Reader activities
Having read this scenario, consider the following:
- What are the key anatomical and physiological concepts for the cardiovascular system?
- In relation to cardiac anatomy, physiology and disease, what other clinical information needs to be ascertained from handover with the theatre team?
Applied anatomy and pathophysiology
In order to competently care for Mrs Raipul, there must be sound understanding of the underlying anatomy, physiology and disease aetiology. This subject area is well explored in a range of texts (Levick 2003; Noble et al. 2005), so a concise introduction to key issues pertinent to the case study follows.
The cardiovascular system has a vital role in delivering oxygen (metabolic substrate) to cells and removing carbon dioxide (metabolic waste). Functioning organs are maintained by pulsatile blood flow from the heart and the linking of pulmonary and systemic circulatory systems. The heart is situated in the mediastinum with the base of the heart (point of origin of great arteries) located behind the sternum at the level of the second intercostal space. The apex of the heart (left ventricle) is approximately located at fifth intercostal space left mid-clavicular line. Considering its vital function, the heart is only the size of a clenched hand and often weighs <0.5 kg/lb (Filer and Hatchett 2007).
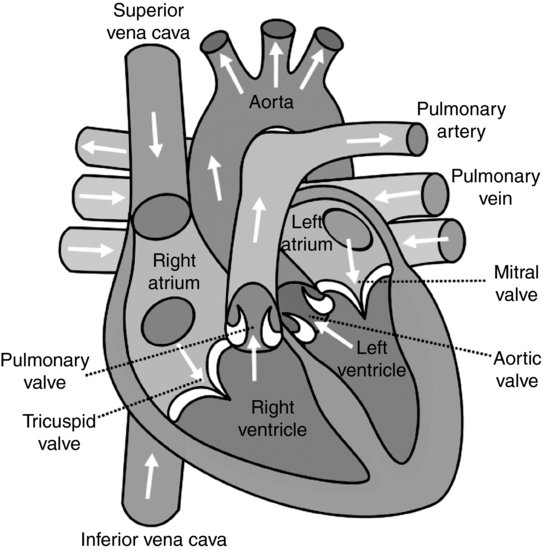
Simplistically, the heart can be visualized as a hollow, fibromuscular organ. The right side of the heart pumps blood to the pulmonary circulation via the main pulmonary artery, and the left side pumps to the systemic circulation via the aorta. The heart comprises four chambers: two thin-walled atria chambers that act to collect blood returning to the heart, and two thicker walled ventricles that act to contract and eject blood out into the systemic and pulmonary circulations via the great arteries. The chambers are separated by a dividing structure, the septum. This ensures that deoxygenated blood in the right side of the heart, and oxygenated blood in the left side, remains isolated. The cardiac valves ensure blood only travels forward through the heart (see Figure 5.2).
The wall of the heart comprises three layers: pericardium, myocardium and endocardium. The outer layer (pericardium) is a two-layered fibrous sac which, attached to the base of the heart, stabilises the heart position whilst allowing free movement of the apex. The myocardium is mainly muscle (myocytes) together with specialised autorhythmic conduction cells. The endocardium is the inner lining of squamous epithelial cells.
Whilst the endocardium can obtain metabolic nutrients from direct blood contact, other layers of the heart require supply via the coronary circulation. There are two main coronary arteries (left coronary and right coronary artery) that arise from anterior and posterior sinuses of the ascending aorta, and from these a diffuse capillary system ensures optimal oxygen supply to the myocardium. The majority of coronary blood drains back to the heart via the cardiac veins into the coronary sinus, located between the right atrium and ventricle.
In considering cardiac physiology, there are three main physiological principles that need attention for any patient with cardiac disease: preload, afterload and contractility. Preload is the volume of blood in the ventricles at the end of diastole (i.e. emptied from the atria). It is therefore affected by circulating volume, atrial contraction, left ventricular compliance and length of time of diastole (relaxation phase of cardiac cycle). Afterload is defined as resistance to ventricular ejection and is affected by the resistance in and around the outflow tract area, and in the peripheries. Contractility is the strength of myocardial contraction. In optimising preload and afterload through manipulation of the autonomic nervous system and inotropic therapy, contractility (and cardiac output) is increased. It is manipulation of the relationship between preload and contractility that is explored in Starling curves.
Assessment
Practitioners first meet critically ill patients through clinical handover. It is such communication between healthcare professionals that has been the focus of recent healthcare policy initiatives (National Institute for Health and Clinical Excellence (NICE) 2007). Information gained in patient transfer is important to understand retrospective events, to promote continuity of care and to inform prospective care planning through anticipation of future patient problems.
The arrival of any patient into a critical care area usually heralds great activity. However, once the patient is monitored and is safe, active listening to patient handover is paramount. In the handover of Mrs Raipul, key essentials (name, age, sex, reason for admission and plan) are outlined, but there is additional useful information to be gained, for example, the site from which the coronary artery grafts were taken and subsequently placed; length of time on cardiopulmonary bypass (CPB) and aortic cross-clamp time. Knowledge of graft donor site will direct post-operative observations and management. Knowledge of coronary arteries grafted will give some indication as to the severity of existing cardiac disease. Furthermore, longer CPB times are associated with increased systemic inflammatory and hematological problems, whilst longer aortic cross-clamp times are associated with increased organ ischaemia (Carter 2003). Additional useful information includes Mrs Raipul’s pre-operative left ventricular function (an indirect measure of contractility) and filling pressures required to come off CPB. Mrs Raipul’s central venous or capillary wedge pressures can be used as indicators of preload (Starling curve) and used post-operatively to optimise cardiac output. Excessive volume loading beyond these preload values could result in reduced cardiac output and pulmonary oedema.
Further useful patient information can be deduced from Mrs Raipul’s past medical history, pre-operative medications and social history. Such detail can be integrated into patient assessment through the use of a history-taking model (Bickley and Szilagyi 2005). Additional past medical history (previous myocardial infarctions, asthma, hypertension, diabetes, renal failure, epilepsy, stroke) is important and may inform Mrs Raipul’s post-operative care. For example, in patients with asthma, the choice of ventilation mode, respiratory observations and bronchodilator medications need consideration. Pre-existing disease will also be reflected in pre-operative medications taken. If Mrs Raipul is prescribed beta-blockers, aspirin/clopidogrel or angiotensin-converting enzyme (ACE) inhibitor drugs, these may influence her post-operative pathway. Pre-existing disease and the use of medications could give rise to an increased risk of bleeding, need for pacing or more complex fluid management. Similarly, if Mrs Raipul has any drug allergies, these must be noted, together with the nature of the reaction.
An understanding of any significant issues in Mrs Raipul’s family or social history can also be useful in planning her care, and this can usually be gained from admission and nursing notes. Details regarding Mrs Raipul’s next of kin/dependents, history of smoking, use of recreational drugs or excessive alcohol and her occupation can be helpful in building a holistic assessment and package of care. This information, integrated with initial patient assessment and routine cardiovascular observations (heart rate, rhythm, systolic/mean/diastolic pressures, central venous pressures, peripheral inspection) can ensure that the three cardiac principles of preload, afterload and contractility can be individually optimised for Mrs Raipul.
Developing scenario – bleeding and hypovolaemia
On undertaking routine observations on Mrs Raipul, you note:
- Heart rate 98 beats per minute. Sinus rhythm.
- Blood pressure 95/55 mmHg.
- Central venous pressure (CVP) 3 mmHg.
- Core temperature has not markedly risen over the past hour (35.8–36.0°C) and Mrs Raipul, who was warm to mid thigh, is now only warm to knee.
- Chest drain losses have risen from 50 mL over the first 2 hours to 100 mL over the past 30 minutes.
Reader activities
Consider the following:
- What physiologic terms describe Mrs Raipul’s clinical signs?
- Why is Mrs Raipul especially at risk of bleeding?
- How can a clotting screen help determine the cause of bleeding?
- What drugs can be administered to reverse clotting abnormalities?
- What other treatment options can be used to minimise blood loss?
Applied pathophysiology
Mrs Raipul’s clinical signs indicate hypotension due to hypovolaemia. Her blood pressure is low, and certainly lower than when it was measured pre-operatively at 145/95 mmHg. Her right-sided filling pressure (CVP) reading is also low, indicating reduced venous return. Hypovolaemia occurs when circulating blood volume is insufficient to fill intravascular spaces. The resultant reduction in venous return to the right heart leads to a decreased cardiac output, and reduced systolic and mean arterial pressure. A 10% reduction in circulating volume will initiate compensatory mechanisms including tachycardia and peripheral vasoconstriction with higher diastolic blood pressure values (Ganong 2005), as evidenced in Mrs Raipul’s observations. Losses resulting in a 20% or more reduction of circulating volume will lead to clinical signs and symptoms of hypovolaemic shock including reduced renal, cerebral and splanchnic perfusion with impaired function.
Any patient who sustains injury or trauma (post surgery, burns, road traffic accident), is dehydrated or has gastrointestinal obstruction is at risk of hypovolaemic shock unless the underlying cause is well managed. Mrs Raipul has undergone cardiac surgery and has a number of risk factors for hypovolaemia. Pre-operatively, Mrs Raipul will have been nil by mouth prior to surgery and will have a degree of dehydration, especially if receiving high dose diuretics. She will have sustained some blood loss peri-operatively and this may not have been fully replaced. Fluid volume deficit could have been further aggravated by the interstitial fluid shifts that occur post-CPB. During cardiac surgery, patients are centrally cooled to 32°C for cerebral and myocardial protection, and when re-warming begins, judicious volume replacement is required to ensure further hypovolaemia does not result.
Hypovolaemia due to blood loss is clearly a key consideration in Mrs Raipul’s case. Factors that increase risk of post-operative bleeding include the pathophysiology of CPB and cardiac surgical techniques. CPB activates whole body inflammatory responses resulting from blood contact with non-endothelial CPB circuitry. Such contact activates plasma protein systems and intrinsic and extrinsic coagulation pathways, with the risk of clotting. To counteract this, intra-operative heparin is administered and this requires reversal post-surgery. CPB also activates platelet activity, decreasing overall platelet numbers and function. Cardiac surgery also requires suturing of key blood vessels and placement of cannulae into major cardiac structures. A sub-optimal surgical technique or excessive force (high systemic pressures) on these sites increases risk of post-operative bleeding. These are all major contributors to risk of post-operative bleeding.
There are many other specific factors that may pre-dispose Mrs Raipul to bleed post-operatively. In line with current secondary prophylactic practice, Mrs Raipul will have received pre-operative aspirin and anti-platelet therapy (clopidogrel) to reduce the risk of myocardial infarction (Antiplatelet Trialists’ Collaboration 1994). Even with discontinuation of these prior to surgery, some residual anti-coagulation effect remains, increasing potential for blood loss. Furthermore, as the patient re-warms post-operatively, residual heparin given during CPB and trapped in vasoconstricted peripheries will be washed back into the systemic circulation and have an anti-coagulation effect (heparin rebound). If autotransfusion is used in theatre, or if blood drained from CPB is transfused (pump blood), these will contain heparin and/or citrate phosphate; whilst volume replacement will restore normovolaemia, further coagulation problems can result.
Critical care assessment and diagnostics
Whilst there are key diagnostics to assist in the treatment of blood loss, patient assessment can also offer pointers for management. Chest drains are routinely placed at the end of surgery to aid the flow of serous and blood losses away from the heart, thereby preventing clot formation. It would be standard for Mrs Raipul to have a minimum of one Portex mediastinal drain but Redivac drains may also be present. Chest drains are initially placed on low suction (2–4 kPa) and frequent observations undertaken on losses from, and patency of, the drains post-theatre (see Figure 5.3). Whilst there is little robust evidence base for the care of such drains, some practice guidelines are available. Clamping of chest drains (especially if air leaks are present) should be avoided, and routine milking or stripping of chest tubing should not occur due to the high negative pressures generated, and the stress placed on surgical anastomoses and healthy clot formation (Thorn 2006).
Blood loss is usually greatest immediately post-surgery and should reduce to near zero 6–12 hours post-surgery. With some local unit variation, acceptable blood loss values are 2 mL/kg/hour for the first 2–3 hours, up to 1 mL/kg/hour for the next 3 hours, and then under 0.5 mL/kg/hour by 12 hours post-surgery. There would be concern if blood loss substantially increased beyond these levels, or if blood loss appeared arterial, or if blood was observed advancing up the drain tubing against gravity. Surgical teams should attend haemodynamically unstable patients, especially those with increasing CVP readings as this may herald the surgical emergency of cardiac tamponade.
In contrast, a generalised coagulopathy is evidenced by increasing blood loss from any incision and/or line insertion sites. In this instance, and in all but the most urgent surgical emergency, clotting studies are an important diagnostic tool. In theatre, activated clotting time (ACT) is routinely measured to determine the impact of heparin whilst on CPB and the effectiveness of protamine to reverse heparin once off CPB. In critical care areas, clotting tests include full blood count (FBC) and full clotting screen (see Table 5.1 for tests of haemostasis). Furthermore, some centres are increasingly using thromboelastograms (TEGs) to give rapid assessment of clotting (Wenker et al. 2000).
Table 5.1 Tests of haemostasis.
| Name of test | Normal value | Clinical significance |
| Haemoglobin (Hb) | Males 13.5–17.5 g/dL Females 11.5–15.5 g/dL | Increased value associated with dehydration Decreased with anaemia, blood loss, vitamin deficiency, etc. |
| Red blood cell (RBC) | Males 4.5–6.5 × 10 12/L Females 3.9–5.6 × 1012/L | As above |
| Packed cell volume (PCV) | Males 40–52% Females 36–48% | As above Test also known as haemotocrit |
| Activated partial thromboplastin time (APTT) | 30–40 seconds | Increased in liver disease, vitamin K deficiency Test used to direct heparin therapy – APTT goal set per patient |
| Prothrombin time (PT) | 10–14 seconds | Increased in liver disease |
| Thrombin time (TT) | 14–16 seconds | Increased in liver disease |
| International normalised ratio (INR) | Test used to direct warfarin therapy Normal value aim with 2.0–3.0 on warfarin | |
| Platelet count | 150–400 × 109/L | Increased in malignant bone marrow disorders Decreased post-bypass, if receiving intra-aortic balloon therapy, in acute leukaemia, cytotoxic drugs Platelets may be dysfunctional if patient on aspirin pre-operatively |
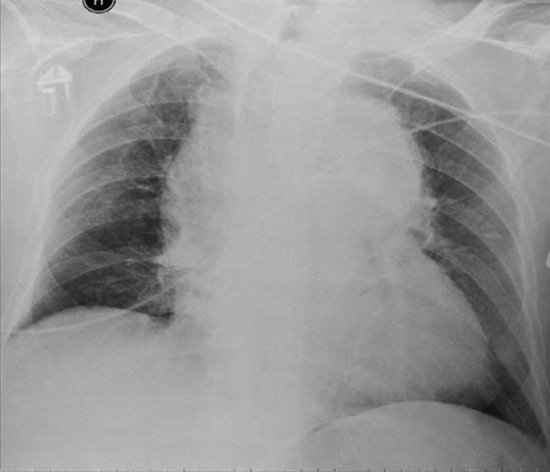
A final diagnostic test to check for bleeding post-cardiac surgery is the chest X-ray. The most common view for chest X-rays is the posteroanterior (PA) film undertaken in the radiology department. In this view the scapulas are rotated away from the lung fields and mediastinal shadowing of the lung fields is reduced. The PA film offers the highest quality chest view; however, it is not always practical to transport critically ill patients to radiology departments and so portable X-rays (anteroposterior (AP) films) are often used. Supine AP films are always used post-operatively. These still yield valuable information, although some caution must be exercised when interpreting mediastinal widening. Either all sequential AP or PA views should be used for comparison purposes (see Fig 5.4 for and example of a chest x-ray).
Excessive blood loss post-cardiac surgery will accumulate in the mediastinal space, producing a widening of the mediastinum (Figure 5.4). A haemothorax (blood in the pleural space) may also be present if one or both pleura were opened peri-operatively. Such collections move freely in the pleural space and shift with the patient’s position. In the supine position, blood will layer out over the posterior surface of the chest, producing a diffuse shadow that makes, by comparison, the unaffected lung look denser in appearance. Echocardiography may also be useful to assess cardiac function/structure in these situations. The transthoracic (TTE) or transoesophageal echocardiography (TOE) approach can be used to give information about cardiac structures and any obstruction to ventricular filling or pericardial collection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree