Reader activities
Having read this scenario, consider the following:
- How do Penelope’s symptoms support a diagnosis of sepsis?
- What stage of the sepsis continuum is Penelope experiencing? Explain this using relevant pathophysiology.
- Consider the possible causes of Penelope’s sepsis. Outline the factors that make her high risk for the development of sepsis.
- Analyse the blood gas presented. Consider why Penelope might have altered gas exchange.
- Explain the possible reasons why Penelope has a raised lactate and hyperglycaemia. Why are these abnormalities of concern?
- What is ScvO2? What is the normal value and why might it be altered in sepsis?
Definitions of sepsis
Sepsis has been defined as a ‘systemic inflammatory response (SIRS) with a presumed or confirmed infectious process’ (Bone et al. 1992). It is one step in a continuum originally described by the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) Consensus Conference in 1991 (see Table 4.2).
Table 4.2 The sepsis continuum.
Source: Adapted from Bone et al. (1992).
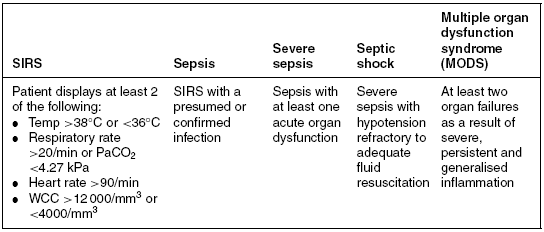
In 2001, an International Sepsis Definitions Conference added additional clinical signs and symptoms to reflect better the clinical response to infection (Levy et al. 2003). These include hyperglycaemia; altered mentation; the presence of considerable oedema; raised inflammatory markers, for example, white cell count (WCC) and C-reactive protein (CRP); altered haemodynamic parameters, for example, altered ScvO2, oliguria and coagulation abnormalities; and finally altered tissue perfusion parameters such as a raised lactate and reduced capillary refill time.
Based on these definitions, Penelope is presenting with severe sepsis, and possibly septic shock. She meets the criteria for SIRS. In addition, she has inadequate tissue perfusion evidenced by a metabolic acidaemia, raised lactate and an elevated SvO2. She is also hypotensive with a mean arterial blood pressure (MABP) <65 mmHg, and her respiratory and renal function appear to be deteriorating (raised urea and creatinine). However, these abnormalities could be due to inadequate fluid resuscitation. This would need to be determined, ideally with the use of advanced haemodynamic monitoring, for example, pulmonary artery catheter, pulse-induced contour cardiac output (PiCCO) or oesophageal Doppler prior to assuming she is in septic shock.
The pathophysiology of sepsis
Sepsis can be caused by a multitude of invading organisms, and the clinical manifestation and outcome vary depending on the individual’s immune response. This in turn will be modulated by factors such as their pre-morbid status, the severity of the infection and the ability to mount an appropriate host response. Although traditionally gram-negative bacteria such as Escherichia coli predominated as a cause of sepsis, gram-positive organisms such as the Staphylococcus aureus organism have now been shown to make up almost half of all cases of severe sepsis (Bochud and Calandra 2003; Vincent et al. 2006). The rates of fungal infections are also increasing in many countries (Bochud and Calandra 2003; Vincent et al. 2006). Additionally, the growing rates of multi-resistant organisms such as multi-resistant Staphylococcus auerus (MRSA) and vancomycin-resistant enterococci (VRE) further contribute to the high mortality seen in sepsis.
Nosocomial infections are a significant cause of sepsis in the critically ill population, with common primary sites being the lungs, abdomen and urinary tract (Vincent et al. 2006). Intubated patients, such as Penelope, have been shown to be at particularly high risk for the development of nosocomial pneumonia or ventilator-associated pneumonia (VAP) as it is commonly known (Pruitt and Jacobs 2006), with mortality rates of 46% compared to 32% in those without VAP (Pruitt and Jacobs 2006). Critically ill patients are also at high risk of bacterial translocation within the gut, especially if they are unable to tolerate enteral nutrition. During gut hypoperfusion, intestinal bacteria multiply, and with protein malnutrition the integrity of the villi is disrupted and mucosal permeability increases. This subsequently allows passive movement of bacteria across the intestinal epithelium into the systemic circulation during periods of gut reperfusion (Pastores et al. 1996; Holland et al. 2005). Penelope is currently receiving no enteral nutrition. A further possible cause for sepsis in Penelope’s case could be the presence of a central venous catheter, as catheter-related bloodstream infections are common in critically ill patients (Pronovost et al. 2006).
Regardless of the causative organism, the resulting process and mortality rates are similar. The pathophysiology associated with sepsis involves activation of inflammatory responses, endothelial dysfunction, disturbances of coagulation and fibrinolysis, and other factors such as the role of nitric oxide, neuro-endocrine activation and mitochondrial dysfunction.
Inflammation
A series of complex mechanisms are activated by the presence of the invading organism. These involve both the innate (non-specific) and specific immune mechanisms (see Thibodeau and Paton (2007) for an account of normal immune responses). Both pro-inflammatory and anti-inflammatory mediators are released, and the normal ability to activate and downregulate the inflammatory response to infection is impaired. The anti-inflammatory response renders the patient immuno-suppressed and thus prone to developing further infection (Hotchkiss and Karl 2003). The pro-inflammatory response leads to an increased metabolic rate and cardiac output, despite a reduction in contractility, increased capillary permeability resulting in loss of fluid from the intravascular space and an associated hypotension due to the effects of systemic vasodilation triggered via release of prostaglandins, bradykinin and nitric oxide. This leads to a reduced systemic vascular resistance (SVR). Initial clinical manifestations are therefore dependent on whether the patient presents with a predominant pro- or anti-inflammatory picture (Hotchkiss and Karl 2003).
These changes can be seen in Penelope’s clinical presentation by the poor gas exchange and associated pulmonary oedema, the systemic hypotension, the warm peripheries and the increased heart rate. Pulses may also be bounding, indicating an increased cardiac output. In some patients, however, intravascular hypovolaemia due to fluid movement into the interstitial spaces and myocardial ischaemia leads to poor contractility and thus a drop in cardiac output. This will reduce oxygen delivery (DO2) and lead to a lower-than-normal SvO2, and an associated metabolic acidaemia.
Disturbance of coagulation and fibrinolysis
Endothelial damage caused by pro-inflammatory mediators such as IL-1, IL-6, IL-8 and tumour necrosis factor (TNF-α) causes the release of tissue factor which stimulates coagulation via both the intrinsic and extrinsic pathways. Thrombin and resultant fibrin clots within the vasculature are then formed. This contributes to poor blood flow, particularly within the micro-circulation and the typical perfusion deficits seen at the extremities. Anti-coagulation and fibrinolysis are also impaired exacerbating the problem. A key player in this pro-coagulant state is thrombin which is responsible for the ultimate conversion of fibrinogen to fibrin, and for the activation of further coagulation, and also for the suppression of anti-coagulant and fibrinolytic activities. Additionally, it has been shown that significant numbers of septic patients have decreased function or availability of protein C (Schulman and Hare 2003). Protein C has an important role in the body’s response to inflammation and mediates many of the harmful responses seen in sepsis (Bernard et al. 2001). Protein C restricts the role of tissue factor on the clotting cascade and blocks thrombin formation by inhibition of factors Va and VIIIa. This prevents platelet and neutrophil activation and blocks the release of pro-inflammatory cytokines TNF-α, IL-1 and IL-6. In addition, it is pro-fibrinolytic through its role in the inactivation of plasminogen activation inhibitor type 1 (PAI-1) (Matthay 2001; Hotchkiss and Karl 2003).
The metabolic acidaemia seen in Penelope’s case would in part be caused by inadequate tissue perfusion due to both the hypotension and reduced micro-circulatory blood flow because of the disruption of normal coagulation and fibrinolysis. Anaerobic metabolism would then ensue, causing the release of lactate, which contributes to hydrogen ion production, low bicarbonate and a drop in the blood pH. Organ perfusion deficits will be evident due to maldistribution of blood flow.
Other factors
In sepsis, inducible nitric oxide (NO) synthase production becomes abnormally elevated. This overproduction of NO causes mitochondrial inhibition, severe vasodilatation and decreased vasopressor responsiveness, which can lead to refractory hypotension. An acute-phase endocrine stress response is also triggered during sepsis involving the release of catecholamines, adrenocorticotrophin (ACTH), glucocorticoids and growth hormone (Trager et al. 2003; Gearhart and Parbhoo 2006). These factors promote hepatic glycogenolysis and gluconeogenesis (Brierre et al. 2004), causing elevated plasma glucose levels as seen in Penelope. Hyperglycaemia is also evident due to insulin resistance and thus the inability of some cells to utilise available glucose (Brierre et al.2004; Gearhart and Parbhoo 2006), whilst other non-insulin-dependent cells become glucose toxic. This contributes to the mitochondrial damage and may explain why cells are unable to utilise oxygen made available to them (Singer 2005), resulting in a higher-than-expected SvO2. Insulin resistance has also been linked with the development of critical illness neuropathy. Finally, some patients show evidence of adrenal insufficiency and thus reduced serum cortisol levels (Brierre et al. 2004).
Tests and investigations
Mixed venous and central venous oxygen saturations (SvO2 and ScvO2)
SvO2 measurements are used to monitor the balance between oxygen delivery (DO2) and oxygen consumption (VO2). Normal values are approximately 75% as normal oxygen consumption is about 25% (Marx and Reinhart 2006). Blood from the pulmonary artery is required to obtain this measurement. However, with a reduction in the number of pulmonary artery catheters that are now used, this has become problematic. A central venous saturation (ScvO2) obtained from the distal lumen of a central venous catheter has been suggested as an alternative. Although values are not transferable in patients with septic shock (Marx and Reinhart 2006), studies have demonstrated that ScvO2 levels are a reliable alternative for monitoring trends with values of >70% associated with a reduction in mortality (Rivers et al. 2001). An ScvO2 of <70% indicates an inadequate DO2, allowing bedside clinicians to attempt to improve this by volume resuscitation, improvement in contractility and oxygen carriage. In sepsis, however, patients often present with higher-than-normal values as the cells become unable to utilise the oxygen made available to them due to the inflammatory process and associated mitochondrial dysfunction. The amount of oxygen which is returned to the right heart is therefore higher than that would be normally expected. This can be clearly seen in the results presented for Penelope.
Lactate
Lactate is produced as a by-product of anaerobic metabolism and is normally converted in the liver to bicarbonate. Thus, although elevated levels of lactate can indicate tissue hypoperfusion from any cause, or hepatic dysfunction, or a combination of factors, a raised lactate (normal lactate levels are considered to be <2 mmol/L) has been shown to be a useful early marker of critical illness. It is now recommended in the sepsis resuscitation guidelines that all patients presenting with suspected sepsis should have their lactate measured and acted upon within the first six hours of recognition and, regularly, thereafter to monitor their ongoing condition (Dellinger et al. 2008). Elevated levels of lactate will cause the patient to present with a metabolic acidosis due to lactic acid. Thus, as seen in Penelope’s results, the pH will be low, and bicarbonate and base excess levels will be reduced as bicarbonate becomes utilised to buffer the acid produced.
Anion gap (AG)
Penelope will also have an increasing anion gap. Normal values for the AG are 8–16 mEq/L (Thelan et al. 1998). The AG represents the concentration of all the unmeasured anions in the plasma. The negatively charged proteins account for about 10% of plasma anions and make up the majority of the unmeasured anion represented by the anion gap under normal circumstances. The acid anions (e.g. lactate, acetoacetate and sulphate) produced during a metabolic acidosis are not measured as part of the usual laboratory biochemical profile. The H+ produced reacts with bicarbonate anions (buffering) and the CO2 produced is excreted via the lungs (respiratory compensation). The net effect is a decrease in the concentration of measured anions (i.e. HCO3−) and an increase in the concentration of unmeasured anions (the acid anions), so the AG increases.
AG can be calculated from the following formula:
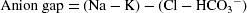
Short synacthen test
Adrenal sufficiency can be affected in septic patients, and is sometimes assessed by administering a small dose (usually 250 μg of adrenocorticotropic hormone (ACTH)) and monitoring to see if a normal serum cortisol concentration response is achieved (Brierre et al. 2004). However, following the CORTICUS study (Sprung et al. 2007), the use of this test prior to commencing steroid therapy is no longer recommended (Dellinger et al. 2008). Indeed, the use of any steroids in sepsis is now limited to those patients who do not respond to vasopressors (Dellinger et al. 2008).
See Chapter 3 for further discussion of investigations relevant to fever.
Developing scenario
Despite fluid resuscitation, guided by haemodynamic monitoring, Penelope’s condition continues to deteriorate. She is now hypotensive with a MABP of 40 mmHg, is oliguric and has a worsening metabolic and respiratory acidaemia. Her cardiac output is 6.8 l/min (normal 4–6 l/min), and she has an SVR of 500 dynes/sec/cm−5 (normal 800–1200). She also has marked systemic oedema and evidence of clotting dysfunction (prolonged activated partial thromboplastin time (aPPT) and low platelets). Penelope is now in established septic shock and has evidence of MODS (see Table 4.2).
Table 4.3 Sepsis care bundles.
Source: Surviving Sepsis Campaign (2009). Reproduced with permission. Copyright © 2009. European Society of Intensive Care Medicine, International Sepsis Forum and Society of Critical Care Medicines.
| Sepsis resuscitation bundle (within 6 hours) | Sepsis management bundle (within 24 hours) |
|
|
Managing septic shock
In 2003 international experts involved with the Surviving Sepsis Campaign developed evidenced-based guidelines for managing patients with sepsis, which were updated in 2007 (Dellinger et al. 2008). These were further developed into two care bundles (Table 4.3), one aimed at sepsis resuscitation during the first 6 hours, and one for ongoing management of the patient with sepsis (Dellinger et al. 2008). These along with the Savings Lives Campaign document which discusses high-impact interventions produced by the Department of Health (DH 2007) provide a template for management of all patients at risk of or diagnosed with sepsis.
Airway and breathing
As Penelope is intubated, she is at high risk of developing a nosocomial pneumonia. Attention must be paid to the avoidance of this as a source of secondary sepsis. She should be nursed in accordance with the ventilator care bundle (Pruitt and Jacobs 2006). Furthermore, guidelines for managing patients with acute lung injury should be followed to reduce ventilator-induced lung injury and further exacerbation of the inflammatory response. A key aspect of this includes pressure-limited ventilation (ARDSnet 2000; Sevransky et al. 2004). Further details of these aspects of management can be found in Chapter 1.
Circulation
One of the key challenges in managing severely septic patients is the struggle to balance fluid requirements for a patient who has systemic hypotension alongside pulmonary fluid overload and deteriorating gas exchange. This problem is evident in the data presented for Penelope, showing respiratory dysfunction alongside hypotension, and associated oliguria, systemic oedema and a low SVR, and a metabolic acidaemia. The overall management aim must be to optimise tissue perfusion.
Initially, fluid resuscitation is paramount to ensure that the goals in Table 4.2 are reached, and during the first 24 hours, the patient will usually require large volumes of fluid (Dellinger et al. 2008). Which fluid should be utilised to do this remains controversial. Studies such as the SAFE study which showed no difference between the use of saline and albumin (Finfer et al. 2004) suggest that a colloid or a crystalloid may be utilised. However, many clinicians continue to prefer the choice of colloid for intravascular fluid repletion due to the reduced volume requirements. Colloid fluid challenges may therefore be used together with a restricted crystalloid maintenance fluid regime to avoid the associated pulmonary complications. The nursing role includes close monitoring of fluid balance and vigilance for signs of hypovolaemia and deteriorating gas exchange, as well as monitoring the effects of administered fluids. A central venous pressure (CVP) of 12 mmHg would be the target for optimal intravascular filling in Penelope as she is receiving positive pressure ventilation (Dellinger et al. 2008). However, the CVP may be unreliable in a patient with pulmonary oedema, and more advanced monitoring should therefore be instigated, for example, a PiCCO, Doppler or pulmonary artery catheter. Fluid loss into the interstitial space due to the increased vascular permeability will also be compounded by insensible losses associated with evaporation during sweating. Additionally, in spontaneously ventilating patients an increased respiratory rate and loss of water vapour in expired air will be evident. These factors, must be taken into consideration during fluid assessment.
The use of blood products is generally restricted with an aim to keep haemoglobin (Hb) 7–9 g/dL as this has been shown to reduce blood viscosity and improve micro-circulatory blood flow (Hebert et al. 1999). However, this has to be considered in relation to optimising DO2, and in some patients, for example, those with acute cardiac insufficiency, a higher level may be more appropriate (Hebert et al. 2001). Examining haemoglobin (Hb) and haematocrit (HCT) levels alongside blood gases will help to ensure optimal DO2 to the tissues is achieved. Additionally, the risk of the development of disseminated intravascular coagulation (DIC) must not be forgotten and the bedside nurse should be vigilant for signs of bleeding or tissue perfusion deficits, for example, poor distal limb perfusion and bruising, which may be associated with this condition. Laboratory data such as platelet count, aPTT and internationalised ratio (INR) should also be monitored closely for this reason.
Vasopressor and/or inotropic drugs will be required to maintain an adequate MABP (>65 mmHg) in a patient such as Penelope where fluid resuscitation alone has not been adequate. Agents of choice appear to be noradrenaline or dopamine for high-output states with a low SVR, with the possible addition of vasopressin, and dobutamine for inotropic support in patients presenting with a low cardiac output following fluid resuscitation (Dellinger et al. 2008). These patients must be nursed in a critical care facility where invasive blood pressure can be monitored and nurses are familiar with the actions and potential problems associated with the agents being used. Double pumping of such drugs is essential to avoid life-threatening periods of hypotension during syringe changes. The best methods of safely managing this have been studied by Morrice et al. (2004) and Trim and Roe (2004).
Both fluid therapy and vasoacative drug therapy should be guided by the use of an invasive haemodynamic device which calculates cardiac output, SVR, DO2 and VO2. Additionally, pulmonary data are available from some devices, for example, extravascular lung water (EVLW) from PiCCO (Cottis et al. 2003) which can be useful in decision making regarding fluid and drug therapy.
Penelope could meet the criteria for the administration of recombinant activated protein C (rhAPC), a drug aimed at restoring normal coagulation and limiting thrombin production. Following a study by Bernard et al. (2001), which showed that rhAPC significantly reduced mortality, it is now recommended for use in patients presenting with severe sepsis and septic shock, who have a high risk of death unless contraindications associated with bleeding are identified (Dellinger et al. 2008). The bedside nurse must ensure a high degree of vigilance during administration of this agent for signs of bleeding. This should include neurological assessment for signs of cerebral haemorrhage. Further, the drug must be administered through a dedicated line, preferably central, and should be withheld for at least two hours prior to and after any invasive procedure being carried out. Laboratory data need to be examined, but it should be noted that although the aPPT is likely to increase, this may not affect the risk of bleeding, so clinical assessment should take priority in deciding whether to stop the infusion before the 96 hours it is usually administered for has elapsed (Schulman and Hare 2003).
The management of fever would also be an important issue to consider as part of the care of Penelope. The data presented suggest that she is currently compromised from both a respiratory and a metabolic perspective. This in itself should justify the use of antipyretics and/or physical cooling interventions in this case (see Chapter 3 for further details of fever management).
Disability of the nervous system
Regular assessment of Penelope’s neurological status and sedation level should be undertaken. Penelope is at high risk of the development of critical illness neuropathy due to insulin resistance and hyperglycaemia. Paralysing agents should be avoided for these reasons, and regular assessment using a peripheral nerve stimulator (with an aim of maintaining one to two twitches) should be undertaken if continuous administration is necessary (Murray et al. 2002).
Strict attention to tight glycaemic control is also vital (Van den Berghe et al. 2001). This may be further complicated as an infusion of steroids to counter any suspected adrenal insufficiency may be in progress (Annane et al. 2002), although use of steroids is controversial with a recent research multi-centre trial (the CORTICUS trial) failing to demonstrate any mortality benefit (Sprung et al. 2007). Problems controlling plasma glucose levels may also arise during attempts to administer enteral nutrition. This is a high priority in the prevention of bacterial translocation and secondary sepsis but the presenting condition, and the use of opiates for sedation and analgesia may impair gut motility leading to enteral feed and absorption rates varying over time, and substitution glucose infusions being utilised. Frequent assessment of plasma glucose levels and appropriate titration of infusions using a validated protocol is vital to prevent undesired hypoglycaemia from occurring. Numerous studies have examined the use of a variety of protocols to try and increase both the effectiveness and safety of such a regime (Wilson et al. 2007). Dellinger et al. (2008) recommend maintaining blood glucose levels <8.3 mmol rather than the 4.4–6.1 mmol suggested by Van den Berghe et al. (2001) to reduce the risks of such problems occurring (see Chapter 7 for more information on altered blood sugar management).
Exposure/environment
A holistic approach to the management of Penelope is vital as many of the above factors will lead to problems associated with poor perfusion such as impaired skin integrity. Eye, oral care and effective positioning are paramount to prevent secondary infection, as is the importance of appropriate psychological care to reduce anxiety and stress, which in turn will reduce the metabolic demands on the body (Frazier et al. 2003; Adam and Osborne 2005). Additionally, continued attempts to establish enteral feeding should be made (Heyland et al. 2003), alongside the use of stress ulcer prophylactic agents to prevent upper gastrointestinal bleeding (Dellinger et al. 2008). The nurse should prioritise strict infection control measures and should be continuously vigilant for potential causes of sepsis, performing septic screens as indicated, and minimising risks wherever possible. This should include appropriate wound management and effective care of invasive lines and tubes. The central venous catheter care bundle (DH 2007) should be applied to Penelope’s management to help prevent catheter-related bloodstream infection. The two elements of this bundle focus on catheter insertion actions and ongoing care. The latter element makes reference to effective hand hygiene, regular catheter site inspection, maintaining intact dry dressings on insertion sites, aseptic technique and chlorhexidine 2% for cleaning ports and guidelines for administration set replacement. It also recommends against routine catheter replacement (DH 2007). The National Patient Safety Agency (NPSA) offers further guidelines for central venous catheter care to prevent bloodstream infection as part of the recent Matching Michigan project initiative (NPSA 2009).
The other relevant high-impact interventions included in the DH (2007) Saving Lives document should also be applied to Penelope’s care. These include guidelines on the management of peripheral lines, urinary catheters and best practice with regards to prevention and management of clostridium difficile, antibiotic use and obtaining blood cultures. As sepsis is a thrombotic disorder, attention should also be paid to effective anti-thrombotic measures such as the use of thrombo-embolic stockings and systemic anticoagulation (Dellinger et al.2008).
Conclusion
This scenario has discussed the complex challenges associated with caring for a patient with severe sepsis.
Key learning points
- Severe sepsis is part of a continuum of illness which starts with a patient’s normal inflammatory response being triggered, which can then become out of control, with the end result being multiple organ dysfunction syndrome and death.
- Management of patients with severe sepsis can be optimised using an evidence-based approach, implementing care bundles such as those related to sepsis, ventilator care and central venous catheter care (DH 2007; Dellinger et al. 2008; NPSA 2009).
- Early recognition of infection and its consequences and prevention is vital.
- Appropriate use and titration of ventilatory support, fluid and drugs within a holistic framework of care which encompasses the DH (2007) high-impact interventions offers the patient the best chance of recovery.
Critical appraisal of research paper
Pronovost et al. (2006) An intervention to decrease catheter related bloodstream infections in the ICU. The New England Journal of Medicine 355(26), 2725–2732.
This American prospective cohort study studied the impact of a number of interventions, one being related to central-line management, in 103 ICUs across 67 hospitals in Michigan. Interventions specific to the reduction of catheter-related bloodstream infection included hand washing, full barrier precautions during central-line insertion, chlorhexidine use to clean line sites, avoidance of the use of the femoral access and removal of unnecessary catheters. Results indicated that following the implementation of the interventions, the median rate of catheter-related bloodstream infection per 1000 catheter days had decreased significantly at both 3 months and 18 months (p < 0.002). Their conclusions suggest that the use of these evidence-based interventions result in a large and sustained reduction of central venous catheter-related infection in both adult and paediatric intensive care.
Reader activities
1. Read the research article written by Pronovost et al. (2006).
2. Using the critical appraisal framework in Appendix I, consider the methodological quality of the paper.
3. Reflect on this aspect of your own practice and the implications for future practice management that this paper raises.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


