Fluid and Electrolyte Replacement
Objectives
• Describe osmolality and tonicity.
• Discuss normal range for serum osmolality and tonicity of intravenous solutions.
• Describe the classifications of intravenous fluids.
• Discuss the functions of major electrolytes.
• Differentiate between major intracellular and extracellular electrolytes.
• Explain the difference between cations and anions.
• Explain the methods used to correct potassium, calcium, and magnesium excess.
Key Terms
anion, p. 224
cation, p. 224
colloid, p. 227
crystalloid, p. 225
electrolytes, p. 224
hypercalcemia, p. 236
hyperchloremia, p. 241
hyperkalemia, p. 231
hypermagnesemia, p. 239
hypernatremia, p. 234
hyperosmolar, p. 225
hyperphosphatemia, p. 241
hypertonic, p. 225
hypocalcemia, p. 236
hypochloremia, p. 241
hypokalemia, p. 230
hypomagnesemia, p. 239
hyponatremia, p. 234
hypo-osmolar, p. 225
hypophosphatemia, p. 241
hypotonic, p. 225
iso-osmolar, p. 225
isotonic, p. 225
osmole, p. 224
osmolality, p. 224
osmolarity, p. 224
tonicity, p. 225
![]() http://evolve.elsevier.com/KeeHayes/pharmacology/
http://evolve.elsevier.com/KeeHayes/pharmacology/
The authors gratefully acknowledge the work of Jane F. Marek, who updated this chapter for the eighth edition.
Fluid and electrolyte balance is necessary to maintain homeostasis. Intake must equal output to maintain fluid balance, and the body constantly strives for equilibrium. The kidneys, with the respiratory, endocrine, gastrointestinal (GI), and integumentary systems, regulate fluid and electrolyte balance.
The total body water of a 70-kg man is approximately 60% (40 L). This percentage varies with age, gender, and percentage of body fat. Neonates have 70% to 80% water, whereas older adults have 45% to 55% water. Women tend to have less body water than men due to effects of hormones and to higher amount of adipose tissue, which contains very little water. Total body water is contained in two major compartments: the intracellular fluid (ICF) compartment and the extracellular fluid (ECF) compartment. The ECF compartment is composed of plasma (intravascular), intrastitial fluid (surrounding cells), and transcellular fluid, also known as the third-space (mucus and GI, cerebrospinal, pericardial, synovial, and ocular fluids). Table 16-1 shows the fluid compartments and percentage of total body water. Fluids move freely between the intracellular and extracellular compartments to maintain balance and homeostasis.
TABLE 16-1
| FLUID COMPARTMENT | PERCENTAGE |
| Intracellular fluid | 40% |
| Extracellular fluid | 20% |
| Interstitial fluid | 15% |
| Intravascular fluid | 5% |
| Transcellular fluid | Approximately 1-2 L total, but generally not included in calculations |
| Total body fluid | 60% |
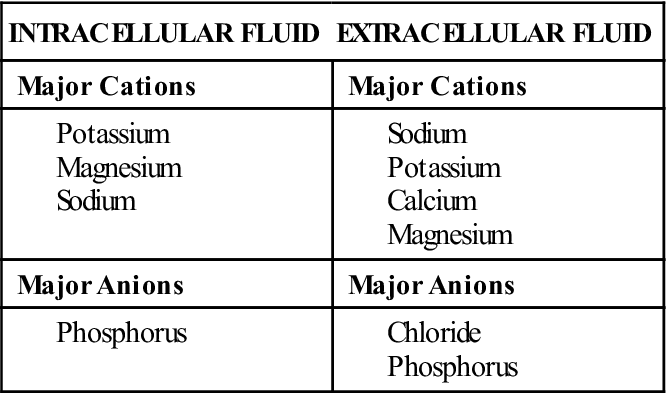
Electrolytes are substances that separate or dissociate into ions (charged particles) in solution; they are abundant in both ICF and ECF. Ions carry either a positive charge (cation) or a negative charge (anion). Major cations and anions are listed in Table 16-2. Electrolyte balance is essential for normal physiologic functioning and is closely linked with fluid balance. In addition, most electrolytes interact with hydrogen ions to maintain acid-base balance. Electrolyte balance must be kept within a narrow range to maintain homeostasis. Many illnesses and body system alterations cause electrolyte imbalances, which must be corrected quickly before serious sequelae ensue.
TABLE 16-2
| CATIONS | ANIONS |
| Potassium (K+) | Chloride (Cl−) |
| Sodium (Na+) | Bicarbonate (HCO3−) |
| Calcium (Ca2+) | Phosphate (PO4−) |
| Magnesium (Mg2+) | Sulfate (SO4−) |
The major electrolytes in body fluids are sodium, potassium, calcium, magnesium, chloride, phosphorus, sulfate, hydrogen and bicarbonate ions, and many proteins. Electrolyte concentration differs in ICF and ECF. Table 16-3 lists the major cations and anions found in body fluid.
TABLE 16-3
ELECTROLYTE CONCENTRATION IN BODY FLUIDS
Administering intravenous (IV) fluids and electrolyte replacements may be thought of as “routine” nursing care but requires as much diligence and critical thinking as administration of any medication. This chapter describes fluid replacement strategies, pharmacologic management of specific electrolyte imbalances, and nursing care of patients with fluid and electrolyte imbalances.
Homeostasis
Two principles stand out when considering homeostasis and fluid and electrolyte balance. The first principle is that anions and cations must be balanced within each compartment and remain electrically neutral. The amount of fluid within each compartment remains constant. Compartments work continuously to maintain fluid balance and replace and exchange ions to maintain neutrality. The second principle is that the fluid compartments remain in osmotic equilibrium (except for transient changes).
Movement of fluid and particles between and within compartments is controlled by a number of processes, including osmosis, diffusion, membrane transport, hydrostatic pressure, membrane permeability, colloidal osmotic pressure (plasma proteins), and size of the particle (ion). The number of solutes in a solution is expressed as a unit of measurement called the osmole, which is particularly useful when referencing osmotic solutions.
Osmolality and osmolarity are two different terms of measurement used to describe the number of osmoles or solutes in a solution. In clinical practice, the difference between the absolute values of these two measurements is negligible. Hence, these terms are often used interchangeably. However, they are two separate measurements. Osmolality refers to the number of osmoles per kilogram of water and is expressed as mmol/kg (SI units) or mOsm/kg (conventional units). Osmolality is the concentration of fluid that affects the movement of water between compartments by osmosis. Osmolarity is the number of osmoles in 1 L of solution and is expressed as mmol/L (SI units) or mOsm/L (conventional units).
Osmolality
Osmolality refers to the number of particles dissolved in the serum, primarily sodium, urea (blood urea nitrogen [BUN]), and glucose. It also is a measure of the concentration of solutes per kilogram in urine. Normal serum osmolality ranges from 280 to 300 mOsm/kg. As the number of particles increases, the concentration of the solution also increases. The change in the concentration of particles will affect chemical behavior and movement of water. Sodium is the primary electrolyte in the ECF and keeps water in this compartment.
The following three types of fluid concentration are based on the osmolality of body fluids:
1. Iso-osmolar fluid has the same proportion of weight of particles (e.g., sodium, glucose) and water.
2. Hypo-osmolar fluid contains fewer particles than water.
Hypo-osmolality of body fluid may be the result of excess water intake or fluid overload (edema) caused by an inability to excrete excess water. Hyperosmolality of body fluid could be caused by severe diarrhea, increased salt and solutes (protein) intake, inadequate water intake, diabetes, ketoacidosis, or sweating.
Osmolality and Tonicity
The terms osmolality and tonicity have been used interchangeably; they are similar but not identical. Osmolality refers to the concentration of particles in a solution and is used in reference to body fluids. Tonicity is used primarily as a measurement of the concentration of IV solutions compared with the osmolality of body fluids.
Fluid Replacement
General Considerations
All routes of fluid intake and loss must be considered when assessing fluid balance. General guidelines can be used as the basis for establishing fluid needs. The recommended water intake for a healthy adult is 3.7 L for a man and 2.7 L for a woman, or approximately 30 to 40 mL/kg/d. Patients lose water daily through various routes: about 350 mL through respiration; 450 mL through skin in perspiration and evaporation; 1400 mL in urine; and 200 mL in feces. Insensible losses (water lost via the lungs, skin, and feces) are not measurable. The minimum urinary output for an adult is 0.5 to 1 mL/kg/h or 35 to 70 mL/h for the 70-kg patient.
Daily water requirements differ according to the patient’s age and medical problems. Fluid maintenance needs for the NPO (nothing by mouth) patient can be calculated as follows: 4 mL/kg/h for first 10 kg of weight; 2 mL/kg/h for second 10 kg of weight; 1 mL/kg/h for every kg of weight thereafter. Using this formula, a 70-kg patient would require 110 mL/h of IV fluid (IVF), or 2640 mL/d.
IVFs are ordered based on an evaluation of the patient’s fluid and electrolyte balance, fluid requirements, and fluid needs. Several questions must be addressed. Is the purpose of IV therapy replacement or maintenance? What are the patient’s water, electrolyte, and protein requirements? The patient’s weight, caloric needs, and body surface area are other important considerations. Illness and surgery increase the amount of fluids lost and affect fluid and electrolyte needs. Ongoing assessment and monitoring the patient’s responses to fluid and electrolyte therapy is vital for patients with fluid and electrolyte imbalances.
Intravenous Solutions
With fluid volume deficit (FVD) from the extracellular body compartment, fluid is lost from the interstitial and vascular spaces. Different types and concentrations of IVFs are available to replace body fluid losses.
Types of Intravenous Solutions
The three general classifications of IV solutions used for fluid replacement are crystalloids (with and without added electrolytes), colloids, and blood and blood products.
Crystalloids
Crystalloid solutions contain fluids and electrolytes and are able to freely cross capillary walls. They do not contain any proteins, which are necessary to maintain the colloidal oncotic pressure that prevents water from leaving the intravascular space. Crystalloids are used to treat dehydration and electrolyte imbalances and as short-term maintenance fluids. Crystalloids cause early plasma expansion but have a shorter duration of action than colloid solutions.
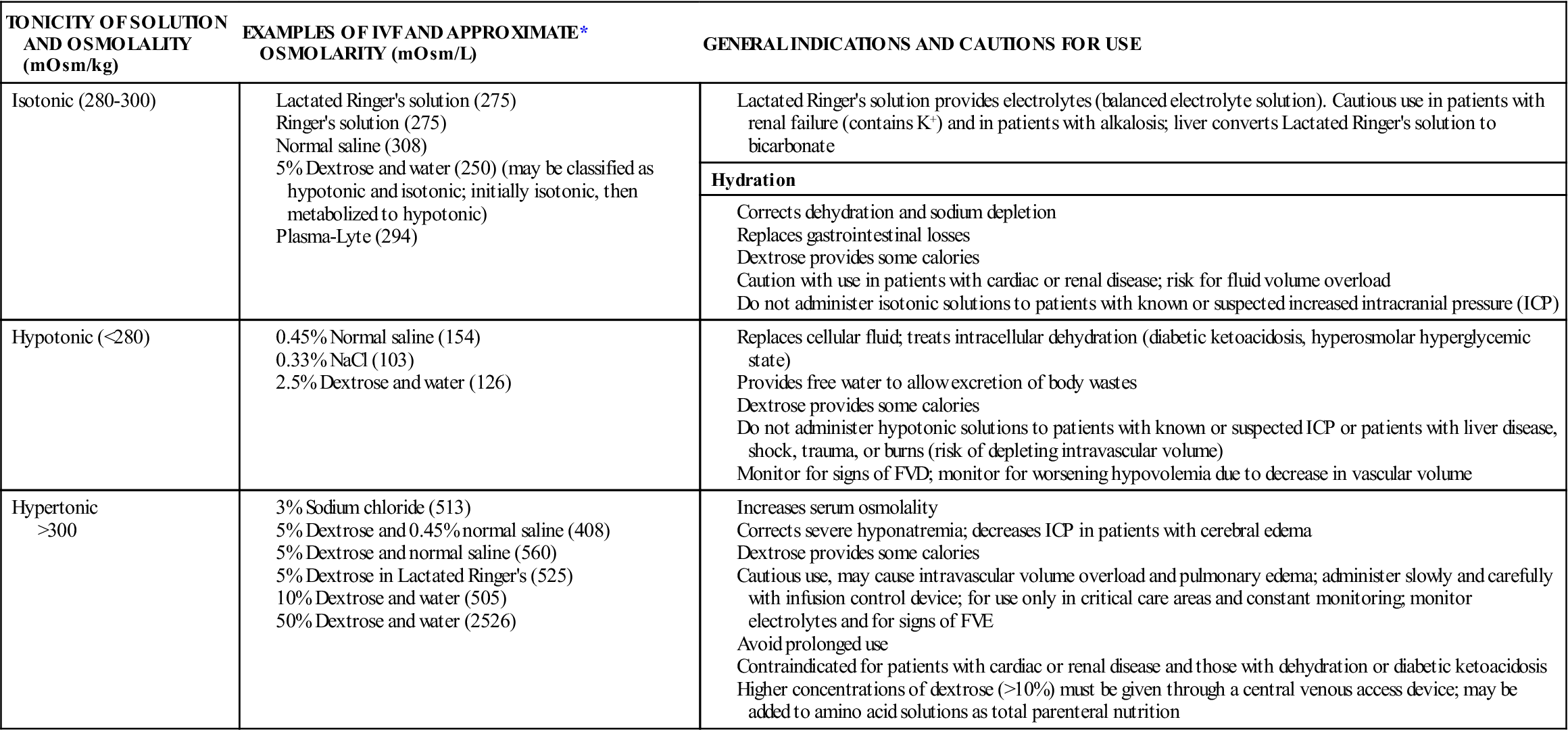
Crystalloid IVFs are classified by their total osmolality when compared with the osmolality of serum. The three major classifications of crystalloid IVF are isotonic, hypotonic, and hypertonic (Table 16-4):
TABLE 16-4
TONICITY OF INTRAVENOUS SOLUTIONS
| TONICITY OF SOLUTION AND OSMOLALITY (mOsm/kg) | EXAMPLES OF IVF AND APPROXIMATE* OSMOLARITY (mOsm/L) | GENERAL INDICATIONS AND CAUTIONS FOR USE |
| Isotonic (280-300) | ||
| Hydration | ||
| Hypotonic (<280) | ||
| Hypertonic >300 |
*Refer to manufacturer’s label on individual bags of IVF for exact osmolarity.
Parenteral solutions containing dextrose are available in different concentrations and in combination with other solutions, such as normal saline and lactated Ringer’s solution. Dextrose solutions provide hydration and some calories and increase glucose levels in the blood. Dextrose solutions are also used to dilute IV medications for administration after compatibility between IVF and the medication has been verified. Dextrose may also affect the stability of some medications; a pharmacist should be consulted if there are questions regarding admixtures. The addition of dextrose to an IV solution affects the tonicity of the solution after it is infused. Five-percent dextrose solutions are hypertonic when added to normal saline or lactated Ringer’s solution, but the remaining solution is isotonic because the dextrose is quickly metabolized to carbon dioxide and water.
The Infusion Nursing Society’s Practice Guidelines recommend that dextrose solutions higher than 10% should be given via a central vein. An exception is 50% dextrose, which may be given in small amounts via a peripheral vein to correct hypoglycemia. Dextrose can be irritating to veins due to the pH of the solution (3.4 to 4). If hypertonic solutions are not diluted and given peripherally, there is a risk of vein irritation, damage, and thrombosis. Long-term use of hypertonic solutions may result in electrolyte depletion, increased intravascular volume, fluid overload, water intoxication, and pulmonary edema. Rapid infusions of dextrose solutions may cause hyperglycemia, which can lead to osmotic diuresis and fluid and electrolyte imbalance. Hypertonic IV solutions should be administered in a critical care unit, where the staffing ratio allows continuous monitoring for complications. To decrease medication errors with hypertonic solutions, hospitals may limit access of these solutions and dispense them only directly from the pharmacy.
Sodium solutions are available in various concentrations and tonicities. Isotonic solutions are primarily used for hydration to expand the ECF and during blood product transfusions. Rapid infusion of isotonic saline solutions may lead to hypernatremia, fluid volume excess (FVE), and electrolyte depletion. Long-term use should also be avoided, as normal saline provides no calories. Hypotonic solutions are also used for hydration and to treat hyperosmolar diabetes. Hypertonic saline solutions are used to treat severe hyponatremia or hypochloremia; careful monitoring of electrolyte levels is important to avoid excess replacement.
Balanced electrolyte solutions, such as lactated Ringer’s solution and Ringer’s solution, contain electrolytes (no magnesium) and minimal calories with the addition of dextrose. Their primary use is hydration and electrolyte replacement. However, they do not provide adequate electrolytes for maintenance therapy for patients with limited or no oral intake. Lactated Ringer’s solution is similar in electrolyte content to plasma. Lactate is added as a buffering agent and is metabolized to bicarbonate. Complications of infusions of Ringer’s and lactated Ringer’s include fluid overload, excess electrolytes, and metabolic acidosis with long-term therapy. Because lactate is metabolized in the liver, lactated Ringer’s is contraindicated for patients with liver disease.
Colloids
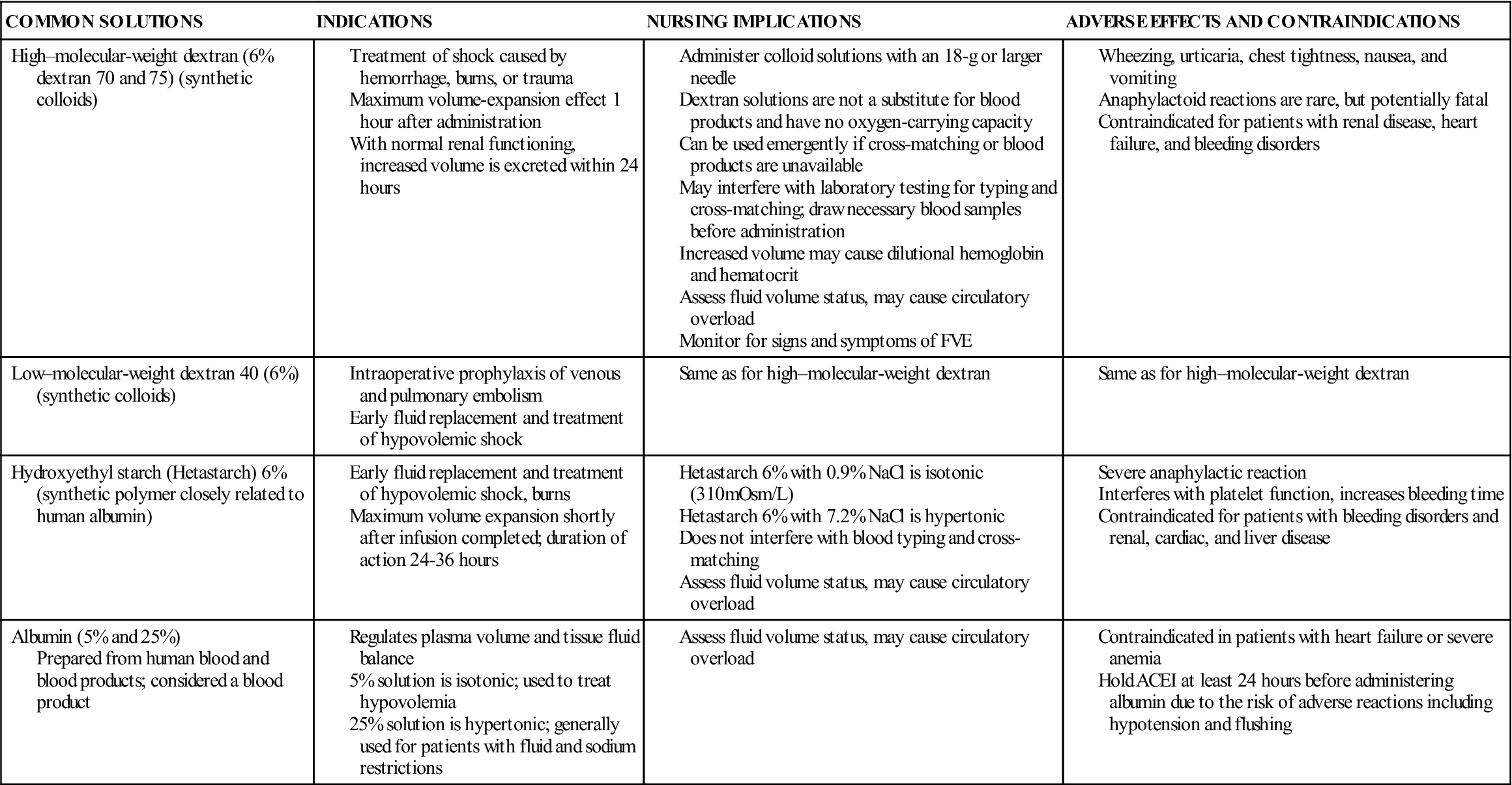
Colloid solutions contain protein or other large molecular substances that increase osmolarity without dissolving in the solution. Because of their size, the particles are unable to pass through the semipermeable membranes of the capillary walls and stay within the intravascular compartment; thus, colloids are also known as plasma expanders. They act by increasing the colloidal oncotic pressure and pulling fluids from the interstitial space into the plasma, increasing blood volume. The composition of colloid solutions includes proteins, carbohydrates, and lipids. Colloids typically have small and large particles, except albumin, whose particles are all one size. Commonly used colloids are shown in Table 16-5.
TABLE 16-5
Blood and Blood Products
Blood and blood products include whole blood and the components that can be processed through centrifugation methods. Blood products include packed RBCs (PRBCs), plasma, platelets, and cryoprecipitate. Plasma can be further processed into volume expanders, coagulation factor concentrates, and immune globins. A unit of PRBCs contains concentrated RBCs with most of the plasma and platelets removed; approximate volume is 350 mL/unit. The approximate volume of a unit of whole blood is 500 mL/unit. Advantages of infusing PRBCs over whole blood are that infusing packed cells allows an increase in oxygen-carrying capacity with a smaller volume, and other portions of the blood can be processed for other therapies. One unit of whole blood elevates the hemoglobin by approximately 0.5 to 1 g/dL, and one unit of packed RBCs elevates the hematocrit by three points.
![]() It is critical for nurses to understand the specific blood component to be infused before beginning the infusion. Reducing errors and increasing patient safety during blood and blood product transfusions is a focus of the Joint Commission on Accreditation of Health Care Organizations and is one of the 2013 National Patient Safety Goals. The National Healthcare Safety Network’s Hemovigilance Module was developed to increase patient safety and decrease costs associated with transfusion-related adverse events.
It is critical for nurses to understand the specific blood component to be infused before beginning the infusion. Reducing errors and increasing patient safety during blood and blood product transfusions is a focus of the Joint Commission on Accreditation of Health Care Organizations and is one of the 2013 National Patient Safety Goals. The National Healthcare Safety Network’s Hemovigilance Module was developed to increase patient safety and decrease costs associated with transfusion-related adverse events.
The maximum rate of infusion is 4 hours per unit, beginning with removal of the unit from the refrigerator. Loop diuretics are often prescribed to patients receiving whole blood or PRBCs to prevent circulatory overload, particularly in patients who are at risk for developing pulmonary edema and FVE. For patients receiving multiple blood transfusions, the serum ionized calcium level should be monitored. Both PRBCs and whole blood are processed using sodium citrate and citric acid for anticoagulation; multiple blood transfusions can result in a decrease in the plasma ionized (free) calcium levels. Practice guidelines are available from the American Red Cross (see Key Websites).
IV lipid emulsions are indicated for patients who are unable to tolerate oral or enteral feedings for 7 days or more. Lipids can supply up to 30% of the patient’s caloric intake. Parenteral nutrition provides essential nutrients intravenously, including proteins, carbohydrates, electrolytes, trace minerals, and vitamins. Lipids are often added to parenteral nutrition formulas; these formulas are referred to as total nutrient admixture. The patient must have a centrally or peripherally inserted vascular access device to receive parenteral nutrition formulas (see Chapter 17).

































