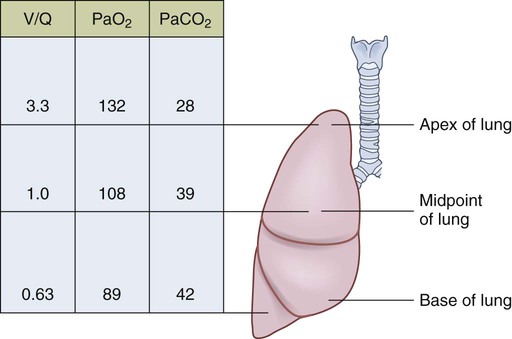
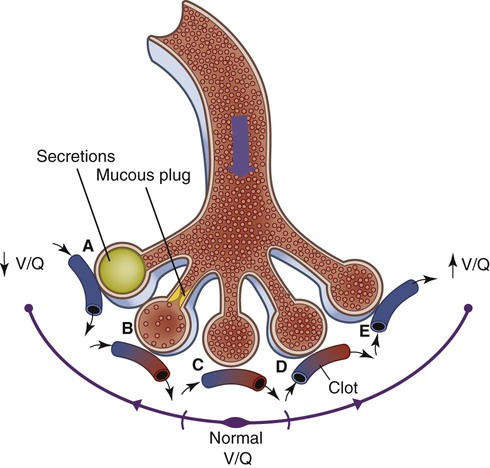
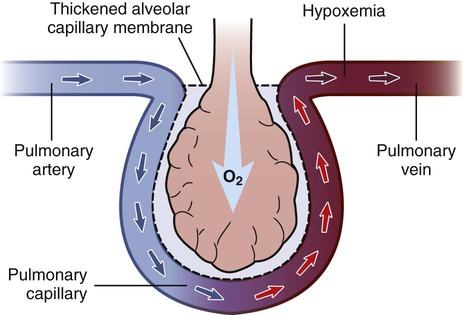
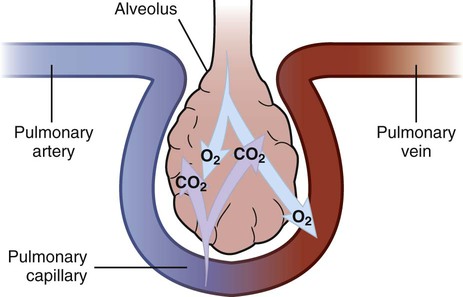
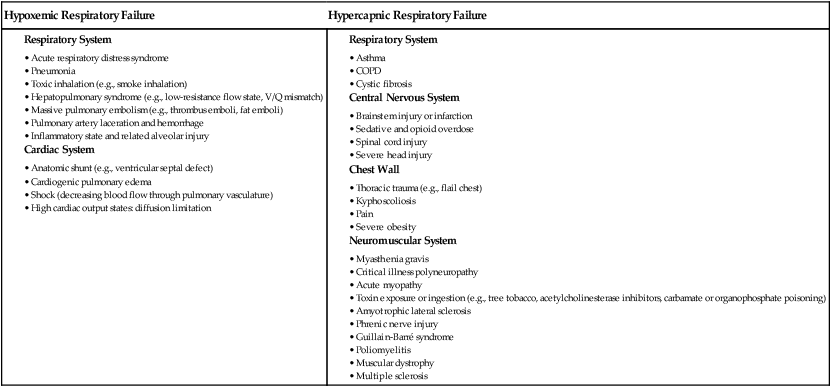
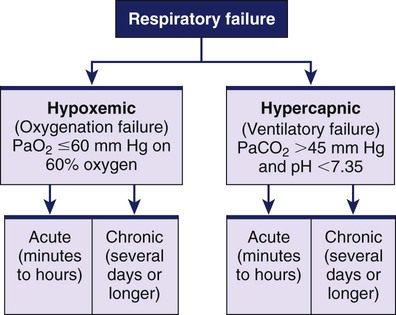
1. Compare the pathophysiologic mechanisms and clinical manifestations that result in hypoxemic and hypercapnic respiratory failure. 2. Differentiate between the nursing and collaborative management of the patient with hypoxemic or hypercapnic respiratory failure. 3. Relate the pathophysiologic mechanisms and the clinical manifestations associated with acute lung injury and acute respiratory distress syndrome (ARDS). 4. Select appropriate nursing and collaborative management strategies for the patient with ARDS. 5. Prioritize measures to prevent or reverse complications that may result from acute respiratory failure or ARDS. The major function of the respiratory system is gas exchange. This involves the transfer of oxygen (O2) and carbon dioxide (CO2) between atmospheric air and circulating blood within the pulmonary capillary bed (Fig. 68-1). Respiratory failure results when one or both of these gas-exchanging functions are inadequate (e.g., insufficient O2 is transferred to the blood or inadequate CO2 is removed from the lungs). Diseases that interfere with adequate O2 transfer result in hypoxemia. This causes a decrease in arterial O2 (PaO2) and saturation (SaO2). Insufficient CO2 removal results in hypercapnia. This causes an increase in arterial CO2 (PaCO2).1–4 eTABLE 68-1 PREDISPOSING FACTORS FOR ACUTE RESPIRATORY FAILURE Respiratory failure is not a disease but a symptom of an underlying pathologic condition affecting lung function, O2 delivery, cardiac output (CO), or the baseline metabolic state. It is a condition that occurs because of one or more diseases involving the lungs or other body systems (Table 68-1 and eTable 68-1 [available on the website for this text]). Respiratory failure is classified as hypoxemic or hypercapnic (Fig. 68-2). Hypoxemic respiratory failure is also referred to as oxygenation failure because the primary problem is inadequate O2 transfer between the alveoli and the pulmonary capillaries. Although no universal definition exists, hypoxemic respiratory failure is commonly defined as a PaO2 less than 60 mm Hg when the patient is receiving an inspired O2 concentration of 60% or more. This definition incorporates two important concepts: (1) the PaO2 level indicates inadequate O2 saturation of hemoglobin, and (2) this PaO2 level exists despite administration of supplemental O2 at a percentage (60%) that is about three times that in room air (21%).5–7 TABLE 68-1 CAUSES OF HYPOXEMIC AND HYPERCAPNIC RESPIRATORY FAILURE* Hypercapnic respiratory failure is also referred to as ventilatory failure because the primary problem is insufficient CO2 removal. Hypercapnic respiratory failure is commonly defined as a PaCO2 greater than 45 mm Hg in combination with acidemia (arterial pH less than 7.35). This definition incorporates three important concepts: (1) the PaCO2 is higher than normal, (2) there is evidence of the body’s inability to compensate for this increase (acidemia), and (3) the pH is at a level where a further decrease may lead to severe acid-base imbalance. (See Chapter 17 for a discussion of acid-base balance.) Numerous disorders can compromise lung ventilation and subsequent carbon dioxide removal (see Table 68-1 and eTable 68-1). Many patients experience both hypoxemic and hypercapnic respiratory failure.6–9 Always interpret data within the context of your assessment findings and the patient’s baseline. For example, a person with chronic lung disease may have a baseline PaCO2 higher than “normal.” Although this example implies that ventilation and perfusion are ideally matched in all areas of the lung, this situation does not normally exist. In reality, some regional mismatch occurs. At the lung apex, V/Q ratios are greater than 1 (more ventilation than perfusion). At the lung base, V/Q ratios are less than 1 (less ventilation than perfusion). Because changes at the lung apex balance changes at the base, the net effect is a close overall match (Fig. 68-3). Many diseases and conditions cause V/Q mismatch (Fig. 68-4). The most common are those in which increased secretions are present in the airways (e.g., chronic obstructive pulmonary disease [COPD]) or alveoli (e.g., pneumonia), and in which bronchospasm is present (e.g., asthma).5–8 V/Q mismatch may also result from alveolar collapse (atelectasis) or as a result of pain. Pain interferes with chest and abdominal wall movement and compromises ventilation. Additionally, it increases muscle tension, producing generalized muscle rigidity. Pain also causes systemic vasoconstriction and activates the stress response. Finally, it increases O2 consumption and CO2 production.10 In this case, increased O2 demand and CO2 production may increase ventilation demands. All these conditions result in limited airflow (ventilation) to alveoli but have no effect on blood flow (perfusion) to the gas exchange units (see Fig. 68-1). The consequence of the imbalance is V/Q mismatch. A pulmonary embolus affects the perfusion portion of the V/Q relationship. The embolus limits blood flow but has no effect on airflow to the alveoli, again causing V/Q mismatch11 (see Fig. 68-4). If large enough, the embolus can cause hemodynamic compromise due to the blockage of a large pulmonary artery. A shunt occurs when blood exits the heart without having participated in gas exchange. A shunt can be viewed as an extreme V/Q mismatch (see Fig. 68-4). There are two types of shunt: anatomic and intrapulmonary. An anatomic shunt occurs when blood passes through an anatomic channel in the heart (e.g., a ventricular septal defect) and bypasses the lungs. An intrapulmonary shunt occurs when blood flows through the pulmonary capillaries without participating in gas exchange. Intrapulmonary shunt is seen in conditions in which the alveoli fill with fluid (e.g., acute respiratory distress syndrome [ARDS], pneumonia). Diffusion limitation occurs when gas exchange across the alveolar-capillary interface is compromised by a process that thickens, damages, or destroys the alveolar membrane or affects blood flow through the pulmonary capillaries (Fig. 68-5). Diffusion limitation is worsened by disease states affecting the pulmonary vascular bed such as severe COPD or recurrent pulmonary emboli. Some disease states cause the alveolar-capillary interface to become thicker (fibrotic), which slows gas transport. These include pulmonary fibrosis, interstitial lung disease, and ARDS.12,13 The classic sign of diffusion limitation is hypoxemia that is present during exercise but not at rest. During exercise, blood moves more rapidly through the lungs, decreasing the time for diffusion of O2 across the alveolar-capillary interface. Diffusion limitation may also occur in a high CO state (e.g., hepatopulmonary syndrome) or other disease states (e.g., inflammatory response seen with pancreatitis or severe brain trauma) unrelated to lung tissue damage. In this situation, CO is markedly elevated and vascular resistance is low. Blood circulates through the pulmonary capillary bed rapidly, allowing less time for gas exchange to occur.14 Alveolar hypoventilation is a generalized decrease in ventilation that results in an increase in the PaCO2 and a consequent decrease in PaO2. Alveolar hypoventilation may be the result of restrictive lung diseases, central nervous system (CNS) diseases, chest wall dysfunction, acute asthma, or neuromuscular diseases. Although alveolar hypoventilation is primarily a mechanism of hypercapnic respiratory failure, it is mentioned here because it can also cause hypoxemia.15 Many different diseases can cause a limitation in ventilatory supply (see Table 68-1 and eTable 68-1). These diseases can be grouped into four categories: (1) abnormalities of the airways and alveoli, (2) abnormalities of the CNS, (3) abnormalities of the chest wall, and (4) neuromuscular conditions.16,17 Patients with asthma, COPD, and cystic fibrosis are at high risk for hypercapnic respiratory failure because the underlying pathophysiology of these conditions results in airflow obstruction and air trapping. Ultimately respiratory muscle fatigue and ventilatory failure occur due to the additional work needed to inspire adequate tidal volumes against increased airway resistance and air trapped within the alveoli.18–21 Various types of neuromuscular diseases may result in respiratory muscle weakness or paralysis (see Table 68-1). For example, patients with Guillain-Barré syndrome, muscular dystrophy, myasthenia gravis (acute exacerbation), or multiple sclerosis are at risk for respiratory failure because the respiratory muscles are weakened or paralyzed as a result of the underlying neuromuscular condition. Therefore they are unable to maintain normal PaCO2 levels.15–17 Tissue O2 delivery is determined by cardiac output and the amount of O2 carried in the hemoglobin. Therefore respiratory failure places the patient at greater risk if there are coexisting heart problems or anemia. Failure of O2 use most commonly occurs in septic shock. Adequate O2 may be delivered to the tissues, but impaired O2 extraction or diffusion limitation exists at the cellular level. This results in an abnormally high amount of O2 returning in the venous blood because it is not used at the tissue level. (Chapter 67 discusses shock.) Acid-base alterations (e.g., alkalosis, acidosis) may also interfere with O2 delivery to peripheral tissues (see Chapter 17). A more gradual change in PaO2 and PaCO2 is better tolerated because compensation can occur. An example is the patient with COPD who develops a progressive increase in PaCO2 over several days after a respiratory tract infection. Because the change occurred over several days, there is time for renal compensation (e.g., retention of bicarbonate), which minimizes the change in arterial pH. The patient will have compensated respiratory acidosis.3,4 (See Chapter 17 for a discussion of renal compensation for acid-base disorders.) You may detect manifestations of respiratory failure that are specific (primary) (arising from the respiratory system) or nonspecific (secondary) (arising from other body systems) (Table 68-2). Understanding the significance of these manifestations is critical to your ability to detect the onset of respiratory failure and evaluate the effectiveness of treatment. TABLE 68-2 MANIFESTATIONS OF HYPOXEMIA AND HYPERCAPNIA*
Nursing Management
Respiratory Failure and Acute Respiratory Distress Syndrome
Acute Respiratory Failure
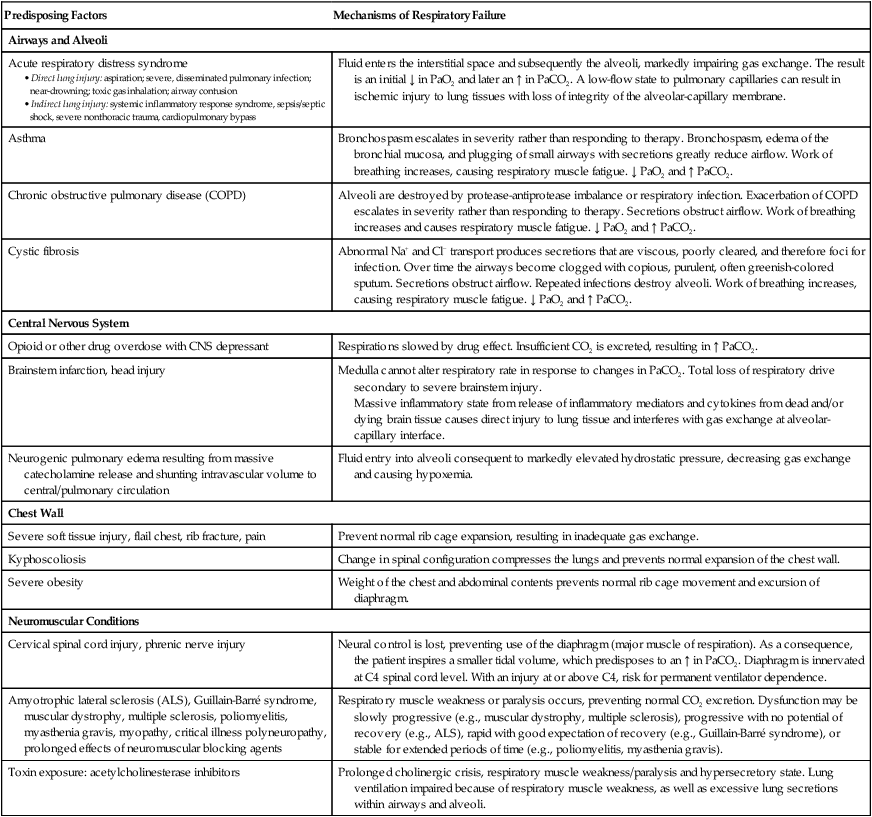
Predisposing Factors
Mechanisms of Respiratory Failure
Airways and Alveoli
Acute respiratory distress syndrome
Fluid enters the interstitial space and subsequently the alveoli, markedly impairing gas exchange. The result is an initial ↓ in PaO2 and later an ↑ in PaCO2. A low-flow state to pulmonary capillaries can result in ischemic injury to lung tissues with loss of integrity of the alveolar-capillary membrane.
Asthma
Bronchospasm escalates in severity rather than responding to therapy. Bronchospasm, edema of the bronchial mucosa, and plugging of small airways with secretions greatly reduce airflow. Work of breathing increases, causing respiratory muscle fatigue. ↓ PaO2 and ↑ PaCO2.
Chronic obstructive pulmonary disease (COPD)
Alveoli are destroyed by protease-antiprotease imbalance or respiratory infection. Exacerbation of COPD escalates in severity rather than responding to therapy. Secretions obstruct airflow. Work of breathing increases and causes respiratory muscle fatigue. ↓ PaO2 and ↑ PaCO2.
Cystic fibrosis
Abnormal Na+ and Cl− transport produces secretions that are viscous, poorly cleared, and therefore foci for infection. Over time the airways become clogged with copious, purulent, often greenish-colored sputum. Secretions obstruct airflow. Repeated infections destroy alveoli. Work of breathing increases, causing respiratory muscle fatigue. ↓ PaO2 and ↑ PaCO2.
Central Nervous System
Opioid or other drug overdose with CNS depressant
Respirations slowed by drug effect. Insufficient CO2 is excreted, resulting in ↑ PaCO2.
Brainstem infarction, head injury
Medulla cannot alter respiratory rate in response to changes in PaCO2. Total loss of respiratory drive secondary to severe brainstem injury.
Massive inflammatory state from release of inflammatory mediators and cytokines from dead and/or dying brain tissue causes direct injury to lung tissue and interferes with gas exchange at alveolar-capillary interface.
Neurogenic pulmonary edema resulting from massive catecholamine release and shunting intravascular volume to central/pulmonary circulation
Fluid entry into alveoli consequent to markedly elevated hydrostatic pressure, decreasing gas exchange and causing hypoxemia.
Chest Wall
Severe soft tissue injury, flail chest, rib fracture, pain
Prevent normal rib cage expansion, resulting in inadequate gas exchange.
Kyphoscoliosis
Change in spinal configuration compresses the lungs and prevents normal expansion of the chest wall.
Severe obesity
Weight of the chest and abdominal contents prevents normal rib cage movement and excursion of diaphragm.
Neuromuscular Conditions
Cervical spinal cord injury, phrenic nerve injury
Neural control is lost, preventing use of the diaphragm (major muscle of respiration). As a consequence, the patient inspires a smaller tidal volume, which predisposes to an ↑ in PaCO2. Diaphragm is innervated at C4 spinal cord level. With an injury at or above C4, risk for permanent ventilator dependence.
Amyotrophic lateral sclerosis (ALS), Guillain-Barré syndrome, muscular dystrophy, multiple sclerosis, poliomyelitis, myasthenia gravis, myopathy, critical illness polyneuropathy, prolonged effects of neuromuscular blocking agents
Respiratory muscle weakness or paralysis occurs, preventing normal CO2 excretion. Dysfunction may be slowly progressive (e.g., muscular dystrophy, multiple sclerosis), progressive with no potential of recovery (e.g., ALS), rapid with good expectation of recovery (e.g., Guillain-Barré syndrome), or stable for extended periods of time (e.g., poliomyelitis, myasthenia gravis).
Toxin exposure: acetylcholinesterase inhibitors
Prolonged cholinergic crisis, respiratory muscle weakness/paralysis and hypersecretory state. Lung ventilation impaired because of respiratory muscle weakness, as well as excessive lung secretions within airways and alveoli.
Etiology and Pathophysiology
Hypoxemic Respiratory Failure.
Ventilation-Perfusion Mismatch.
Shunt.
Diffusion Limitation.
Alveolar Hypoventilation.
Hypercapnic Respiratory Failure.
Airway and Alveoli Abnormalities.
Neuromuscular Conditions.
Tissue Oxygen Needs.
Clinical Manifestations