Drugs for Women’s Reproductive Health and Menopause
Objectives
• Recognize that successful contraception is essential to the health and well-being of women.
• Differentiate between types of medications used for osteoporosis.
Key Terms
amenorrhea, p. 847
anovulation, p. 853
breakthrough bleeding, p. 847
chloasma, p. 847
dysmenorrhea, p. 847
dyspareunia, p. 856
ethinyl estradiol, p. 846
hormone therapy, p. 862
menarche, p. 846
menopause, p. 846
menorrhagia, p. 847
mestranol, p. 846
metrorrhagia, p. 851
mittelschmerz, p. 847
oligomenorrhea, p. 856
osteopenia, p. 868
osteoporosis, p. 851
pelvic inflammatory disease, p. 859
progestin, p. 846
withdrawal bleeding, p. 848
The authors gratefully acknowledge the work of Marcia Welsh, who updated this chapter for the eighth edition.
Women have specific health care needs throughout their reproductive and postreproductive life cycle. Women’s reproductive life cycle begins with menarche, the start of spontaneous menstruation, and continues through menopause, the permanent cessation of menstruation. Successful contraception is essential to the health and well-being of sexually active women of reproductive age. Successful adaptation to menopause, control of menopausal symptoms, and continued sexual wellness is essential to the health and well-being of older women. This chapter reviews pharmacologic products that may be used throughout the reproductive and menopausal life cycle of women.
In 2011, the Department of Health and Human Services classified contraception as a preventative service under the Affordable Care Act. For many women, this law will reduce the cost of contraception methods and improve access to health care providers.
Estrogen-Progestin Combination Products
All estrogen-progestin combination products (also known as combined hormone contraception [CHC] products) contain a synthetic version of estrogen and a compound known as progestin. Ethinyl estradiol (EE) is the most commonly used synthetic estrogen found in CHC products. An older form of estrogen, mestranol, is found in higher-dose (≥50 mcg) oral combination products. Mestranol is converted into ethinyl estradiol in the body. Contraception products with high levels of ethinyl estradiol or mestranol are prescribed only in certain circumstances. Newer CHC products, including low-dose contraception pills, contain only EE as the estrogen component. Progestins are natural or synthetic hormones that have progesterone-like effects. Progesterone is the naturally occurring sex hormone produced in the ovaries of women. Progestogen refers to any synthetically produced progesterone compound. Almost all progestins are derivatives of testosterone, a steroid hormone classified under the androgen group. The term progestin will be used to describe the compound used in CHC products. Not only do progestins have contraceptive properties, they serve to balance out the effects of estrogen.
One of eight different types of progestins is used in CHC products. Norethindrone, norethindrone acetate, and ethynodiol diacetate are first-generation progestin compounds that were the earliest progestin formulations to be used in contraception pills. Second-generation progestins include noresthisterone and levonorgestrel (LNG). LNG is the most commonly used progestin worldwide. Third-generation progestins, also referred to as new generation progestins, include desogestrel (etonogestrel), gestodene, and norgestimate. The new generation progestins have a higher efficacy rating and fewer effects on lipid and carbohydrate metabolism when compared to their earlier counterparts. They also have fewer androgenic side effects, which are described later in this chapter.
Drospirenone (DSRP) is a new-generation progestin derived from spironolactone. Because spironolactone is a potassium-sparing diuretic hormone, it can increase serum potassium levels in women taking CHC pills with DSRP, altering water and electrolyte balance. A progestin that comes in an injectable form is medroxyprogesterone acetate (MPA).
Amount of estrogen and type of progestin determine bioactivity and possible side effects of CHC products. The combination of estrogen and progestin causes the products to have estrogen-like activity or estrogenic activity, progesterone-like activity or progestational activity, and androgen-like activity or androgenic activity. The combination of estrogen and the selected progestin also has an effect on the uterine endometrium. The lowest effective dose that successfully prevents conception should be used.
Mechanism of Action
The estrogen component of combined hormone contraceptive products inhibits ovulation by preventing the formation of a dominant follicle. When a dominant follicle does not mature, estrogen remains at a consistent level and is unable to reach the peak level needed to stimulate the luteinizing hormone (LH) surge. The progestin component also suppresses the LH surge. When the LH surge is suppressed, ovulation is prevented and pregnancy does not occur. Any cycle, whether induced by drugs or naturally occurring, in which ovulation does not occur is called an anovulatory cycle. Therefore, CHCs produce drug-induced anovulatory cycles. The estrogen component of CHC products also stabilizes the uterine endometrium, inhibiting proliferation and secretory changes and decreasing occurrence of irregular and/or heavy bleeding. The progestational effects of progestin change the endometrium to make it less favorable for implantation of a fertilized ovum. In addition, progestins have an effect on the quantity and viscosity of the cervical mucus, making it thick and hostile to sperm penetration. Progestins alter the motility of both the muscles of the fallopian tube and the cilia within the tube, impeding the movement of the ovum through the tube.
Route of Delivery
There are several routes of administration for combined hormone contraception products. Most women are familiar with oral contraception, in which a pill is ingested daily, absorbed by the gastrointestinal tract, and metabolized by the liver. CHC products can also be administered through a transvaginal and transdermal route, under the trade names of NuvaRing and the Ortho-Evra transdermal contraceptive patch, respectively. The advantage of these alternative sites for administration is avoiding gastrointestinal absorption and the initial metabolism by the liver, or the “first-pass” effect. Theoretically, side effects such as nausea and vomiting, heart and circulatory risks, and noncompliance with a daily dosage regime may be avoided. Intramuscular and subcutaneous routes of administration for combined hormone contraception are not available in the United States.
Combined Oral Contraception Products
Combined oral contraception (COC) products are one of the most commonly used methods of reversible contraception in the world because of their ease of use, high degree of effectiveness, and relative safety. The theoretical effective rate (absolute correct use) for COC is 99.3%, while typical use effective rate (accounting for patient error) is around 92%. This means that the COC is 92% to 99.3% effective for contraception. When the pill was approved for use by the U.S. Food and Drug Administration (FDA) in 1960, little was known about the best combination of estrogen and progestin or their optimum effective doses. In the 1970s, research provided evidence that the adverse side effects, particularly heart and circulatory effects, were directly related to the dose of estrogen in the oral contraception product. The higher dose of estrogen increased risk for venous thromboembolism (VTE), myocardial infarction (MI), and stroke. Subsequently, “low-dose” oral contraception pills and, more recently, “very low-dose” contraception pills have been introduced. Low-dose COC pills greatly reduce the risk for dangerous side effects. A low-dose COC product contains ≤35 mcg of ethinyl estradiol or ≤50 mcg or less of mestranol and a progestin. Very low-dose CHC pills contain ≤20 mcg of EE and a progestin. Research continues to focus on actual and potential short- and long-term benefits and risks associated with use of low-dose oral contraceptives, particularly in the areas of heart and circulatory risks as well as carcinogenesis. Clinical studies also continue to investigate the venous circulatory effects of new-generation progestins.
COC formulations are differentiated based on the strength of the estrogen component, type of progestin used, and whether estrogen or progesterone (and androgen) activity predominate. Increased estrogenic activity may include side effects such as cyclic breast changes, dysmenorrhea (painful periods), menorrhagia (heavy periods), chloasma (hyperpigmentation of the skin), and VTE, while decreased estrogenic activity may cause amenorrhea (absence of periods) or spotting at certain points in the cycle. Increased progestational activity may cause weight gain, depression, fatigue, and decreased libido, while lack of progestational activity may cause breakthrough bleeding (BTB) and headaches. BTB is an episode of bleeding that occurs during the active pill cycle of COC products. It is more common at the start of COC use, when a woman changes her COC type of pill, and with progestin-only preparations of contraception. There is no evidence that an episode of BTB is associated with a decrease in the COC’s effectiveness as long as the patient continues to take the pill on daily basis and as prescribed. Increased androgenic activity may cause acne, hirsutism, edema, and cholestatic jaundice. The estrogens and progestins in oral contraception pills also have an effect on the uterine endometrium, which may cause changes in the patient’s periods, such as irregular bleeding, heavy or light periods, or spotting between periods. The undesirable side effects of hormonal contraception products are discussed later in this chapter.
Most women on COC products experience shorter, lighter periods. Other advantages with COC are decreased blood loss and uterine cramps, elimination of mittelschmerz (mid-cycle pain usually associated with ovulation), reduction of symptoms in many forms of benign breast disorders, and prevention of physiologic ovarian cysts. COC products also reduce the incidence of pelvic inflammatory disease (PID) and ectopic pregnancy, reduce endometrial and ovarian cancer risk, and reduce deaths from colorectal cancer. COC products do not reduce the incidence of sexually transmitted infections (STIs).
The goal of therapy is to identify the product that offers the best contraceptive protection while producing the fewest unwanted side effects as a result of either the estrogen or the progestin component. Note: the effectiveness of oral contraceptives can also be compromised by concurrent use of some medications (e.g., antibiotics) or herbal products (Herbal Alert 56-1).
Types of Combined Oral Contraception Products
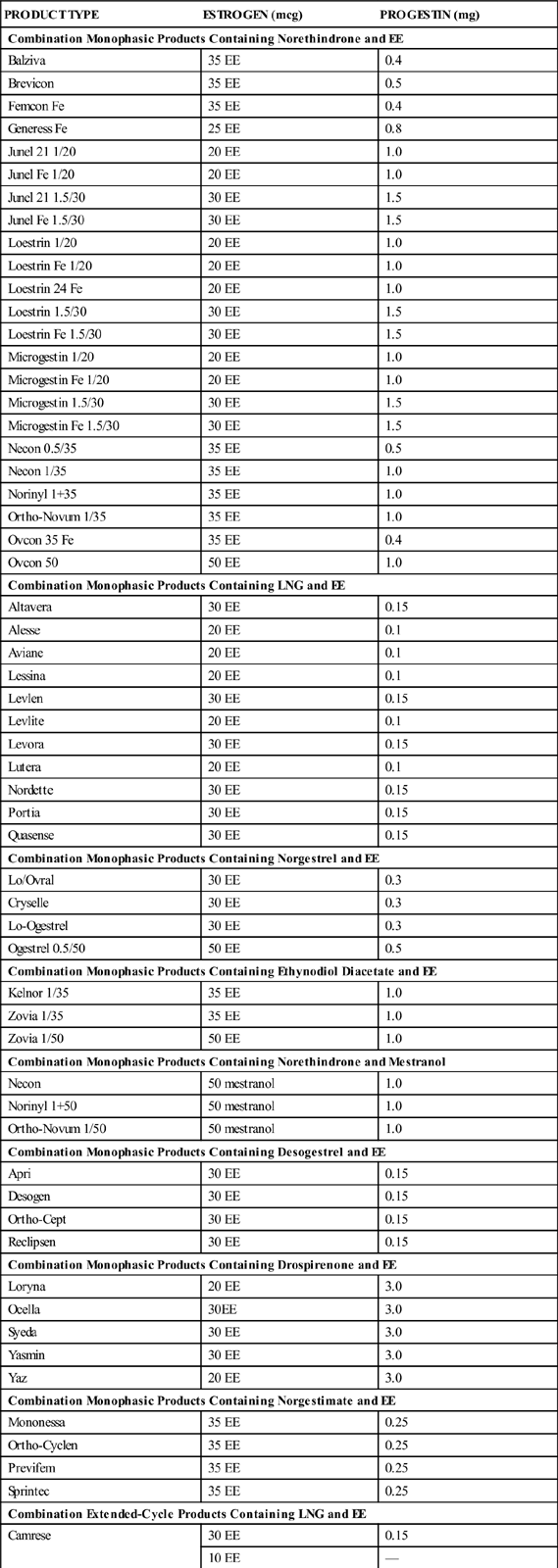
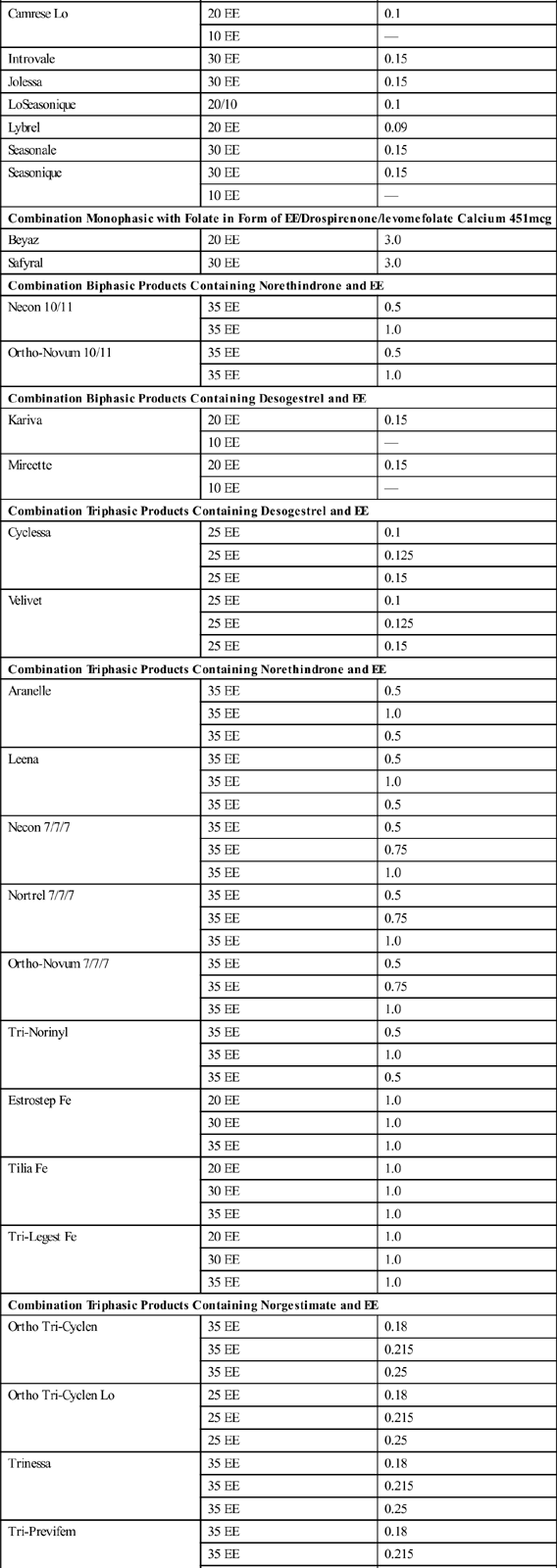
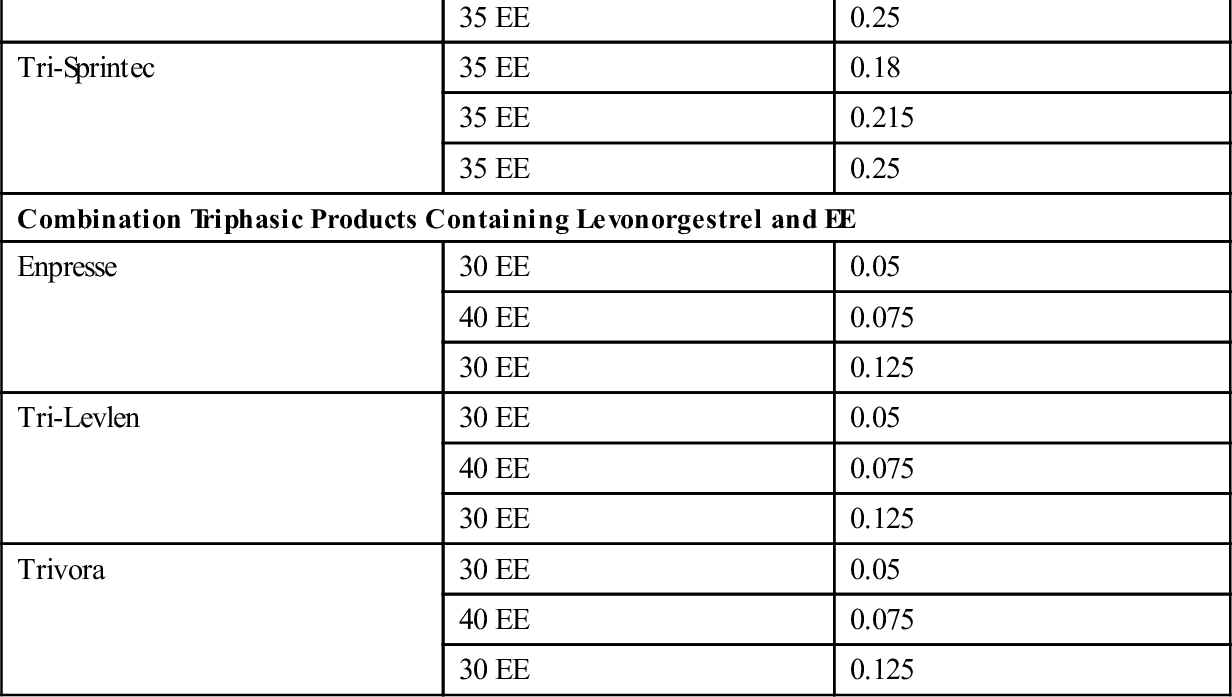
There are three types of combined oral contraception pill products: monophasic, biphasic, and triphasic. These types of COC pills have also been categorized as “phasic” and “nonphasic” pills. The monophasics, or nonphasic pills, provide a fixed ratio of estrogen to progestin throughout the menstrual cycle. In biphasics, a phasic pill, the amount of estrogen is fixed throughout the cycle, but the amount of progestin varies; reduced in the first half to provide for some proliferation of the endometrium and increased in the second half to promote secretory development of the endometrium. This simulates the normal physiologic process of menstruation while still inhibiting ovulation. Ortho-Novum 10/11 is an example of a biphasic pill (10 days of norethindrone 0.5 mg and EE 35 mcg followed by 11 days of norethindrone 1.0 mg and EE 35 mcg). The triphasics are the newest form of phasic COC products delivering low doses of both hormones with minimal side effects, including breakthrough bleeding. With triphasics, the amount of either estrogen or progesterone varies throughout the cycle in different ratios during three phases. Ortho Tri-Cyclen is an example of a triphasic pill that varies the dosage of progestin (7 days of norgestimate 0.180 mg and EE 35 mcg; 7 days norgestimate 0.215 mg and EE 35 mcg; 7 days of norgestimate 0.250 and EE 35 mcg). In Ortho Tri-CyclenLo, the progestin change is the same through the three phases as in Ortho Tri-Cyclen, but the dose of EE is lowered to 25 mcg, making this a low-dose pill option for women. Estrostep is an example of a triphasic pill that varies the dosage of estrogen (7 days of EE 20 mcg and norethindrone acetate 1.0 mg; 7 days of EE 30 mcg and norethindrone acetate 1.0 mg; and 7 days of EE 35 mcg and norethindrone acetate 1.0 mg). Ortho Tri-Cyclen, Ortho Tri-CyclenLo, and Estrostep have low androgenic activity and are FDA approved for the treatment of acne. Table 56-1 shows brand-name forms of COC, amounts of EE and type of progestin, and generic equivalents.
TABLE 56-1
ORAL CONTRACEPTION COMBINATION PRODUCTS: LISTED BY TYPE OF PROGESTIN
| PRODUCT TYPE | ESTROGEN (mcg) | PROGESTIN (mg) |
| Combination Monophasic Products Containing Norethindrone and EE | ||
| Balziva | 35 EE | 0.4 |
| Brevicon | 35 EE | 0.5 |
| Femcon Fe | 35 EE | 0.4 |
| Generess Fe | 25 EE | 0.8 |
| Junel 21 1/20 | 20 EE | 1.0 |
| Junel Fe 1/20 | 20 EE | 1.0 |
| Junel 21 1.5/30 | 30 EE | 1.5 |
| Junel Fe 1.5/30 | 30 EE | 1.5 |
| Loestrin 1/20 | 20 EE | 1.0 |
| Loestrin Fe 1/20 | 20 EE | 1.0 |
| Loestrin 24 Fe | 20 EE | 1.0 |
| Loestrin 1.5/30 | 30 EE | 1.5 |
| Loestrin Fe 1.5/30 | 30 EE | 1.5 |
| Microgestin 1/20 | 20 EE | 1.0 |
| Microgestin Fe 1/20 | 20 EE | 1.0 |
| Microgestin 1.5/30 | 30 EE | 1.5 |
| Microgestin Fe 1.5/30 | 30 EE | 1.5 |
| Necon 0.5/35 | 35 EE | 0.5 |
| Necon 1/35 | 35 EE | 1.0 |
| Norinyl 1+35 | 35 EE | 1.0 |
| Ortho-Novum 1/35 | 35 EE | 1.0 |
| Ovcon 35 Fe | 35 EE | 0.4 |
| Ovcon 50 | 50 EE | 1.0 |
| Combination Monophasic Products Containing LNG and EE | ||
| Altavera | 30 EE | 0.15 |
| Alesse | 20 EE | 0.1 |
| Aviane | 20 EE | 0.1 |
| Lessina | 20 EE | 0.1 |
| Levlen | 30 EE | 0.15 |
| Levlite | 20 EE | 0.1 |
| Levora | 30 EE | 0.15 |
| Lutera | 20 EE | 0.1 |
| Nordette | 30 EE | 0.15 |
| Portia | 30 EE | 0.15 |
| Quasense | 30 EE | 0.15 |
| Combination Monophasic Products Containing Norgestrel and EE | ||
| Lo/Ovral | 30 EE | 0.3 |
| Cryselle | 30 EE | 0.3 |
| Lo-Ogestrel | 30 EE | 0.3 |
| Ogestrel 0.5/50 | 50 EE | 0.5 |
| Combination Monophasic Products Containing Ethynodiol Diacetate and EE | ||
| Kelnor 1/35 | 35 EE | 1.0 |
| Zovia 1/35 | 35 EE | 1.0 |
| Zovia 1/50 | 50 EE | 1.0 |
| Combination Monophasic Products Containing Norethindrone and Mestranol | ||
| Necon | 50 mestranol | 1.0 |
| Norinyl 1+50 | 50 mestranol | 1.0 |
| Ortho-Novum 1/50 | 50 mestranol | 1.0 |
| Combination Monophasic Products Containing Desogestrel and EE | ||
| Apri | 30 EE | 0.15 |
| Desogen | 30 EE | 0.15 |
| Ortho-Cept | 30 EE | 0.15 |
| Reclipsen | 30 EE | 0.15 |
| Combination Monophasic Products Containing Drospirenone and EE | ||
| Loryna | 20 EE | 3.0 |
| Ocella | 30EE | 3.0 |
| Syeda | 30 EE | 3.0 |
| Yasmin | 30 EE | 3.0 |
| Yaz | 20 EE | 3.0 |
| Combination Monophasic Products Containing Norgestimate and EE | ||
| Mononessa | 35 EE | 0.25 |
| Ortho-Cyclen | 35 EE | 0.25 |
| Previfem | 35 EE | 0.25 |
| Sprintec | 35 EE | 0.25 |
| Combination Extended-Cycle Products Containing LNG and EE | ||
| Camrese | 30 EE | 0.15 |
| 10 EE | — | |
| Camrese Lo | 20 EE | 0.1 |
| 10 EE | — | |
| Introvale | 30 EE | 0.15 |
| Jolessa | 30 EE | 0.15 |
| LoSeasonique | 20/10 | 0.1 |
| Lybrel | 20 EE | 0.09 |
| Seasonale | 30 EE | 0.15 |
| Seasonique | 30 EE | 0.15 |
| 10 EE | — | |
| Combination Monophasic with Folate in Form of EE/Drospirenone/levomefolate Calcium 451 mcg | ||
| Beyaz | 20 EE | 3.0 |
| Safyral | 30 EE | 3.0 |
| Combination Biphasic Products Containing Norethindrone and EE | ||
| Necon 10/11 | 35 EE | 0.5 |
| 35 EE | 1.0 | |
| Ortho-Novum 10/11 | 35 EE | 0.5 |
| 35 EE | 1.0 | |
| Combination Biphasic Products Containing Desogestrel and EE | ||
| Kariva | 20 EE | 0.15 |
| 10 EE | — | |
| Mircette | 20 EE | 0.15 |
| 10 EE | — | |
| Combination Triphasic Products Containing Desogestrel and EE | ||
| Cyclessa | 25 EE | 0.1 |
| 25 EE | 0.125 | |
| 25 EE | 0.15 | |
| Velivet | 25 EE | 0.1 |
| 25 EE | 0.125 | |
| 25 EE | 0.15 | |
| Combination Triphasic Products Containing Norethindrone and EE | ||
| Aranelle | 35 EE | 0.5 |
| 35 EE | 1.0 | |
| 35 EE | 0.5 | |
| Leena | 35 EE | 0.5 |
| 35 EE | 1.0 | |
| 35 EE | 0.5 | |
| Necon 7/7/7 | 35 EE | 0.5 |
| 35 EE | 0.75 | |
| 35 EE | 1.0 | |
| Nortrel 7/7/7 | 35 EE | 0.5 |
| 35 EE | 0.75 | |
| 35 EE | 1.0 | |
| Ortho-Novum 7/7/7 | 35 EE | 0.5 |
| 35 EE | 0.75 | |
| 35 EE | 1.0 | |
| Tri-Norinyl | 35 EE | 0.5 |
| 35 EE | 1.0 | |
| 35 EE | 0.5 | |
| Estrostep Fe | 20 EE | 1.0 |
| 30 EE | 1.0 | |
| 35 EE | 1.0 | |
| Tilia Fe | 20 EE | 1.0 |
| 30 EE | 1.0 | |
| 35 EE | 1.0 | |
| Tri-Legest Fe | 20 EE | 1.0 |
| 30 EE | 1.0 | |
| 35 EE | 1.0 | |
| Combination Triphasic Products Containing Norgestimate and EE | ||
| Ortho Tri-Cyclen | 35 EE | 0.18 |
| 35 EE | 0.215 | |
| 35 EE | 0.25 | |
| Ortho Tri-Cyclen Lo | 25 EE | 0.18 |
| 25 EE | 0.215 | |
| 25 EE | 0.25 | |
| Trinessa | 35 EE | 0.18 |
| 35 EE | 0.215 | |
| 35 EE | 0.25 | |
| Tri-Previfem | 35 EE | 0.18 |
| 35 EE | 0.215 | |
| 35 EE | 0.25 | |
| Tri-Sprintec | 35 EE | 0.18 |
| 35 EE | 0.215 | |
| 35 EE | 0.25 | |
| Combination Triphasic Products Containing Levonorgestrel and EE | ||
| Enpresse | 30 EE | 0.05 |
| 40 EE | 0.075 | |
| 30 EE | 0.125 | |
| Tri-Levlen | 30 EE | 0.05 |
| 40 EE | 0.075 | |
| 30 EE | 0.125 | |
| Trivora | 30 EE | 0.05 |
| 40 EE | 0.075 | |
| 30 EE | 0.125 | |
EE, Ethinyl estradiol; LNG, levonorgestrel; mcg, microgram; mg, milligram.
CHCs, Pregnancy category X; progestin-only products, pregnancy category X.
A new-generation progestin, Yasmin is a monophasic combination pill with 30 mcg of ethinyl estradiol and 3 mg of drospirenone (DSRP). While the progestin found in other combination oral contraceptives is structurally similar to androgens, drospirenone is the only progestin derived from spironolactone, which is structurally similar to progesterone. As noted, use of drospirenone may increase serum potassium, which can alter water and electrolyte balances in women using this product. Consequently, Yasmin is contraindicated in women with kidney, liver, or adrenal insufficiency and in women who require daily long-term treatment with any of the following medications: NSAIDs (e.g., ibuprofen) taken long-term and daily for arthritis or other diseases or conditions, potassium-sparing diuretics (e.g., spironolactone), potassium supplementation, ACE inhibitors (e.g., Accupril, Lotensin), angiotensin-II receptor antagonists (e.g., Cozaar, Diovan), and heparin.
Femcon Fe (norethindrone 0.4 mg and EE 35 mcg) and Generess Fe (norethindrone 0.8 mg and EE 25 mcg) are mint-flavored, chewable birth control pills for women who have difficulty swallowing pills. They can also be swallowed whole.
Withdrawal Bleeding
Most of the monophasic, biphasic, and triphasic combined oral contraception products are packaged in both 21-day tablet packs and 28-day tablet packs. In the 21-day tablet packs there are 21 days of active pills (pills that contain estrogen and progestin) followed by a 7-day “pill-free” period. A new pack of pills is started after the 7 day pill-free period. In the 28-day tablet pack, there are 21 days of active pills followed by 7 days of inert pills, called counters. The patient takes one pill daily and begins a new pack the day after the last counter pill is taken. During the hormone-free period (counters) or the 7-day pill-free period, the level of estrogen and progestin decreases allowing for a breakdown of the endometrial lining. This causes a pseudomenstruation known as withdrawal bleeding or withdrawal menses. The withdrawal bleeding is not a true menstrual period and the bleeding experienced by a woman can vary in amount and duration.
There are 28-day oral contraception pills that do provide medication during the 7-day hormone-free period. Estrostep Fe, Loestrin Fe 1.5/30, Loestrin Fe 1/20, Loestrin 24 Fe, and Generess Fe contain ferrous fumarate, an iron compound, to provide iron supplementation during the phase of withdrawal bleeding. This promotes healthy iron stores in women and protection against menstrual-associated iron-deficiency anemia. Another formulation is Mircette (desogestrel 0.15 mg and EE 20 mcg), which supplies 2 inert pills and 5 pills with 10 mcg of ethinyl estradiol during the counter phase. The use of low-dose estrogen during the “inert” phase of the pill cycle provides a greater degree of ovarian suppression. Another benefit may be the decrease in headaches associated with estrogen withdrawal. Patients who experience headaches associated with the menstrual cycle may benefit from Mircette.
Withdrawal bleeding periods are scheduled monthly to mimic a normal 28-day menstrual cycle; however, researchers have established that a monthly episode of withdrawal bleeding is not necessary to maintain a healthy uterus. There are now FDA-approved formulations of estrogen-progestin combination pills that do one of the following: (1) shorten the period of withdrawal bleeding by extending the number of active pills and decreasing the number of inert pills in the 28-day pill cycle, (2) decrease the number of withdrawal menses per year by having 81 to 84 continuous days of active pills and 7 days of less active pills, resulting in four withdrawal menses per year, or (3) eliminate withdrawal bleeding altogether by continuous oral administration of active pills.
Extended-Cycle COC Products
Loestrin 24 Fe is a 24-day monophasic hormonal regimen of 20 mcg of ethinyl estradiol tablets and 1 mg of norethindrone acetate, plus 4 ferrous fumarate tablets. It provides 24 days of active hormonal therapy and 4 days of hormone-free pills containing an iron supplement. The active pill days are extended, while the number of inert pill days are reduced, which shortens the period of withdrawal bleeding that the patient will experience. The shorter period of hormone-free tablets increases the contraceptive’s efficacy.
Like Yasmin, Yaz (drospirenone 3 mg and EE 20 mcg), another extended-cycle combined hormone pill, contains the progestin drospirenone (DRSP), a spironolactone analogue with antimineralocorticoid properties. The 24-day active hormone pill regimen of Yaz combined with the 30-hour half-life of drospirenone results in less hormonal fluctuation between cycles, compared with traditional oral contraceptives with 21 days of active pills and 7 days of placebos. Preclinical studies indicate that drospirenone also provides an antiandrogenic effect, which means that it counteracts the effects of male hormones in the body. Yaz is FDA approved for the treatment of premenstrual dysphoric disorder (PMDD) as well as acne. It has the same serum potassium precautions as Yasmin. As with Loestrin 24 Fe, women experience a shorter and lighter withdrawal bleeding period with increased ovarian suppression.
Beyaz, with 24 pills containing 3 mg DSRP, 20 mcg of EE, and 0.451 mg of levomefolate calcium (a folic acid supplement), and with 4 pills of 0.451 mg of levomefolate calcium in the inert phase, is also available in the United States. Produced by the same pharmaceutical company as Yasmin and Yaz, Beyaz contains DSRP and has the same precautions as Yasmin and Yaz; however, it also contains a form of folic acid throughout the pill cycle promoting healthy folic acid levels for reproductive-age patients. Therefore, Beyaz may help prevent open neural tube defects, should the patient plan to become pregnant. Beyaz is also approved for use in the treatment of PMDD. In April 2012, the FDA issued an updated warning that women taking combined oral contraception products containing drospirenone may have a twofold increased risk for venous blood clot formation compared with women taking CHC products that do not contain DSRP. Women considering a contraceptive product containing DSRP should be made aware of this risk.
Continuous Dosing COC Products
Seasonale (Jolessa) is a continuous dosing combined hormone contraception pill. The 91-day regimen includes 84 days of active pills and 7 days of inert pills. Seasonale (Jolessa) causes withdrawal bleeding to occur just four times per year. The active hormone pills in Seasonale (Jolessa) contain 30 mcg of ethinyl estradiol (EE) and 0.15 mg of levonorgestrel (LNG). Seasonale (Jolessa) is the drug predecessor of Seasonique.
Seasonique is a continuous dosing contraception pill that causes withdrawal bleeding four times per year. It cycles 84 days of tablets that contain 30 mcg of EE and 0.15 mg of LNG, followed by 7 days of tablets that contain 10 mcg EE. During the 7 days of low-dose estrogen pills, women usually experience withdrawal menses. However, by adding very low levels of estrogen in the 7-day “break” period, instead of inert pills, Seasonique provides additional benefits such as a reduction in breakthrough bleeding. LoSeasonique contains 20 mcg of EE and 0.1 mg of LNG, followed by 7 days of 10 mcg of EE, offering a very low-dose continuous CHC product option. Users of extended-cycle birth control pills are more likely to experience bleeding or spotting between periods. The continued progestin dose causes extreme atrophy of the endometrial lining. The atrophic endometrium subsequently breaks down, and the patient experiences uterine bleeding in an irregular pattern.
Lybrel is the first continuous dose combined oral contraception pill to be FDA approved. Lybrel comes in a 28-day pack and contains 20 mcg of EE and 90 mcg of LNG. Lybrel is taken daily and continuously without interruption for withdrawal menses.
Although these products are more commonly known for their contraceptive value, women with menstrual disorders such as menorrhagia (heavy periods), metrorrhagia (irregular bleeding between periods, usually heavy), endometriosis, dysmenorrhea, premenstrual syndrome (PMS), and physiologic ovarian cyst formation may benefit from continuous-cycle COC products because of their ability to suppress ovarian function and limit uterine bleeding.
Ortho-Evra Transdermal Patch
The Ortho-Evra patch is a weekly form of combined hormone contraception, consisting of 750 mcg of ethinyl estradiol and 6 mg of the progestin norelgestromin (NGMN) delivered through a transdermal system. It is a thin plastic patch placed on the skin of the buttocks, stomach, upper outer arm, or upper torso. The patch is placed once a week for 3 weeks in a row. The fourth week is patch-free to allow for withdrawal bleeding. It should be placed on clean, dry skin; placement on or near the breasts should be avoided because of the estrogen component. Site of patch placement should be rotated to avoid skin irritation. If the patch partially or completely detaches from the skin, a new patch should be placed. When used correctly, the patch protects against pregnancy on a monthly basis. The theoretical and typical use effective rate for the patch is 99.3% and 92%, respectively, making it 92% to 99.3% effective at preventing pregnancy.
The patch works in a similar manner to COC pills by inhibiting ovulation, thickening cervical mucus to prevent sperm penetration, and preventing a fertilized egg from implanting in the uterus. The patch avoids the first-pass through the liver effect. Advantages include not having to remember to take a pill daily. As with combined oral contraception products, the ability to become pregnant returns quickly when its use is discontinued. There is less menstrual flow and cramping, acne, iron-deficiency anemia, excess body hair, premenstrual symptoms, and vaginal dryness with the patch. As with COC pills, the patch reduces the risk for ovarian and endometrial cancers, PID, breast cysts, ovarian cysts, and osteoporosis (loss of bone mass predisposing the patient to fractures). With the patch, there are fewer occurrences of ectopic pregnancy.
Disadvantages of the patch include skin reaction at the site of application, menstrual cramps, and a change in vision or the inability to wear contact lenses; it is not as effective for women who weigh more than 198 lb. In 2005, the FDA approved an addition to the labeling of the Ortho Evra transdermal patch stating that it exposes patients to higher levels of estrogen. In theory, this indicated that women using the patch for contraception may be at an increased risk for venous thromboembolism (VTE). With CHC pills, peak serum estrogen levels are reached rapidly after ingestion and then steadily decline. With the patch, peak serum estrogen levels when reached are 25% less than in women taking the pill, however, these peak levels remain throughout the patch placement week, and then decline, exposing women to 60% more estrogen than with the CHC pill. Ongoing research is necessary to demonstrate exact risk for VTE as well as other cardiovascular complications (particularly heart attack and stroke) in women using the Ortho Evra transdermal patch. Women being prescribed the transdermal route of CHC should be notified of potential risks, and the patch should be used with extreme caution in any patient with increased risk for VTE. Women who are older than 35 years and smoke should not use the Ortho Evra transdermal patch. Other side effects include temporary irregular bleeding, weight gain or loss, breast tenderness, and nausea.
NuvaRing Transvaginal Contraception
NuvaRing is a 2-inch–diameter flexible indwelling ring inserted into the vagina. It is nonbiodegradable, transparent, and colorless to almost colorless. NuvaRing releases 15 mcg of EE and 120 mcg of the progestin etonogestrel per day, similar to the quantities of estrogen and progestin found in lower-dose COC products. Etonogestrel is a biologically active metabolite of desogestrel and a new-generation progestin, which may be associated with an increased risk for VTE. Also, NuvaRing has an extended placement schedule, which may mean that the estrogen peak rate, although lower than that of CHC pills, remains sustained longer because of the 3-week ring placement. As with the Ortho Evra transdermal patch, this may expose the patient to higher levels of estrogen. Studies have been inconclusive, and the FDA has placed no additional warning on NuvaRing.
Theoretical effectiveness and typical effectiveness rates are 98% and 92%, respectively, reflecting a rate similar to other leading CHC contraceptive methods. The patient inserts the ring during the first 5 days of the menstrual cycle. She then removes the ring after 3 weeks, remains “ring-free” for 1 week (for withdrawal menses), and then inserts a new ring. Backup contraception is recommended during the first 7 days after the first ring is placed. During this time, the hormones reach an appropriate protective level. After this, contraceptive effects are expected to be continuous provided the ring is correctly inserted. Correct insertion involves placing the ring into the middle or upper third of the vagina. Unlike the diaphragm, it does not need to be placed near or over the cervix. It is the close proximity of the ring to the vaginal mucosa that causes absorption of steroid hormones to occur. The ring remains in place during intercourse, tampon use, or the administration of intravaginal medications. If the ring slips out, it can be rinsed with lukewarm water and reinserted into the vagina. It should be reinserted within 3 hours after becoming dislodged. If the ring remains out for more than 3 hours, additional contraception is required until the ring has been in place for 7 days. Possible side effects include vaginal discharge, irritation, or infection. Other associated risks are the same as for low-dose COC products and are increased in patients who smoke.
Progestin-Only Contraception Products
Progestin-only contraception products do not contain estrogen. The estrogen component of contraceptives increases the risk of circulatory disorders; therefore, these products allow contraception to be available for women who cannot take estrogen-progestin combination products. Advantages of progestin-only contraception products include relative safety, ease of use, spontaneity of sexual intercourse, and reversibility. However, because the estrogen component is missing, these products have a higher incidence of irregular bleeding and spotting, as well as the possibility of depression, mood changes, decreased libido, fatigue, and weight gain. Progestin-only contraception products do not protect women against STIs. Women who cannot take estrogen but may be candidates for progestin-only contraception products include patients with personal or strong family history of VTE or heart disease, breastfeeding patients, smokers older than 35 years of age, and women with uncontrolled hypertension. Women who have an untoward response to estrogenic effects such as chloasma, migraine headaches, or changes in lipid profiles may also be candidates for progestin-only products. Progestin-only contraceptive methods are available in oral, intramuscular, subcutaneous, and implantable routes of delivery.
Progestin-Only Oral Contraception Pill
The progestin-only oral contraception pill (POP), called the minipill, has four mechanisms of action: (1) alteration in cervical mucus, making it thick and viscous, which blocks sperm penetration; (2) interference with the endometrial lining, which makes implantation difficult; (3) decreased peristalsis in the fallopian tubes, slowing the transport of ovum; and (4) in approximately 50% of cycles, interference with the LH surge inhibiting ovulation. POPs that contain 0.35 mg of norethindrone as the progestin include Micronor, Nor-QD, Aygestin and their generic equivalents, Errin, Jolivette, Nora-BE and Camila. Ovrette is a POP that contains 0.075 mg of norgestrel. The minipill is taken continuously, without a break for withdrawal bleeding. Patients should be instructed to take the minipill daily, within a 3-hour window.
The theoretical effective and typical use effective rates for the first year of use are similar to that of COC products even though patient adherence to dosage schedule with POPs is more specific. It takes 4 to 6 hours for the progestin to thicken the cervical mucus to prevent sperm penetration, and the duration of the effect of the progestin on cervical mucus lasts just over 24 hours. Risk for pregnancy will increase if a patient misses a pill because POPs do not suppress the release of follicle-stimulating hormone (FSH) and LH to the same degree as COC products. If the minipill is taken more than 3 hours late, a backup contraceptive method should be used for 48 hours. There are no placebo pills in a pack of progestin-only pills. All 28 pills contain active hormones, so the patient continuously takes one active pill daily. Because the endometrial lining is altered, an increase in the amount of irregular bleeding is noted.
Depo-Provera
Depo-Provera is a highly effective, long-acting injectable progestin in the form of depot-medroxyprogesterone acetate (DMPA or MPA), with a theoretical and typical use efficacy rate of 99% and 97%, respectively. This makes Depo-Provera, the brand name for DMPA, one of the most effective hormonal methods of contraception at 97% to 99%. It appeals to women because of its confidentiality of use and convenient dosing schedule. DMPA is popular for adolescents for these reasons. Depo-Provera is administered in a flexible dosing schedule, every 11 to 13 weeks. The mechanism of action of Depo-Provera relies on the progestational activities: thickening of the cervical mucus, thinning of the uterine endometrium, and decrease in fallopian tube motility. Because the progestin in Depo-Provera reaches a higher circulating level than with POPs, DMPA inhibits both FSH and LH secretion from the anterior pituitary gland. This results in both anovulation (lack of ovulation) and amenorrhea. Because FSH and LH secretion is inhibited, any formation of a dominant follicle is prohibited and the production of estrogen in the body is greatly decreased. The patient experiences a hypoestrogen state, which can affect bone mineral density (BMD).
The depot-medroxyprogesterone vial or prefilled syringe should be vigorously shaken just prior to administration to ensure a uniform medication suspension. Depo-Provera, 150 mg, is given by deep IM injection into the gluteal, ventrogluteal, or deltoid muscle. Depo-Provera is more effective if given in the ventrogluteus muscle, although research has not supported this. The site should not be massaged after injection. The injection site is documented so that sites can be rotated. The patient is given a personalized calendar for subsequent doses and should return for another injection within 13 weeks. If the patient is late for her injection (13 weeks and 1 day), pregnancy should be ruled out before she receives another injection.
As with oral contraceptives, there is no protection against STIs. There is concern that MPA may cause a loss of bone mineral density, and results of research studies are still controversial. Most recent evidence suggests that at least partial or full recovery occurs in BMD of the spine, with partial recovery occurring in BMD of the hip after the patient discontinues Depo-Provera use. Until ongoing research is conclusive, the FDA recommends that Depo-Provera be discontinued after 2 years of continuous use unless other methods of contraception are inadequate. Many professional provider organizations agree that the concerns of the 2-consecutive-year limit given by the FDA and the BMD effects of DMPA should not prevent the practitioner from considering the benefit-risk ratio for each individual patient. The American College of Obstetricians and Gynecologists (ACOG) states in a committee opinion that “the possible adverse effects of DMPA must be carefully balanced against the significant personal and public health impact of unintended pregnancy.” The benefits, risks, and alternatives, as well as the prevention of bone mineral density loss while on Depo-Provera, must be discussed with the patient before administration of the product.
Women taking Depo-Provera should be instructed to increase calcium and vitamin D intake to the daily recommended allowance for their age, as well as to participate in regular weight-bearing exercises. Depo-Provera is safe to receive immediately postpartum. Women can breastfeed while using this contraceptive without affecting milk supply. The most common side effects include initially irregular uterine bleeding or spotting. Menstruation may cease about 1 year after starting Depo-Provera. In addition, Depo-Provera has been shown to cause progressive weight gain in some women. Other side effects include breast tenderness and an increase in depression. The drug is contraindicated in cases of undiagnosed vaginal bleeding and known or suspected pregnancy. Caution should be used in giving DMPA postpartum in women who are at risk for or have a history of postpartum depression.
Depo-subQ Provera 104 is available as an injectable suspension. It contains the same progestin in Depo-Provera, MPA, but it is administered in a subcutaneous injection. The dose is 104 mg/0.65 mL, and it is given to women every 11 to 13 weeks. Depo-subQ Provera 104 has the same mechanism of action, benefits, and risks as Depo-Provera, and women should be counseled about the potential loss of bone mineral density. Depo-Provera and Depo-subQ Provera 104 have a slower return to fertility than other hormonal methods of contraception.
Implantable Progestins
Nexplanon is a single-rod device containing 68 mg of the progestin etonogestrel; it is placed in the same location as Implanon. It is bioequivalent to Implanon, lasting for up to 3 years and removed in the same manner. Training is also provided for practitioners for the insertion and removal of Nexplanon. The difference is that Nexplanon contains barium, a radio-opaque substance that can help locate the device on two-dimensional x-ray, ultrasound, magnetic resonance imaging (MRI) and computed tomography (CT) scanning if necessary. Also, the device comes in a new, preloaded application system that will reduce insertion errors. Nexplanon may not be as effective in women who have a BMI greater than 30 (obese) or are on medications that induce liver enzymes. Patient education will be the same as that for Implanon.
Theoretical and typical effectiveness rates for implantable progestins are the same, at 99.6%.
Pharmacokinetics: Estrogen-Progestin Combination Products
EE is rapidly absorbed orally. It undergoes significant first-pass metabolism and elimination via the liver. Mestranol is converted in the liver to EE, which is 97% to 98% bound to plasma proteins. The half-life varies from 6 to 20 hours. Excretion is via bile and urine in a conjugated form. Some enterohepatic recirculation occurs. The steroid hormones in NuvaRing are absorbed through the vaginal mucosa into veins that flow directly into the inferior vena cava. The steroid hormones pass through the circulatory system and directly to the uterus, causing a “first-uterine” pass. The etonogestrel in NuvaRing has a bioavailability of 100% when administered vaginally, compared with EE bioavailability of 55%. With the Ortho-Evra patch, absorption is through the skin into venous circulation, also bypassing the hepatic portal system. Avoiding the liver first-pass effect decreases adverse enterohepatic reactions. The norelgestromin in the patch binds to albumin. Levels of serum steroid hormones in the patch reach constant levels of contraceptive efficacy within 48 hours. It is suggested that the alternative routing may increase the incidence of venous thromboembolitic events because peak levels of serum EE, although lower than that of oral ingestion, remain in the body for a longer duration. Alternative routing may decrease the incidence of irregular bleeding and nausea (NuvaRing only). Also, the avoidance of first-pass through the liver has the potential to decrease adverse drug interactions. Serum hormone levels are rapidly reached and blood levels do not fluctuate as much as is seen with oral contraception products.
Pharmacokinetics: Progestin-Only Products
Progestins are also well absorbed orally. Peak plasma levels occur from 0.5 to 4 hours after ingestion, depending on the particular compound. Norethynodrel and ethynodiol diacetate are converted to norethindrone. Levonorgestrel is bioavailable and does not undergo first-pass liver metabolism; norethindrone undergoes first-pass metabolism and is 65% available. The progestins are bound to plasma proteins and to sex-hormone–binding globulin. The half-life of norethindrone varies from 5 to 14 hours; the half-life of levonorgestrel is 11 to 45 hours. MPA is without androgenic and estrogenic activity. Depot-MPA is crystalline suspension. DMPA is not soluble in lipid or water. The crystalline deposits in body tissues and then is reabsorbed. Depo-Provera provides higher peak levels of progestin than POPs and Implanon. Once injected, the levels of MPA increase for 3 weeks, and then remain stable. MPA is not detected in the blood between 120 and 200 days after injection. Nexplanon is a sustained-release system that releases progestin at a level of 60 to 70 mcg/day during the first six weeks after insertion and 35 to 45 mcg/day during the first year. This decreases to 30 to 40 mcg/day after 2 years of implantation and 25 to 30 mcg/day by the end of the third year. Once the rod is inserted, effective contraceptive levels are reached within 8 hours.
Start Date and Dosing Schedule
There are three ways to implement the start of hormonal contraception products unless otherwise indicated by the pharmaceutical company manufacturing the product.
With the first-day start method, the contraception product is initiated on the first day of menstruation, or the first day a women experiences bleeding. The first day of bleeding is day 1 of the menstrual cycle. Days are then counted 2, 3, 4, 5, 6, and so on, until the first-day bleeding begins again, usually around day 28. Most methods of contraception can be safely started on day 1 through day 5 of the menstrual cycle, when it is less likely that the patient has an early undiagnosed pregnancy. No backup method of contraception is needed when the product is started on the first through fifth day of menstruation. (A backup method is a second method of contraception that is used until the primary method reaches its peak level of contraceptive effectiveness.) Usually this is a barrier method, such as a condom or diaphragm.
Many products require a Sunday start (patient starts the tablets or patch on the Sunday after the first day of menstruation). If menstruation actually starts on Sunday, the patient starts her tablet or patch on that day. The Sunday start aids a woman in remembering the first day of her contraception cycle. If a patient starts her method of contraception later than day 5 of her menstrual cycle, a backup form of contraception should be used for 7 days.
The quick-start method of initiating contraception starts the method on the day the patient receives the prescription regardless of where she might be in her menstrual cycle. This method increases patient compliance and resolves the risk for becoming pregnant while waiting for a menstrual period to begin to start the method. Pregnancy should be ruled out prior to the quick-start method, but there is a risk that the patient could have an early pregnancy undetectable by screening. A backup method of contraception must be used for 7 days if the quick-start method is employed after the first 5 days of the menstrual cycle. Both estrogen-progestin and progestin-only contraception methods are contraindicated in pregnancy (Category X). Nonetheless, there is no evidence of fetal risks associated with these medications when inadvertently used in pregnancy. If the patient does not get a withdrawal menses when planned, a pregnancy test is administered.
Special Considerations
Depo-Provera and NuvaRing should be started within the first 5 days of the menstrual cycle. (Sunday start and quick-start methods are off-label.) Seasonale (Jolessa) and Seasonique use a Sunday start only. Lybrel uses a first-day start. If the patient is on a 21-day combined oral contraception regimen, she restarts her next pack following the 7-day break whether or not her bleeding has stopped. With 28-day packs, a pill is taken daily without stopping regardless of bleeding pattern. Usually, withdrawal menses occur in a cyclic fashion. In biphasic and triphasic preparations, the day 1 pill is clearly marked and the tablets are taken in the order noted. A difference in the color of the tablets delineates the change in dose of estrogen or progestin through the phases. With the POP, a pill is taken daily without a break. To increase effectiveness, all oral contraception pills should be taken at the same time daily. With the POP, women should strictly adhere to this instruction.
Missed Doses
Table 56-2 presents guidelines for missed doses of oral contraceptives. All patients who are prescribed oral methods of contraception should also be instructed in the use of emergency contraception.
TABLE 56-2
GUIDELINES FOR MISSED DOSES OF ORAL CONTRACEPTIVES
| MISSED DOSE | RECOMMENDATIONS |
| Combination Products | |
| One tablet | Take tablet as soon as realized. Take next tablet as scheduled. |
| Two tablets | Take 2 tablets as soon as realized and 2 tablets the next day Use backup method of contraception for rest of cycle. |
| Three tablets | Discontinue present pack, and allow for withdrawal bleeding. Start new package of tablets 7 days after last tablet taken. Use another form of contraception until tablets have been taken for 7 consecutive days. |
| Progestin-Only Products | |
| One or more tablets | Take tablet as soon as realized, and follow with next tablet at regular time, PLUS use backup method of contraception for 48 h |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


