Research evidence has greatly expanded since the 1990s as numerous quality studies in nursing, medicine, and other healthcare disciplines have been conducted and disseminated. These studies are commonly communicated via journal publications, the Internet, books, conferences, and television. The expectations of society and the goals of healthcare systems are the delivery of high-quality, cost-effective health care to patients, families, and communities nationally and internationally. To ensure the delivery of quality health care, the care must be based on the current, best research evidence available. Healthcare agencies are emphasizing the delivery of evidence-based health care, and nurses and physicians are focused on evidence-based practice (EBP). With the emphasis on EBP over the last 2 decades, outcomes have improved for patients, healthcare providers, and healthcare agencies (Brown, 2009; Craig & Smyth, 2012; Doran, 2011; Gerrish et al., 2011; Higgins & Green, 2008; Melnyk & Fineout-Overholt, 2011; Sackett, Straus, Richardson, Rosenberg, & Haynes, 2000). Evidence-based practice (EBP) is an important theme in this textbook and was defined earlier as the conscientious integration of best research evidence with clinical expertise and patient values and needs in the delivery of quality, cost-effective health care (see Chapter 1) (Craig & Smyth, 2012; Institute of Medicine, 2001; Sackett et al., 2000). Best research evidence is produced by the conduct and synthesis of numerous high-quality studies in a selected health-related area. The concept of best research evidence was described in Chapter 2, and the processes for synthesizing research evidence (systematic review, meta-analysis, meta-synthesis, and mixed-methods systematic review) are defined. The greatest benefits of EBP are improved outcomes for patients, providers, and healthcare agencies. Organizations and agencies nationally and internationally have promoted the synthesis of the best research evidence in thousands of healthcare areas by teams of expert researchers and clinicians. These research syntheses, such as systematic reviews and meta-analyses, have provided the basis for developing strong evidence-based guidelines for practice. These guidelines identify the best treatment plan or gold standard for patient care in a selected area to promote quality health outcomes. Healthcare providers have easy access to numerous evidence-based guidelines to assist them in making the best clinical decisions for their patients. These evidence-based syntheses and guidelines are communicated by presentations and publications and can be easily accessed online through the National Guideline Clearinghouse (NGC, 2012b) in the United States (http://www.guidelines.gov/), Cochrane Collaboration (2012) in England (http://www.cochrane.org/), and Joanna Briggs Institute (2012) in Australia (http://www.joannabriggs.edu.au/). Individual studies, research syntheses, and evidence-based guidelines assist students, educators, registered nurses (RNs), and advanced practice nurses (APNs) to provide the best possible care. Expert APNs, such as nurse practitioners, clinical nurse specialists, nurse anesthetists, and nurse midwives, are resources to other nurses and facilitate access to evidence-based guidelines to ensure patient care is based on the best research evidence available (Gerrish et al., 2011). Nurse researchers and APNs are involved in the development of systematic reviews, meta-analyses, meta-syntheses, and evidence-based guidelines to manage patient health problems, prevent illnesses, and promote health. Healthcare agencies are highly supportive of EBP because it promotes quality, cost-effective care for patients and families and meets accreditation requirements. The Joint Commission revised their accreditation criteria to emphasize patient care quality achieved through EBP. Approximately 25% of chief nursing officers (CNOs) identified the movement toward evidence-based nursing practice as their number one priority (Nurse Executive Center, 2005; The Joint Commission, 2012). Many CNOs and healthcare agencies are trying either to obtain or to maintain Magnet status, which documents the excellence of nursing care in an agency. Approval for Magnet status is obtained through the American Nurses Credentialing Center (ANCC). The national and international healthcare agencies that currently have Magnet status can be viewed online at the ANCC (2012) website (http://www.nursecredentialing.org/FindaMagnetHospital.aspx). The Magnet Recognition Program® recognizes EBP as a way to improve the quality of patient care and to revitalize the nursing environment. Selection criteria for Magnet status that require healthcare agencies to promote the conduct of research and the use of research evidence in practice follow. These selection criteria include critical elements for EBP, especially financial support for and outcomes related to research activities. Important research-related outcomes to be documented by agencies for Magnet status include nursing studies conducted and professional publications and presentations by nurses. For each study, the following needs to be documented: title of the study, principal investigator or investigators, role of nurses in the study, and study status (Horstman & Fanning, 2010). In working toward EBP, nurses are encouraged to embrace the benefits of EBP, use the evidence-based guidelines available, synthesize current research evidence, and support or participate in the research needed to determine the effectiveness of selected nursing interventions. Barriers to the EBP movement have been both practical and conceptual. One of the most serious barriers is the lack of research evidence available regarding the effectiveness of many nursing interventions. EBP requires synthesizing research evidence from randomized controlled trials (RCTs) and other types of intervention studies, and these types of studies are still limited in nursing. Mantzoukas (2009) reviewed the research evidence in 10 high-impact nursing journals, including Nursing Research, Research in Nursing & Health, Western Journal of Nursing Research, Journal of Nursing Scholarship, and Advances in Nursing Science, between 2000 and 2006 and found that the studies were 7% experimental, 6% quasi-experimental, and 39% nonexperimental. However, RCTs and quasi-experimental studies conducted to determine the effectiveness of nursing interventions continue to increase. Systematic reviews and meta-analyses conducted in nursing have been limited compared with other disciples. In addition, nurse authors of these research syntheses have sometimes indicated there is inadequate research evidence to support using certain nursing interventions in practice (Craig & Smyth, 2012; Mantzoukas, 2009). Bolton, Donaldson, Rutledge, Bennett, and Brown (2007, p. 123S) conducted a review of “systematic/integrative reviews and meta-analyses on nursing interventions and patient outcomes in acute care settings.” Their literature search covered 1999-2005 and identified 4000 systematic/integrative reviews and 500 meta-analyses covering the following seven topics selected by the authors: staffing, caregivers, incontinence, elder care, symptom management, pressure ulcer prevention and treatment, and developmental care of neonates and infants. The authors found a limited association between nursing interventions and processes and patient outcomes in acute care settings. Their findings included the following. Extensive evidence has been generated through nursing research, but additional studies are needed that focus on determining the effectiveness of nursing interventions on patient outcomes (Bolton et al., 2007; Craig & Smyth, 2012; Doran, 2011; Mantzoukas, 2009). Identifying the areas where research evidence is lacking is an important first step in developing the evidence needed for practice. Well-designed experimental and quasi-experimental studies are needed to test selected nursing interventions and to use that understanding to generate sound evidence for practice (see Chapter 14). Nurses also need to be more active in conducting quality syntheses (systematic reviews, meta-analyses, and meta-syntheses) of research evidence in selected areas (Finfgeld-Connett, 2010; Higgins & Green, 2008; Rew, 2011; Sandelowski & Barroso, 2007). The next section of this chapter provides guidelines to direct different types of research syntheses. Another concern is that the research evidence is generated based on population data and then is applied in practice to individual patients. Sometimes it is difficult to transfer research knowledge to individual patients, who respond in unique ways or have unique needs (Biswas et al., 2007). More work is needed to promote the use of evidence-based guidelines with individual patients. The National Institutes of Health (NIH) is supporting translational research to improve the use of research evidence with different patient populations in various settings. Patients who have poor outcomes when managed according to an evidence-based guideline need to be reported, and, if possible, their circumstances should be published as a case study. Electronic patient records now make it possible to determine patient outcomes of care delivered using EBP guidelines. Best research evidence is generated mainly from RCTs and other intervention studies with limited focus on the contributions of descriptive-correlational studies, qualitative research, mixed-methods studies, and theories. These types of studies do make contributions to the research evidence in many areas and need to be synthesized for use in practice (Mantzoukas, 2009) (see Chapter 10 for mixed-methods studies). Qualitative researchers have developed several synthesis processes for qualitative studies, and these are discussed later in this chapter. Another concern of the EBP movement is that the development of evidence-based guidelines has led to a “cookbook” approach to health care. Health professionals are expected to follow these guidelines in their practice as developed. However, the definition of EBP describes it as the conscientious integration of best research evidence with clinical expertise and patient values and needs. Nurse clinicians have a major role in determining how the best research evidence will be implemented to achieve quality care and outcomes. For example, a nurse practitioner uses the national evidence-based guidelines for the treatment of patients with hypertension (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [JNC 7]) (Chobanian et al., 2003) but also makes clinical decisions based on the needs and values of individual patients. If a patient has a dry, persistent, irritating cough when taking angiotensin-converting enzyme inhibitor medications, this type of medication would not be used to manage the patient’s high blood pressure if possible. If a patient refuses a treatment based on cultural or religious reasons, these reasons would be taken into consideration in developing the patient’s treatment plan. Evidence-based guidelines provide the gold standard for managing a particular health condition, but the healthcare provider and patient individualize the treatment plan. Another serious barrier is that some healthcare agencies and administrators do not provide the resources necessary for nurses to implement EBP. Their lack of support might include the following: (1) inadequate access to research journals and other sources of synthesized research findings and evidence-based guidelines, (2) inadequate knowledge on how to implement evidence-based changes in practice, (3) heavy workload with limited time to make research-based changes in practice, (4) limited authority to change patient care based on research findings, (5) limited support from nursing administrators or medical staff to make evidence-based changes in practice, (6) limited funds to support research projects and research-based changes in practice, and (7) minimal rewards for providing evidence-based care to patients and families (Butler, 2011; Eizenberg, 2010; Gerrish et al., 2011). The success of EBP is determined by all involved including healthcare agencies, administrators, nurses, physicians, and other healthcare professionals. We all need to take an active role in ensuring that the health care provided to patients and families is based on the best research available. In this section, guidelines are provided for conducting systematic reviews, meta-analyses, meta-syntheses, and mixed-methods systematic reviews to guide you in synthesizing research evidence for nursing practice. Numerous research syntheses have been conducted in nursing and medicine, so be sure to search for an existing synthesis or review of research in an area before undertaking such a project. More recent data suggest that at least 2500 new systematic reviews are reported in English and indexed in MEDLINE each year (Liberati et al., 2009). Table 19-1 identifies some common databases and EBP organizational websites for nurses to search for nursing syntheses of research. The Cochrane Collaboration library of systematic reviews is an excellent resource with more than 11,000 entries relevant to nursing and health care (http://www.cochrane.org/cochrane-reviews). In 2009, the Cochrane Nursing Care Field was developed to support the conduct, dissemination, and use of systematic reviews in nursing. The Joanna Briggs Institute also provides resources for locating and conducting research syntheses in nursing (see Table 19-1). If you can find no synthesis of research for a selected nursing intervention or the review you find is outdated, you might use the following guidelines to conduct a systematic review of relevant research. TABLE 19-1 Evidence-Based Practice Resources A systematic review is a structured, comprehensive synthesis of the research literature to determine the best research evidence available to address a healthcare question. A systematic review involves identifying, locating, appraising, and synthesizing quality research evidence for expert clinicians to use to promote an EBP (Bettany-Saltikov, 2010a; Craig & Smyth, 2012; Higgins & Green, 2008; Liberati et al., 2009; Rew, 2011). Systematic reviews are often conducted by two or more researchers or clinicians (or researchers and clinicians) in a selected area of interest to determine the best research knowledge in that area. Systematic reviews need to be conducted with rigorous research methodology to promote the accuracy of the findings and minimize the reviewers’ bias. Table 19-2 is a checklist for critically appraising the steps of a systematic review, and these steps are discussed in the following section. These steps are based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and other relevant sources to guide nurses in conducting systematic reviews (Bettany-Saltikov, 2010a, 2010b; Higgins & Green, 2008; Rew, 2011). The PRISMA Statement was developed in 2009 by an international group of expert researchers and clinicians to improve the quality of reporting for systematic reviews and meta-analyses. The PRISMA Statement includes 27 items, which can be found at http://prisma-statement.org/ and are detailed in the article by Liberati et al. (2009). If the review process is clearly detailed in the report, others can replicate the process and verify the findings (Rew, 2011). A systematic review conducted by Goulding, Furze, and Birks (2010) is presented as an example with the discussion of the review steps outlined in Table 19-2. Goulding et al. conducted a systematic review of only RCTs to determine the best interventions to use in changing maladaptive illness beliefs of people with coronary heart disease (CHD). TABLE 19-2 Checklist for Critically Appraising Published Systematic Reviews A systemic review or meta-analysis is best directed by a relevant clinical question that focuses the review process and promotes the development of a quality synthesis of research evidence. Formulating the question involves identifying a relevant topic, developing a question of interest that is worth investigating, deciding if the question will generate significant information for practice, and determining if the question will clearly direct the review process and synthesis of findings. A well-stated question will define the nature and scope of the literature search, identify keywords for the search, determine the best search strategy, provide guidance in selecting articles for the review, and guide the synthesis of results (Bettany-Saltikov, 2010a, 2010b; Higgins & Green, 2008; Liberati et al., 2009). The question developed might focus on an intervention or therapy, health promotion action, illness prevention strategy, diagnostic process, prognosis, causation, or experiences (Bettany-Saltikov, 2010a). One of the most common formats used to develop a relevant clinical question to guide a systematic review is the PICO or PICOS format described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins & Green, 2008). PICOS format includes the following elements: P—Population or participants of interest (see Chapter 15 on sampling) I—Intervention needed for practice (see Chapter 14 on intervention research) C—Comparisons of the intervention with control, placebo, standard care, variations of the same intervention, or different therapies (see Chapter 14) O—Outcomes needed for practice (see Chapter 13 on outcomes research and Chapter 17 on measurement methods) S—Study design (see Chapter 11 on types of study designs) Goulding et al. (2010) noted that interventions to change maladaptive illness beliefs were beneficial to people with CHD because positive illness representations may lead to improved lifestyle behaviors of exercise, smoking cessation, and balanced diet. What was not known was “[w]hich types of intervention to change illness cognitions (e.g. counseling, education, or cognitive behavioural) are most effective” (Goulding et al., 2010, p. 948) for people with CHD. The population was people with CHD, and the intervention was focused on changing maladaptive illness beliefs of these individuals. The different types of this intervention, including counseling, education, and cognitive behavioral therapy, were compared. The intervention group was compared with groups receiving standard care, no treatment, or a variation of the treatment. The primary outcome measured was the change in beliefs about CHD at follow-up. The study design included synthesis of only RCTs using guidelines from the Cochrane Collaboration handbook (Higgins & Green, 2008) (see Chapters 11 and 14 about conducting RCTs). The study design (RCTs) clearly focused the literature review but might have eliminated some important studies that could have expanded the knowledge related to the intervention of changing illness beliefs. Most systematic reviews of research include a purpose and specific aims or objects to guide the synthesis process (Bettany-Saltikov, 2010a; Rew, 2011). The purpose identifies the major goal or focus of the review. Goulding et al. (2010, p. 948) identified their purpose as follows: “This systematic review was therefore necessary to collate and present evidence of the effectiveness of maladaptive belief change interventions for people with CHD.” The specific aims direct the remaining steps of the research synthesis and become the focus of the discussion of the findings. Goulding et al. (2010) identified the following. Researchers conducting a systematic review or meta-analysis need to identify the inclusion and exclusion criteria to be used to direct their literature search. The PICOS format might be used to develop the search criteria with more detail being developed for each of the elements. These search criteria might focus on the following: (1) type of research methods, such as quantitative, qualitative, or outcomes research; (2) the population or type of study participants; (3) study designs, such as description, correlational, quasi-experimental, experimental, qualitative, or mixed methods; (4) sampling processes, such as probability or nonprobability sampling methods; (5) intervention and comparison interventions; and (6) specific outcomes to be measured. The PICOS format is effective in identifying the key terms to be included in the search process. The search criteria also need to indicate the years for the review, language, and publication status. The focus of the review might be narrowed by limiting the years reviewed, the language to English, and only studies in print (Bettany-Saltikov, 2010b; Higgins & Green, 2008; Rew, 2011). Often searches have been limited to published sources in common databases, which excludes the grey literature from the research synthesis. Grey literature refers to studies that have limited distributions, such as theses and dissertations, unpublished research reports, articles in obscure journals, articles in some online journals, conference papers and abstracts, conference proceedings, research reports to funding agencies, and technical reports (Benzies, Premji, Hayden, & Serrett, 2006; Conn, Valentine, Cooper, & Rantz, 2003). Most grey literature is difficult to access through database searches and is often not peer-reviewed with limited referencing information. These are some of the main reasons for not including grey literature in searches for systematic reviews and meta-analyses. However, excluding grey literature from these searches might result in misleading, biased results. Studies with significant findings are more likely to be published than studies with nonsignificant findings and are usually published in more high-impact, widely distributed journals that are indexed in computerized databases (Conn et al., 2003). Studies with significant findings are more likely to have duplicate publications that need to be excluded when selecting studies to include in a research synthesis. Benzies et al. made the following recommendations related to including grey literature in a systematic review or meta-analysis. 1. Review the references of identified studies for additional studies. These are ancestry searches to use citations in relevant studies to identify additional studies. 2. Hand search certain journals for selected years, especially for older studies that were not identified in the electronic search. 3. Identify expert researchers in an area and search their names in the databases. 4. Contact the expert researchers regarding studies they have conducted that have not been published yet. 5. Search thesis and dissertation databases for relevant studies. 6. Review abstracts and conference reports of relevant professional organizations. 7. Search the websites of funding agencies for relevant research reports (Bettany-Saltikov, 2010b; Conn et al., 2003; Liberati et al., 2009). Often it is best to construct a table that includes the search criteria so that they can be applied consistently throughout the search process (Liberati et al., 2009) (see Chapter 6). Goulding et al. (2010) used the PICOS format to determine inclusion criteria for the studies in the search. The participants had to be adults with at least one of the following: angina, CHD, myocardial infarction, or eligible for or recently received revascularization by percutaneous coronary intervention or coronary artery bypass graft surgery. The studies needed to focus on an intervention to change knowledge, attitudes, perceptions, and misconceptions about CHD. The interventions to change maladaptive beliefs were compared with different interventions, usual care, or no intervention. The primary outcome was change in beliefs about CDH, and the secondary outcomes were focused on quality of life, behavior change, anxiety level, depression, psychological well-being, and modifiable risk factors. The study design was limited to only RCTs that included a comparison of the intervention group with a control group or another intervention. Goulding et al. (2010) designed their literature search strategies and their protocol for conducting their systematic review using sources such as the Cochrane Collaboration handbook (Higgins & Green, 2008) and the Quality of Reporting of Meta-analyses (QUOROM) Statement (Moher et al., 1999). No date restriction was applied to the search for studies, but because of the lack of funds, only studies reported in English were identified. The databases searched are discussed in Step 4. The researchers did not include grey literature in their review and recognized this as a limitation in their discussion section. The next step for conducting a systematic review or meta-analysis requires an extensive search of the literature focused by the criteria and strategies identified in Step 3. The different databases searched, date of the search, and search results need to be recorded for each database (see Chapter 6 for details on conducting and storing searches of databases). Table 19-1 identifies common databases that are searched by nurses in conducting syntheses of research and in searching for evidence-based guidelines. Usually the key search terms are identified in the report. Sometimes authors of systematic reviews provide a table that identifies the search terms and criteria. The PRISMA Statement recommends presenting the full electronic search strategy used for at least one major database such as CINAHL or MEDLINE (Liberati et al., 2009). The search strategies used to identify grey literature and other unpublished studies need to be identified. Goulding et al. (2010) searched the following electronic databases: MEDLINE, EMBASE, CINAHL, BNI, PsychINFO, The Cochrane Library (including the Cochrane Database of Systematic Reviews, CENTRAL, and DARE), and Web of Knowledge. They provided an extensive table that detailed their electronic search of the five databases identified. The search criteria included the participants with different types of CHD; interventions of education and cognitive and behavioral therapies; comparisons with control, standard care, and placebo groups; outcomes of health knowledge, attitudes, and illness perceptions; and study design of RCT. The authors noted that the electronic search identified 3526 citations and that they obtained one source from an expert researcher and seven by reviewing the references of other studies. The selection of studies for inclusion in the systematic review or meta-analysis is a complex process that initially involves review and removal of duplicate sources. The abstracts of the remaining studies are reviewed by two or more authors and sometimes an external reviewer to ensure they meet the criteria identified in Step 3. The abstracts might be excluded based on the study participants, interventions, outcomes, or design not meeting the search criteria. Sometimes the abstracts are not in English, are incomplete, or are of studies not attainable. If contacting the authors of the abstracts cannot produce essential information, often the abstracts are excluded from the review (Bettany-Saltikov, 2010b; Higgins & Green, 2008; Liberati et al., 2009). After the abstracts meeting the designated criteria are identified, the next step is to retrieve the full-text citation for each study. It is best to enter these studies into a table and document how each study meets the eligibility criteria. If studies do not meet criteria, they need to be removed with a rationale provided. Two or more authors of the review need to examine the studies to ensure that the eligibility or inclusion criteria are consistently implemented. Often the study selection process includes all members of the review team. This selection process is best demonstrated by a flow diagram that was developed by the PRISMA Group (Liberati et al., 2009). Figure 19-1 shows this diagram, which has four phases: (1) identification of the sources, (2) screening of the sources based on set criteria, (3) determining if the sources meet eligibility requirements, and (4) identifying the studies included in the review. Goulding et al. (2010) provided the following description of their section of sources and a flow diagram (see Figure 19-2) that documented the final results of the 13 RCTs included in their systematic review. An initial critical appraisal of the methodological quality of the studies occurs during the selection of the studies to be included in the systematic review. Once the studies are selected, a more thorough critical appraisal takes place. This critical appraisal is best done by constructing a table describing the characteristics of the included studies, such as the purpose of the studies, population, sampling method, sample size, sample acceptance and attrition rates, design, intervention (independent variable), dependent variables, measurement methods for each dependent variable, and major results (Bettany-Saltikov, 2010b; Higgins & Green, 2008; Liberati et al., 2009). It is best if two or more experts independently review the studies and make judgments about their quality. The authors of the review usually contact the study investigators if needed to obtain important information about the study design or results not included in the publication. Chapter 18 provides guidelines for critically appraising quantitative and qualitative studies. The critical appraisal of the studies is often difficult because of the differences in types of participants, designs, sampling methods, intervention protocols, outcome variables and measurement methods, and presentation of results. The studies are often rank-ordered based on their quality and contribution to the development of the review (Bettany-Saltikov 2010b; Liberati et al., 2009). Goulding et al. (2010) developed a detailed table of essential content from the 13 studies included in the systematic review and labeled the headings of the columns in the table as (1) authors, year, and country of the study; (2) participants and setting; (3) design, sample size per group, and follow-up; (4) intervention; (5) control or comparison group; (6) study outcomes; and (7) results with statistical significance set at alpha = 0.05. Two reviewers independently assessed the quality of each study. They provided a detailed description of the appraisal process and their findings for each study. You may want to access this systematic review to view their table of studies and examine their critical appraisal process. Some systematic reviews include published meta-analyses as sources in the review. Because a meta-analysis involves the use of statistics to summarize results of different studies, it usually provides strong, objective information about the effectiveness of an intervention or solid knowledge about a clinical problem. Other authors conduct a meta-analysis in the synthesis of sources for their systematic review (Liberati et al., 2009). The authors of the review need to provide a rationale for conducting the meta-analysis and detail the process they used to conduct this analysis. For example, a meta-analysis might be conducted on a small group of similar studies to determine the effect of an intervention. The next section provides more details on conducting a meta-analysis. The systematic review conducted by Goulding et al. (2010) did not include a meta-analysis as a source, and a meta-analysis was not conducted during the review process. A meta-analysis was probably not appropriate because of the limited number of studies that had been conducted to examine the effectiveness of the intervention to change maladaptive illness beliefs in people with CHD. The results of the authors’ reviews need to include a description of the study participants, types of interventions, and outcomes. The results of the different types of interventions might be best summarized in a table that includes the following: (1) study source; (2) structure of the intervention (stand-alone or multifaceted); (3) specific type of intervention such as physiological treatment, education, counseling, or behavioral therapy; (4) delivery method such as demonstration and return demonstration, verbal, video, or self-administered; and (5) statistical difference between the intervention and the control, standard care, placebo, or alternative intervention groups (Liberati et al., 2009). The specific outcomes, including primary and secondary outcomes, of the studies might also be best summarized in a table. This table might include (1) the study source; (2) outcome variable, with an indication as to whether it was a primary or secondary outcome in the study; (3) measurement method used for each study outcome variable; and (4) the quality of the measurement methods, such as the reliability and validity of a scale or the precision and accuracy of a physiological measure (see Chapter 16). Goulding et al. (2010, p. 955) described their participants for their 13 studies as “male and female adults of all ages with a diagnosis of CHD (including people diagnosed with MI [myocardial infarction] or angina or those receiving revascularization). There was no clear link between patient group and effectiveness of interventions to change beliefs.” These authors also provided two detailed tables: one addressing the types of interventions and the other identifying the measurement methods of the study outcomes and quality of these methods. Numerous outcome measures were used in the 13 studies, and some had poor reliability and validity, which limit the results of this systematic review. Goulding et al. also summarized key findings related to the effectiveness of the interventions and reached the following conclusion. In a systematic review or meta-analysis, the discussion of the findings needs to include an overall evaluation of the types of interventions implemented and the outcomes measured. The methodological issues or limitations of the review also need to be addressed. The discussion section needs to include a theoretical link back to the studies’ frameworks to indicate the theoretical implications of the findings. Lastly, the discussion section needs to provide recommendations for further research, practice, and policy development (Bettany-Saltikov, 2010b; Higgins & Green, 2008; Liberati et al., 2009). Goulding et al. (2010) provided the following discussion of their findings; review limitations; and recommendations for research, practice, and policy development. The PRISMA Group developed a 27-item checklist to use in developing the final reports for systematic reviews and meta-analyses. This PRISMA Group checklist is presented in Table 19-3 and discussed on PRISMA Group website (http://www.prisma-statement.org) and in the articles by Liberati et al. (2009) and Moher, Liberati, Tetzlaff, Altman, and PRISMA Group (2009). This checklist is an excellent guide to use in developing the final report of a systematic review or meta-analysis for publication. This checklist was developed to improve the quality, completeness, and consistency of the syntheses developed and published in nursing, medicine, and other healthcare professions. TABLE 19-3 Checklist of Items to Include When Reporting a Systematic Review or Meta-Analysis
Evidence Synthesis and Strategies for Evidence-Based Practice
![]() http://evolve.elsevier.com/Grove/practice/
http://evolve.elsevier.com/Grove/practice/
Benefits and Barriers Related to Evidence-Based Nursing Practice
Benefits of Evidence-Based Practice in Nursing
Barriers of Evidence-Based Practice in Nursing
Guidelines for Synthesizing Research Evidence
Resource
Description
Electronic Databases
CINAHL (Cumulative Index to Nursing and Allied Health Literature)
CINAHL is an authoritative resource covering the English-language journal literature for nursing and allied health. Database was developed in the U.S. and includes sources published from 1982 forward
MEDLINE (PubMed—National Library of Medicine)
Database was developed by the National Library of Medicine in the U.S. and provides access to >11 million MEDLINE citations back to the mid-1960s and additional life science journals
MEDLINE with MeSH
Database provides authoritative medical information on medicine, nursing, dentistry, veterinary medicine, the healthcare system, preclinical services, and more
PsychINFO
Database was developed by the American Psychological Association and includes professional and academic literature for psychology and related disciplines from 1887 forward
CANCERLIT
Database of information on cancer was developed by the U.S. National Cancer Institute
National Library Sites
Cochrane Library
Cochrane Library provides high-quality evidence to inform people providing and receiving health care and people responsible for research, teaching, funding, and administration of health care at all levels. Included in the Cochrane Library is the Cochrane Collaboration (2012), which has many systematic reviews of research. Cochrane Reviews are available at http://www.cochrane.org/reviews/
National Library of Health (NLH)
NLH is located in the United Kingdom. You can search for evidence-based sources at http://www.evidence.nhs.uk/
Evidence-Based Practice Organizations
Cochrane Nursing Care Network
Cochrane Collaboration includes 11 different fields, one of which is the Cochrane Nursing Care Field (CNCF), which supports the conduct, dissemination, and use of systematic reviews in nursing; see http://cncf.cochrane.org/
National Guideline Clearinghouse (NGC)
Agency for Healthcare Research and Quality (AHRQ) developed NGC to house the thousands of evidence-based guidelines that have been developed for use in clinical practice. The guidelines can be accessed online at http://www.guidelines.gov
National Institute for Health and Clinical Excellence (NICE)
NICE was organized in the United Kingdom to provide access to the evidence-based guidelines that have been developed. These guidelines can be accessed at http://nice.org.uk
Joanna Briggs Institute
This international evidence-based organization, originating in Australia, has a search website that includes evidence summaries, systematic reviews, systematic review protocols, evidence-based recommendations for practice, best practice information sheets, consumer information sheets, and technical reports; see Search the Joanna Briggs Institute (2012) at http://www.joannabriggs.edu.au/Search.aspx
Guidelines for Implementing and Evaluating Systematic Reviews
Systematic Review Steps
Step Complete (Yes or No)
Comments: Quality and Rationale
1. Was the clinical question clearly expressed and significant? Was the PICOS (participants, intervention, comparative interventions, outcomes, and study design) format used to develop the question and focus the review?
2. Were the purpose and objectives or aims of the review clearly expressed and used to direct the review?
3. Were the search criteria clearly identified? Was the PICOS format used to identify the search criteria and were the years covered, language, and publication status of sources identified in the search criteria?
4. Was a comprehensive, systematic search of the literature conducted using explicit criteria identified in Step 3? Were the search strategies clearly reported with examples? Did the search include published studies, grey literature, and unpublished studies?
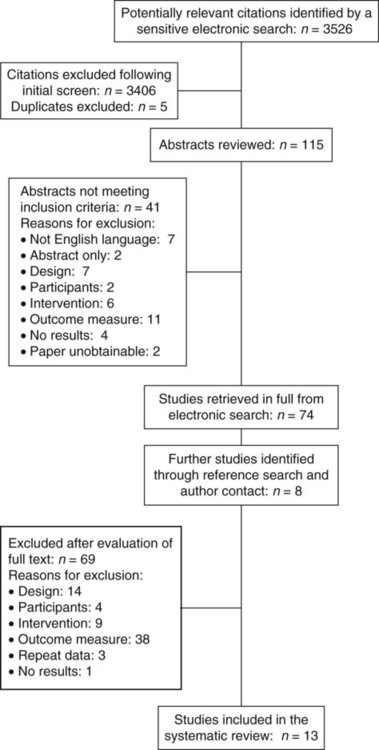
5. Was the process for the selection of studies for the review clearly identified and consistently implemented? Was the selection process expressed in a flow diagram such as Figure 19-1?
6. Were key elements (population, sampling process, design, intervention, outcomes, and results) of each study clearly identified and presented in a table?
7. Was a quality critical appraisal of the studies conducted? Were the results related to participants, types of intervention, outcomes, outcome measurement methods, and risks of bias clearly discussed related to each study (i.e., in table and narrative format)?
8. Was a meta-analysis conducted as part of the systematic review? Was a rationale provided for conducting the meta-analysis? Were the details of the meta-analysis process and results clearly described?
9. Were the results of the review clearly described (i.e., in narrative and table)? Were details of the study interventions compared and contrasted in a table? Were the outcome variables clearly identified and the quality of the measurement methods addressed?
10. Did the report conclude with a clear discussion section?
a. Were the review findings summarized to identify the current best research evidence?
b. Were the limitations of the review and how they might have affected the findings addressed?
c. Were the recommendations for further research, practice, and policy development addressed?
11. Did the authors of the review develop a clear, concise, quality report for publication? Was the report inclusive of the items identified in the PRISMA Statement (Liberati et al., 2009)?
Step 1: Formulate a Relevant Clinical Question to Direct the Review
Step 2: State the Purpose and Objectives or Aims of the Review
Step 3: Identify the Literature Search Criteria and Strategies
Step 4: Conduct a Comprehensive Search of the Research Literature
Step 5: Selection of Studies for Review
Step 6: Critical Appraisal of the Studies Included in Review
Step 7: Conduct a Meta-Analysis If Appropriate
Step 8: Results of the Review
Step 9: Discussion Section of the Review
Step 10: Development of the Final Report for Publication
Section/Topic
No.
Checklist Item
Reported on Page No.
Title
Title
1
Identify the report as a systematic review, meta-analysis, or both
Abstract
Structured summary
2
Provide a structured summary including, as applicable, background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number
Introduction
Rationale
3
Describe the rationale for the review in the context of what is already known
Objectives
4
Provide an explicit statement of questions being addressed with reference to PICOS (participants, interventions, comparisons, outcomes, and study design)
Methods
Protocol and registration
5
Indicate if a review protocol exists; if and where it can be accessed (e.g., Web address); and, if available, provide registration information including registration number
Eligibility criteria
6
Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale
Information sources
7
Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched
Search
8
Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated
Study selection
9
State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis)
Data collection process
10
Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators
Data items
11
List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made
Risk of bias in individual studies
12
Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis
Summary measures
13
State the principal summary measures (e.g., risk ratio, difference in means)
Synthesis of results
14
Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree