Ronald A. Kline, MD, FACS, FAHA
R. Gary Sibbald, BSc, MD, MEd, FRCPC (Med Derm),
MACP, FAAD, MAPWCA, D.Sc. (Hons)
Gregory Ralph Weir, MBChB, MMed (Surg), CVS
Objectives
After completing this chapter, the reader will:
- identify the structure and explain the function of the lower-extremity arterial system
- assess the signs and symptoms of lower-extremity arterial disease and ulcers
- select appropriate vascular laboratory diagnostic testing for lower-extremity arterial disease
- evaluate medical and surgical treatment options for lower-extremity arterial disease
- design appropriate patient education for prevention and appropriate lifestyle change.
Scope of the Problem
The designation, peripheral vascular disease, commonly refers to arterial problems in the legs. The reader should be aware that some authors also include diseases of the venous and lymphatic systems in their definition of peripheral vascular disease.
Leg and foot ulcers may have several different etiologies, including arterial, venous, and lymphatic disease along with trauma, infections, inflammatory diseases, and malignancy. This chapter describes the arterial component, including anatomy and physiology, and examines the treatment of lower-extremity arterial ulcers.
Approximately 8% to 10% of patients in some series with leg and foot ulcers have pure arterial insufficiency.1 It’s estimated that between 1% and 22% of the population over age 60 suffers from lower-extremity skin ulcers.2–5 One patient survey suggested the current estimate may be low because high numbers of patients indicated that they cared for their own ulcers without consulting a healthcare provider.6 The principal etiology of leg ulcers is chronic venous disease, whereas foot ulcers are much more commonly caused by arterial disease.1,7–9 Peripheral arterial ischemia is also the most common vascular consult for limited ability to walk.7 Although chronic wounds have physical, financial, and psychological effects, it’s difficult to measure this impact on a patient’s activities of everyday living.10 It’s also difficult to obtain accurate etiological information about leg ulcers because no ulcer etiology documentation exists in about one-third of medical records.
Vascular Anatomy and Physiology
Vascular anatomy includes the arterial, venous, and lymphatic systems. Vascular ulcers may develop in any of these systems from a variety of causes. For the purposes of this chapter, we will confine our discussion to the arterial system.
Arterial System
Lower-extremity arterial perfusion begins with adequate cardiac performance. As blood exits the left ventricle, it begins its downward course through the descending thoracic aorta meeting several arterial branches. The intercostal arteries, which arise from the descending thoracic aorta, are the first important collaterals to perfusion in the legs. These become important when they are the sole collaterals in distal aortic occlusive disease. As the aorta exits the thorax and enters the true abdominal cavity, its caliber begins to decrease after every major arterial branch. Its greatest reduction in size occurs distal to the renal arteries.
Lumbar arteries usually arise as paired vessels at each vertebral level in the abdomen. The lumbar arteries become important collateral pathways to the lower extremities in distal aortic occlusions or severe aortoiliac occlusive disease. At the level of the umbilicus, the abdominal aorta bifurcates into the common iliac arteries that in turn branch into internal and external iliac arteries. The internal iliac arteries perfuse the lower sigmoid colon and rectum. They also, by way of the gluteal and pudendal branches, provide another collateral pathway to perfusion of the legs. The external iliac artery becomes the common femoral artery at the level of the inguinal ligament. It’s at this level that one can first appreciate the quality of the pulse wave by palpating the femoral artery.
Aspects of the Femoral Artery
The common femoral artery bifurcates into the superficial femoral artery and the deep femoral artery. The deep femoral artery is the single most important collateral pathway for perfusion of the lower portion of the leg. Its muscular perforators allow reconstitution of the popliteal artery in superficial femoral artery occlusions. The superficial femoral artery becomes the popliteal artery after it exits the adductor hiatus, also known as Hunter’s canal (Fig. 15-1).
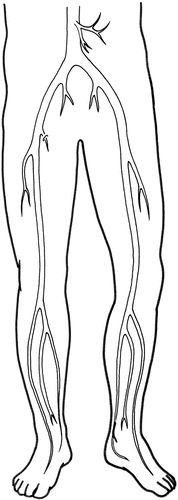
Figure 15-1. Arterial system. This illustration shows the major arteries of the lower extremities.
The superficial femoral artery is the most commonly occluded artery in the legs of patients with peripheral vascular occlusive disease. Its occlusion infrequently results in significant ischemia to the lower leg. Below the knee, the popliteal artery bifurcates into the tibioperoneal trunk and the anterior tibial artery. The anterior tibial artery proceeds from the popliteal fossa through the interosseous membrane, which connects the tibia and fibula; it then courses down the anterior muscle compartment into the foot. The tibioperoneal trunk (also known as the tibiofibular trunk) at a variable distance then bifurcates into the peroneal (also known as the fibular) artery and the posterior tibial artery.
The peroneal artery courses down toward the ankle in the deep muscular compartment, whereas the posterior tibial artery descends into the foot in a more superficial fashion. The peroneal artery provides important muscular profusion branches. It’s commonly patent even in the presence of severe lower-extremity peripheral vascular occlusive disease.
Aspects of the Tibial Arteries
The anterior and posterior tibial arteries proceed into the foot with the anterior tibial artery becoming palpable as it becomes the dorsalis pedis artery. The posterior tibial artery then courses behind the medial malleolus and at this level also becomes palpable. The posterior tibial artery provides both deep and superficial components to the plantar arch. Perforators from the plantar arch provide arterial perfusion to the heel, sole, and branches to the digits.
The anterior tibial artery, which becomes the dorsal pedal artery and is palpable on the dorsum of the foot, eventually communicates with the plantar arch, forming a complete circuit in the foot. The peroneal artery, although it stops above the level of the ankle joint, does provide medial and lateral tarsal branches that communicate with the distal-most portions of the anterior and posterior tibial arteries. This is another important collateral pathway for revascularization of the plantar arch in patients with occlusive disease. Vascular surgeons can perform bypass operations to any of these named vessels, with modern procedures successfully bypassing more distal vessels.
Arterial Wall Architecture
The arterial wall typically consists of three laminae. The outer lamina, the adventitia, is a layer of loose connective tissue that provides moderate strength to the arterial wall. The media, or middle layer, contains both elastic and muscular fibers and is responsible for arterial strength, elasticity, and contractility. The intima, the innermost layer, is the endothelial lining of an artery and a few cell layers thick. As the arterial tree descends from the heart to the periphery, muscular functions become more evident. Vessels below the common femoral artery have a greater propensity for rapid vasoconstriction or vasodilation in direct relationship to perfusion. The tibioperoneal vessels can quickly accommodate changes in perfusion by relaxation or dilation.
Arteries are capable of increasing in size (vasodilation) to maintain constant shear stress when atherosclerotic accumulation decreases luminal surface area. However, once a stenosis reaches 50% of the vessel diameter, the artery loses its ability to dilate any further, and any increase in atherosclerotic accumulation impedes arterial perfusion. Further restriction in flow through this stenotic area results in a decrease in the diameter of the artery distal to the stenosis in order to accommodate diminished blood flow. Compliance of an artery decreases as the arterial wall becomes more rigid with calcific atherosclerosis (Fig. 15-2).
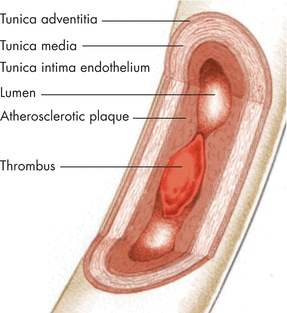
Figure 15-2. Arterial wall. In the layers of the arterial wall shown here, the plaque formation and thrombus significantly reduce blood flow through the vessel.
Arterial Perfusion
As blood descends through successively smaller arterial conduits, it eventually reaches the arteriolar level. Blood flow (rheological factors) in this precapillary bed plays an important role in perfusion. Blood is a non-Newtonian thixotropic fluid (thick or stable at rest but becoming more fluid with motion). The viscosity of blood is inversely proportional to its shear rate or the velocity of blood flow. The slower blood is propulsed, the more viscous it becomes. The primary determinant of whole blood viscosity at any given shear rate is the hematocrit. As red cell mass increases, blood viscosity markedly increases and the flow decreases.
Dehydration and polycythemia (increased number of red blood cells) are two examples of many disease states that increase whole blood viscosity and can result in blood sludging (sticking together of the red blood cells) in the precapillary bed and a resultant decrease in arterial tissue perfusion. In many elderly patients with arterial occlusive disease, even mild dehydration can result in poor extremity perfusion. Simple rehydration can reduce the red cell mass and allow for better perfusion. In other cases of increased blood viscosity, such as multiple myeloma, plasmapheresis may be necessary to remove the abnormal concentrations of proteins. Nonetheless, in the “normal” atherosclerotic patient, it’s the red cell mass, measured by hematocrit, that is the primary determinant of viscosity.
As blood proceeds into the capillary bed, the diameter of the vessel approaches that of the red cell—approximately 8 μm (microns) in diameter. Red cells pass through capillaries sequentially. Red cell deformability plays a role in perfusion at this level. In conditions in which the red cell membrane is relatively rigid, tissue perfusion decreases because of increased transit time for a red cell to pass from the precapillary to postcapillary level. Although nutrient and oxygen extraction are increased by this increase in transit time, the per-unit perfusion of the tissues is decreased overall. Medications such as pentoxifylline may facilitate red cell deformability, thereby potentially increasing the per-unit perfusion of tissues.7,8 Unfortunately, pentoxifylline has limited efficacy in arterial disease, but cilostazol (inhibiting platelet aggregation and an arterial vasodilator) improves both pain-free and maximal treadmill walking distance.11
In normal states, arterial tissue perfusion is well above minimal requirements, but certain tissues, such as muscle, can change their metabolic requirements. Muscle becomes more efficient under anaerobic conditions (this is called the Cori cycle)—for example, a trained long-distance runner. The process of improving efficiency is gradual, but training to increase muscle capacity is useful in patients with claudication. A regular exercise program can increase the distance walked before claudication occurs. The skin does not have the same kind of compensatory mechanism where exercise can gradually increase blood flow.
Arterial Ulcer Pathophysiology
The pathophysiology of vascular ulcers varies according to the type of ulcer. Arterial ulcers are wounds that will not heal due to compromised or inadequate arterial blood flow or ischemia. Hypoxia due to anemia can exacerbate ischemia. Precipitating events for arterial ulcers vary. Limbs with arterial compromise may have minimal but adequate blood flow to maintain tissue viability. Ischemic lower-extremity ulcers are often precipitated by trauma or infection.12
The location of traumatic ulcers varies depending on the cause and site of injury, but these wounds are commonly found on the foot or on the anterior tibial area of the lower leg. Traumatic ulcers may be caused by an acute physical injury, such as blunt trauma (e.g., bumping into a piece of furniture or dropping a heavy object on the foot), or by acute or chronic pressure (such as the continual pressure from ill-fitting footwear). Several other conditions may be responsible for tissue breakdown, including thermal extremes, chemicals, or a localized clot or embolus that can also lead to decreased cellular nutrition from impaired arterial flow. Regardless of the cause, when ischemia is present, wound healing is inhibited. It is also postulated that the ischemic–reperfusion injury cycle may be more detrimental to ulcer healing than previously suspected and may be even more damaging than prolonged ischemia.13 Although some wounds heal in the presence of ischemia, arterial inflow must usually be improved for healing to occur.14,15 Injury repair requires more than baseline oxygen consumption and increased tissue nutritional need. Diminished arterial flow causes tissue hypoxia, and with injury, this will further aggravate the arterial insufficiency and can eventually lead to gangrene or tissue necrosis (Fig. 15-3).
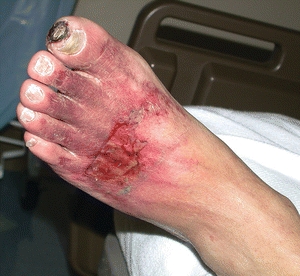
Figure 15-3. Ischemic forefoot. This photograph shows an ischemic forefoot.
Diagnosing Vascular Ulcers
Vascular disease and ulcer etiology can be determined by obtaining a thorough patient history and performing a physical examination. A focused vascular history includes a clear description of the presenting complaint, past medical history for vascular and related conditions, current and previous medications, and risk factors. Symptoms and signs of lower-extremity vascular disease may include pain or loss of protective sensation, tissue compromise, or change in appearance. Noninvasive vascular laboratory testing is required to identify the location of vascular pathology.
The first question to ask every patient is about his or her history of allergies. This question is important if there is a known sensitivity to medications that may be ordered or to dyes used for angiography. The next question should be about the patient’s medications and then about his or her occupation.14
The important points to remember about a patient’s history include remembering the ABCDEs,14 all of which are increased risk factors for arterial disease. They are:
- A1C: Hemoglobin A1C detects the average blood sugar over a period of 90 days. Values below 6% are normal, those between 6.1% and 6.5% represent prediabetes, and those above 6.5% represent diabetes. The higher the HbA1C, the greater the incidence of arterial disease. There is often a personal or family history of diabetes or arterial disease. Arterial disease often manifests at an earlier age in males and in individuals who smoke or have other risk factors.
- Blood pressure: Find out if it is elevated and if the patient is on medications.
- Cholesterol: Elevated cholesterol is a risk factor, and the use of statin cholesterol-lowering agents may reduce this risk.
- Diet and obesity: Increased weight, especially a body mass index above 25, indicates an increased risk for heart and peripheral vascular disease as well as diabetes.
- Exercise: Individuals who exercise regularly have a lower risk of peripheral vascular disease and can build up a greater tolerance to overcome compromised circulation. In general, individuals with leg pain at rest or when recumbent in bed have rest pain that equates to severe ischemia, those who have pain or claudication (aching and throbbing calf muscles) with walking up a few stairs or less than 50 yards have moderate disease, and individuals with symptoms after walking one or two blocks have mild disease.
- Smoking: One cigarette decreases circulation by 30% for 1 hour, and the more pack-years of accumulated smoking history, the greater the risk. Ask patients how many cigarettes they smoke a day and how many years they have been smoking (e.g., 30 years of smoking a half a pack a day is 15 pack-years [30 × 0.5 = 15 pack-years]).
Other risk factors include increased levels of homocysteine and hypothyroidism.14 If peripheral vascular disease is present, it is also more common to have a history of coronary artery disease and previous stroke.14
Physical Examination
Skin inspection, palpation, and auscultation are all an important part of the physical examination. It includes examining the distal extremities for taut or shiny, atrophic skin that’s present with arterial disease. Because skin color may indicate arterial perfusion, each toe should be noted and compared with the other foot and toes. Arterial insufficiency causes ischemic tissue to first become pale, progressing to a mottled netlike appearance (livedo reticularis) and subsequently to a dark purple hue and finally black (Fig. 15-4). Elevating the foot at a 45-degree angle causes the ischemic limb to become pale. Immediately after positioning the ischemic foot in a dependent position, it becomes dark red or ruddy (dependent rubor or redness). This finding is the reactive hyperemia of ischemic tissue. There may be a loss of hair distally, and the nails may lose their luster and become thickened. Make sure to distinguish nail changes from changes that occur with a fungal infection (about half of all abnormal nails), psoriasis, or other nail disorders.
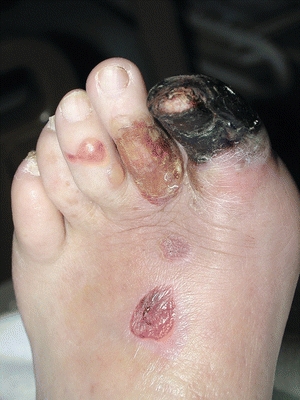
Figure 15-4. Arterial ulcer. This photograph shows a necrotic great toe with blisters on the toes and foot, representing arterial insufficiency.
Palpate the skin for temperature changes. The skin of the distal part of an ischemic limb feels cool or cold, with temperature demarcation that correlates to the diseased artery. Infrared thermometers are now available for under $100 and should be utilized by every wound care clinician.16 These less costly thermometers have been validated to the gold standard.16 The thermometer will also identify repetitive trauma/deep inflammation in the neuropathic foot along with deep and surrounding infection.17
Capillary refill time is determined by compressing the skin (dorsum of the foot or toe pad) with the thumb to remove the local profusion leading to a local blanching of color. Then, release the thumb to observe the capillary refill and return of color as a good indicator of arterial skin perfusion. Perform this test with the foot slightly elevated. Normal capillary refill time is less than 3 seconds from pallor to normal skin color.
Palpate pulses for presence, rate, regularity, strength, and equality. The most common objective physical finding is the presence or absence of pulses. Care must be taken when palpating pedal pulses. It’s common to mistake a contracting tendon for the presence of a pulse. No universal consensus exists regarding a pulse grading system. According to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II),18 pulses are graded as 0 (absent), 1 (diminished), or 2 (normal). The American Heart Association’s guideline adds a grade of 3 for bounding pulses. To ensure consistency, adhere to local policy. A high degree of interobserver variability exists in determining the presence or absence of pulses. It can be confusing if clinicians report 2+ or 3+ pulse examinations. Communication is better facilitated if pulses are recorded simply as present or absent. However, even this seemingly obvious assessment parameter may not always be accurate. One study found only a 50% chance that two observers would agree with a third observer about the presence or absence of dorsalis pedis or posterior tibial pulses.18 This same study identified the dorsalis pedis pulse congenitally absent in 4% to 12% of subjects and the posterior tibial pulse absent in 0.24% to 12.8%.18 Additional descriptors of pulse, such as “weak” or “bounding,” can be added to clarify your findings.
Practice Point
The best way to document pulses is to use descriptor terms, such as present or absent, rather than numerical ratings, such as 2+ or 3+. Use modifier words, such as weak or bounding, to further describe and clarify the pulse findings.
Although pulses in the foot may be present at rest, they may disappear with exercise. A patient who presents with claudication but has clearly discernable pulses should have an exercise test performed in the vascular laboratory. Clinicians are often tempted to skip the assessment of the elusive popliteal pulse, particularly when the dorsalis pedis and posterior tibial pulses are strong. While good pedal pulses indicate foot perfusion, finding bounding popliteal pulses may indicate a popliteal aneurysm. Popliteal aneurysms can be a source of emboli to the lower leg with resulting tissue or limb loss.
Patients with foot or leg ulcers should be tested for neuropathy. This is a common finding in persons with diabetes, but there are several other causes associated with a loss of protective sensation. Approximately 2/3s of patients with neuropathy have diabetes, but the other 1/3 of neuropathies are from other etiologies. For example, neuropathy commonly obscures a traumatic or pressure-induced wound in an ischemic limb. Lack of protective pain sensation and injury awareness prevents the patient with diabetes from seeking appropriate care early. Evaluate neuropathy by testing light touch with monofilaments for the sensory component, examining for dry skin as part of the autonomic component, and eliciting reflexes for the motor component. You can remember to assess for neuropathy with the mnemonic SAM (Sensory, Autonomic, and Motor). An objective assessment of significant neuropathy is best performed by using the 5.07 Semmes-Weinstein monofilament.19 To perform this assessment, ask the patient to close the eyes and indicate when he or she feels the monofilament. Test the areas over the plantar aspect of the first, third, and fifth toe; the first, third, and fifth metatarsal head; both sides of the plantar aspect of the midfoot; the plantar heel; and, lastly, the dorsum of the foot. Place the monofilament on the test position until it bends slightly, and then move it to the next position. Record the number of negative sites the patient reports; if there are four or more negative sites, then neuropathy is present, which indicates a loss of protective sensation (see chapter 16, Diabetic Foot Ulcers, and Figure 16-2).
Practice Point
Use the mnemonic SAM to assess for neuropathy:
Sensory
Autonomic
Motor
Arterial Signs and Symptoms
Arterial insufficiency is commonly associated with complaints of pain19 resulting from atherosclerotic arterial changes interrupting blood flow to tissues.20–22 Claudication pain is pain occurring with exercise and is relieved by rest that is usually located in the muscle group distal to the stenosed or occluded artery. While the calf is the most common location for claudication, it can also occur in the buttocks, thighs, or feet and is predictable and reproducible. Claudication is described by patients as muscle cramping, aching, or weakness. The distance the patient is able to walk until the claudication first develops is referred to as the initial claudication distance. The distance the patient is able to walk before he or she has to stop is called the absolute claudication distance (Fig. 15-5). The period of time the patient needs for the pain to subside after he or she is forced to stop is referred to as the recovery time.

Figure 15-5. Absolute claudication distance.
 Practice Point
Practice Point
When taking a history, it’s important to find out exactly how far the patient can walk before he or she needs to stop; a shorter distance indicates more severe atherosclerosis. Reported changes in ambulatory distance may indicate progressive atherosclerotic disease.
The patient with leg ulcers and poorly perfused tissue commonly seeks care because of sharp, severe, and possibly constant pain at the ulcer site and the distal extremity. Pain that occurs at rest represents inadequate perfusion and is a sign of threatened limb viability or critical limb ischemia. This pain is referred to as rest pain. The patient may describe waking at night with pain across the distal metatarsal area of the foot. In an attempt to relieve the pain, the patient will get out of bed and lower the foot to the ground that has the effect of gravity to increase blood flow and improve tissue perfusion. The patient may even ambulate. The ischemic pain may be relieved by the small contribution of blood flow from collateral vessels if the limb is placed in a dependent position. The patient with pain at rest may begin sleeping with the legs in the dependent position that will predispose the distal leg to edema accumulation. Elevation of this edematous limb will further exacerbate rest pain, distinguishing it from venous insufficiency and other causes of edema.

Practice Point
Rest pain represents end-stage arterial insufficiency and usually requires revascularization.15,21
Patients with extensive sensory neuropathy—for example, those with diabetes—may not experience pain even with severely ischemic ulcers. On the other hand, these patients may experience such intense hyperesthesia associated with the neuropathy that they cannot bear the light touch of stockings. Ulcers in patients with neuropathy are typically found on the plantar side of the foot and are surrounded by calluses from long-term local pressure. These patients may describe the sensations of burning, stinging, shooting, and stabbing pain (neuropathic pain) rather than the more characteristic gnawing, aching, throbbing, and tender pain (nociceptive pain) associated with an acute injury of peripheral vascular disease.
When obtaining a history, note previous arterial surgery for vascular disease, including coronary artery disease and cerebrovascular disease. Vascular disease isn’t limited to any one organ but can occur in all body systems: 60% of patients with peripheral arterial disease will have coronary artery disease, and 40% will have cerebrovascular disease. Document all medications, especially vasoconstrictor drugs. Ischemic symptoms are exacerbated by nicotine. Patients with symptomatic vascular disease may aggravate their symptoms by using tobacco, nicotine gum, or nicotine patches for smoking cessation.
Another arterial finding upon examination is gangrene. In ischemic tissue, gangrene initially appears pale, then blue-gray, followed by purple, and, finally, black. Gangrenous tissue eventually becomes black, hard, and mummified. The hardened tissue isn’t painful, but significant pain may be present at the line of demarcation between the gangrene and the live but ischemic tissue. Gangrene may be a small skin lesion or extend to an entire limb depending on the location of the arterial lesion. If a small patch of skin is affected, the skin will dry and fall off, producing a skin ulcer or a healed tissue deficit. Large areas of gangrene may require debridement, skin graft, or potential amputation. Do not attempt this prior to revascularization.18
Additional physical findings are local patches of color change that may appear as small black or dark purple dots, circular areas found on the distal toes, or localized infarcts around the toe nail beds (Fig. 15-6). Tissue changes found in these areas are caused by tissue ischemia from arteriosclerosis or by atheromatous debris embolizing from a proximal artery. Arterial erosions (loss of epidermis with an epidermal base) or deeper ulcers (loss of epidermis with a dermal or deeper base) may also be found between the toes, starting as a small, moist, macerated spot on the skin surface extending deep into the bony structure of the foot. This may also be caused by pressure due to ill-fitting footwear.
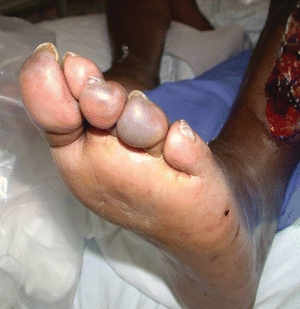
Figure 15-6. Blue toe syndrome. This photograph shows “blue toe syndrome” in the second toe caused by tissue ischemia from arteriosclerosis.
Arterial ulcers typically have distinct borders with a pale-gray or yellow-dry base. They may contain exposed tendons, fascia, fat, muscle, bone, or joint structures in their base. In contrast, venous ulcers most often have granulation tissue in their base and seldom demonstrate other structures found in the base of an arterial ulcer. The surrounding tissue may appear pale compared with skin elsewhere on the body, or it may be reddened if the leg is dependent. Chronic ischemic skin may appear thin and shiny. Foot elevation will produce skin pallor. The red or ruddy color of a dependent ischemic limb is called dependent rubor or reactive hyperemia (Fig. 15-7). Even in a person of color, the difference in hue is discernible when the ischemic limb is compared with the contralateral well-perfused limb.
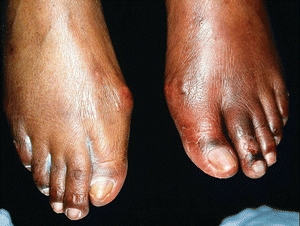
Figure 15-7. Dependent rubor. Foot elevation produces skin pallor in patients with ischemic skin (Buerger’s sign or test). When dependent, the ischemic limb will have a red or ruddy color, as shown here in the patient’s right leg. This is called dependent rubor or reactive hyperemia (Goldflam’s sign).
Arterial pressure is one of the most reliable physical findings in peripheral arterial disease.21,22 However, lower-extremity blood pressures aren’t obtained as a part of the routine physical examination. Bilateral brachial pressures should always be obtained on the initial examination to identify whether a discrepancy exists between them. The correct pressure is always the higher of the two pressures. This pressure is used to determine the ankle–brachial index (ABI) when assessing lower-extremity perfusion.
Vascular Testing
Although an experienced vascular clinician can make a vascular diagnosis based on history and physical examination alone, vascular laboratory studies help pinpoint the diagnosis. The presence, location, and severity of arterial disease are confirmed by vascular laboratory procedures. Information obtained by vascular studies can predict potential ulcer healing (healable ulcer) when the cause is arterial insufficiency.7 Laboratory tests differentiate among conditions contributing to a non-healing ulcer.
Noninvasive vascular testing is divided into direct tests that image the vessel itself and indirect tests that demonstrate changes distal to the diseased vessel. These tests include segmental arterial Doppler ultrasound with pressures, arteriogram, ankle–brachial index (ABI), transcutaneous pressure of oxygen (TcPO2), and toe pressures.23
Handheld Doppler Ultrasound
A Doppler ultrasound transmitting probe sends a signal that is reflected from an object to the receiving probe. If the signal strikes a moving object such as blood cells, a frequency shift is detected and reflected as sound (Doppler principle). The audible signals of arterial flow patterns can then be determined. The handheld Doppler is used to detect an audible signal on the dorsum of the foot or ankle (dorsalis pedis artery and posterior tibial artery). A blood pressure cuff is then placed around the lower calf and inflated until the audible signal disappears. The cuff is then slowly deflated, and when the signal returns, the systolic pressure is determined from the reading on the cuff gauge.
Arteriogram
An arteriogram is an invasive test used to identify an operative lesion in the arterial system by outlining the patent arterial lumen (Fig. 15-8). Indications for a surgical procedure include incapacitating claudication, rest pain, nonhealing ulcers, and gangrene. An arteriogram is not indicated unless a bypass or dilation procedure is required. It’s also not indicated when the patient is too ill for surgery or is refusing surgical intervention.24
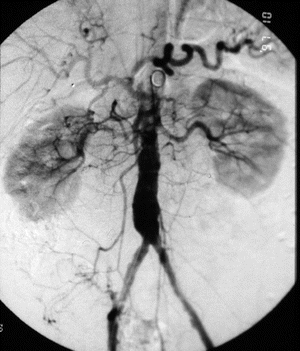
Figure 15-8. Arteriogram. The arteriogram below shows iliac stenosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


