping, and increased tearing and sweating. All doses of AChE inhibitors should be administered on time, because late administration of the drug could result in muscle weakness.
Underdosing can result in myasthenia crisis, and overdosing can result in cholinergic crisis. Edrophonium chloride (Tensilon) is an ultra–short-acting AChE inhibitor that may be used to distinguish between myasthenia crisis and cholinergic crisis. These two different crises have a similar major symptom: severe muscle weakness. After edrophonium is administered, if the symptoms are alleviated because of an increase in ACh, the cause is myasthenia crisis. However, if the muscle weakness becomes more severe, the cause is cholinergic crisis due to drug overdosing.
Edrophonium may also be used to diagnose MG. Its ultra-short duration of 5 to 20 minutes increases muscle strength immediately. If ptosis is immediately corrected after administration of this drug, the diagnosis is most likely MG.
Side Effects and Adverse Reactions
Side effects and adverse reactions of AChE inhibitors include GI disturbances (nausea, vomiting, diarrhea, abdominal cramps) and increased salivation and tearing. Other side effects include miosis (constricted pupil of the eye), blurred vision, bradycardia, and hypotension.
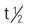 : PO: 3.5-4 h; IV: 2 h
: PO: 3.5-4 h; IV: 2 h
 , half-life; t.i.d., three times a day; UK, unknown.
, half-life; t.i.d., three times a day; UK, unknown. , half-life; t.i.d., three times a day; UK, unknown; <, less than; >, greater than.
, half-life; t.i.d., three times a day; UK, unknown; <, less than; >, greater than.





