BP 140/80 mmHg
Core temperature 37.5°C via tympanic thermometer
Full power in both legs and arms
Pupils equal and reacting to light (PEARL)
BM 5.8 mmol/L
Reader activities
Having read the scenario, consider the following:
- What type of seizure has Simon experienced using current ILAE (International League Against Epilepsy) classification?
- Is the GCS a valid and reliable tool for assessing Simon’s conscious level? Which other methods could be used and what are their possible advantages/disadvantages?
- What further assessments/investigations would be important for Simon in view of his seizure activity?
Related pathophysiology
An epileptic seizure is a transient occurrence of signs and/or symptoms due to abnormal, excessive or synchronous neuronal activity in the brain (Varelas and Spanaki 2006), causing an abrupt and temporary altered state of cerebral function (Hickey 2008; Fuller and Mansfold 2010; Barker 2008). The abnormal neuronal discharge results from an imbalance between excitation and inhibition within the central nervous system. Excessive excitation or inhibition can occur in focal areas of the cerebral cortex, causing focal seizures or throughout the cerebral cortex, causing generalised seizures (Hickey 2008; Barker 2008). The presentation of seizures depends on whether the seizure is focal (local) or generalised but both seizure types can cause alterations in sensation, behaviour, movement, perception or consciousness (Hickey 2008; Fuller and Mansfold 2010; Barker 2008).
Seizures may be caused by an acute insult, such as a head injury, or during an acute disorder of the central nervous system, such as encephalitis, as in Simon’s case. Isolated seizures can also occur following drug toxicity, drug or alcohol withdrawal or metabolic disturbances (Barker 2008). In such cases, resolution of the underlying disorder usually leads to cessation of any further seizure activity; however, in some patients seizures may continue for no apparent reason. Simon is at risk of seizure activity due to his acute encephalitis. He has had an observed episode of ‘twitching’ in his right arm followed by a reduction in his conscious level and he will require further close observation as this event may not be an isolated one.
Seizure classification
The classification of seizures can be complex as it involves two interrelated classifications: the classification of seizure type and classification of epilepsy syndromes (Hickey 2008; NICE 2004; Fuller and Mansfold 2010). It is recommended that confirmed epilepsy should be classified by epilepsy syndrome criteria (Barker 2008). Epilepsy is suspected following the occurrence of two confirmed seizures not arising from any other obvious, underlying cause (Fisher et al. 2005). As Simon has had a single seizure, most likely as a consequence of his encephalitis, he would not be diagnosed or treated for epilepsy at this time (NICE 2004). The risk of recurrence following a single seizure is variable but generally thought to be about 20–30%; however, recurrence may rise to 90% in patients with confirmed abnormal brain imaging or EEG results (Berg 1991; NICE 2004; Fuller and Mansfold 2010) and these patients may require treatment with anti-epileptic drugs (AEDs). Most neurologists would probably not recommend commencing anti-convulsants for Simon after a single seizure unless there was evidence of previous seizure activity, structural brain damage or a confirmed abnormal EEG recording (NICE 2004).
Classification of seizure type
Nurses need to be conversant with the classification of seizure type and the specific signs evident in a patient having a seizure (Mantri 2007). Seizures can manifest in a number of ways, such as involuntary changes in body movement or function, altered sensations, awareness or behaviour. The classification of seizures is currently undergoing review by the ILAE; consequently, some of the terminology cited in this chapter may be subject to imminent change. The most recent classifications are those issued by the ILAE in 2002 and NICE (2004) (see Table 14.2). The DH (2005) supports the use of this classification system in the UK.
Table 14.2 Classification of seizure type.
Source: Adapted from Commission on Classification and Terminology of the International League Against Epilepsy classification of seizure 1981.
| Partial (focal, local) seizures |
Simple partial seizures (consciousness not impaired) with any of the following symptoms:
Complex partial seizures (with impairment of consciousness):
Partial seizures evolving to secondary generalized seizures (generalized tonic–clonic, tonic or clonic):
|
| Generalized seizures (convulsive or non-convulsive) |
|
Unclassified seizures
|
Seizures are categorised as being either partial or generalised. Partial seizures (sometimes called focal or local) begin in a specific area of the brain with focal discharges. Partial seizures are additionally classed as being simple partial seizures, complex partial seizures or partial with secondary generalisation (Gambrell and Flynn 2004; Marthaler 2004). Simple partial seizures are those that do not cause the patient to lose consciousness and occur in a single hemisphere of the brain. The person will remain fully aware but may display behavioural and/or motor, sensory, experiential phenomena, clonic jerking of a body part, localised pain, auras or déjà vu (Marthaler 2004). Manifestations of simple partial seizures correspond to the area of the brain involved and the actions it controls. Seizures arising in the temporal lobe, for example, may cause alterations in memory and emotions or may lead to sensations of smells, tastes or sounds. Frontal lobe seizures commonly lead to uncontrolled movement of an extremity or changes in speech. The manifestations of these types of seizure may involve relatively subtle changes in behaviour or abnormal movements, which may be highly localised (e.g. eye twitching and involuntary movement of a finger or hand) and consequently overlooked or not regarded as significant. Nurses should therefore be vigilant in their observation of patients for any abnormal neurological activity, however slight.
The ictal phase (the seizure event) in simple partial seizures is frequently brief, making it difficult to confirm the diagnosis without continuous EEG monitoring. Simple partial seizures may sometimes progress to a complex partial seizure and it is, therefore, important for the nurse to observe the patients closely until they are fully recovered (Marthaler 2004).
Complex partial seizures impair consciousness and although the patients may appear to be awake, they will be unable to respond to commands and will not remember the event (Gambrell and Flynn 2004). This type of seizure originates mainly in the temporal lobe and common characteristics include automatisms (involuntary behaviours) such as chewing or picking or bizarre behaviours such as uncontrollable laughter, wanderings, hallucinations or unusual epigastric sensations (Gambrell and Flynn 2004). These seizures are often missed entirely as they are unobserved and the patient does not remember having them. They also have a minimal post-ictal phase and the person may return to normal behaviour rapidly. In addition, it is important to be aware that a partial seizure may sometimes develop into a generalised seizure, termed a partial seizure with secondary generalisation (Barker 2008).
Generalised seizures are classified as either non-convulsive or convulsive. Non-convulsive seizures include absence (originally termed petit mal) and myoclonic types. Absence seizures may manifest in staring, eye blinking or lip smacking and tend to occur with great frequency but are often undetected as they are short-lived events (Gambrell and Flynn 2004). Myoclonic seizures involve the jerking of a particular muscle group for a brief period. Non-convulsive seizures occur almost exclusively in children and adolescents; although they may sometimes happen in older patients, they are unlikely to be the type of seizures evident in Simon’s case.
Convulsive generalised or tonic–clonic seizures cause the patient to lose consciousness, fall and have muscle spasms. Tonic spasms are characterised by contractions in which the arms flex and the legs extend. Clonic seizures lead to alternating contraction and relaxation of muscles. Tonic–clonic events often occur with little warning, and the patients will fall from standing, their breathing will be impaired during the event and they may lose bowel or bladder control or occasionally bite their tongue (Gambrell and Flynn 2004). There is a tonic phase in which the patients have violent convulsive muscular contractions and will appear pale, sweaty and tachycardic with an elevated blood pressure, sometimes becoming cyanotic during this period. As the seizure progresses, clonic muscle relaxations lengthen and gradually become more frequent as the seizure nears its end (Hickey 2008). A tonic–clonic seizure of more than five-minute duration is considered a serious event and requires a medical review of the patients, even if they are known to have seizures (NICE 2004). There is often a lengthy post-ictal phase following a tonic–clonic seizure, during which the patient lies still, is exhausted and may sleep for many hours. They will not remember the event and assessment for any traumatic injuries from the fall or convulsions should be undertaken (Gambrell and Flynn 2004).
A thorough understanding of the classification of seizures enables the nurse to identify the specific type of seizure their patient is experiencing and, consequently, promotes appropriate ongoing patient assessment and nursing management (Mirski and Varelas 2003). Witnessed seizures mean that nurses can document and report specific symptoms enabling confirmation of the type of seizure and its duration. The symptoms of Simon’s seizure suggest that he has had a simple partial seizure that has progressed to a complex partial seizure. His seizure was initially partial (focal/local) as it did not involve a loss of consciousness and was manifested by clonic jerking of his right arm only, indicating that a single (the left) hemisphere of the brain was involved. Simon’s seizure, however, progressed to a complex partial seizure in which his conscious level became impaired but the origin was still in a single hemisphere as only his right arm was involved.
Assessment tools/skills
Glasgow Coma Score and neurological assessment
The GCS is the most widely used neurological assessment tool worldwide. In critical care, it is also the cornerstone of many of the prognostic injury severity scoring systems forming a key part of the Trauma Score (TS), the Acute Physiology and Chronic Health Evaluation (APACHE) and the Trauma and Injury Severity Score (TISS) that are currently used to provide global assessment of critically ill patients in the ICU (Wayne et al. 1998).
Accurate GCS, neurological assessment and detection of seizure activity can be complex in many critically ill patients as they may be heavily sedated or receiving neuromuscular blockade (NMB), which will compromise their GCS score. Sedated and NMB patients cannot be assessed for eye opening, motor or verbal responses adequately, and ventilated patients who have endotracheal or tracheostomy tubes in situ will be unable to give verbal responses. Where it is important that accurate GCS scores are obtained, clinical studies have found that this can be achieved, in the absence of a verbal response in intubated patients, by calculating the verbal score from the motor and eye score using a specific mathematical formula (Wayne et al. 1998). Nurses should also bear in mind that pupil responses remain intact in the patient undergoing NMB and sedation, and consequently, regular observations of these are vital in the early detection of altered neurological status.
Electroencephalogram (EEG) and Continuous EEG (cEEG) monitoring
Continuous EEG (cEEG) technology utilises the placement of sensitive scalp electrodes to provide a detailed picture of brain activity. cEEG monitoring allows for the prompt detection and diagnosis of abnormal events, especially non-convulsive seizures (NCsz) that are impossible to detect by neurological assessments or observations (Arbour 2003). Non-convulsive seizures occur relatively frequently in the ICU patient and, if undetected, can lead to serious complications or the development of life-threatening SE (Hirsch 2004; Friedman et al. 2009). cEEG is recommended for patients who are heavily sedated, comatose or receiving NMB and those with severe traumatic brain injury (TBI), with CNS infection or with prior NCsz activity or non-convulsive status epilepticus (NCSE) (Hirsch 2004; Friedman et al. 2009). Simultaneous cEEG monitoring for a number of patients in a single ICU is now feasible as the large amounts of clinical data generated can be collated and analysed rapidly due to improved computer technologies (Friedman et al. 2009).
Simon does not fulfil a need for cEEG monitoring as he is awake and can be assessed by standard neurological assessment and GCS, but should he deteriorate and require sedation, his previous seizure activity might place him in need of cEEG monitoring.
Bispectral index monitoring (BIS)
The EEG-based BIS technology is increasingly being utilised in the ICU setting to monitor levels of sedation and arousal states in patients receiving NMB, bedside procedures or deep sedation during controlled ventilation and drug-induced coma, and would be indicated for a patient at risk of seizures who is sedated or on NMB (Arbour 2003). BIS is discussed more fully in Chapter 11 on managing pain and sedation.
Developing scenario
Three hours following his initial seizure, Simon has a generalised tonic–clonic seizure lasting two minutes. This is followed ten minutes later by a second tonic–clonic seizure of the same duration. During the seizure Simon appears pale, clammy and sweaty and he is incontinent of urine.
Nursing assessment and interventions
Airway/breathing
During a seizure it is not advisable to try and force anything into the mouth, such as airway adjuncts, as there is a high risk of damaging teeth or inducing gagging or choking. Simon should be given high-flow oxygen therapy during the seizure to relieve cerebral hypoxia as brain oxygen consumption may increase by 60% and cerebral blood flow by 250% during a seizure event (Hickey 2008).
On cessation of the seizure and during the post-ictal phase, Simon should be positioned on his side to ensure airway patency and facilitate drainage of secretions. Oral suctioning may be required if Simon has bitten his tongue or if he has hypersalivation and excess secretions (Gambrell and Flynn 2004). Monitoring of respiratory rate, depth and rhythm should commence. Most patients will return to an adequate spontaneous respiratory pattern following a seizure, provided airway patency is maintained, but oxygen therapy should be continued and titrated to achieve oxygen saturation (SpO2) >94%. Should any patient not return to spontaneous breathing or become pulseless, cardiopulmonary resuscitation should commence without delay.
Circulation
A general assessment of Simon’s circulatory system should be undertaken, although serious circulatory events are unlikely in seizures that do not progress to SE. Blood pressure and heart rate should be recorded and Simon’s heart rhythm should be monitored. Seizures initiate sympathetic responses leading to vasoconstriction and an elevation in blood pressure, during the post-ictal phase; however, this ceases and Simon’s blood pressure may dip, requiring re-assessment of his haemodynamic status and administration of appropriate fluid therapy. Simon is known to have encephalitis and monitoring of his temperature should continue two to four hourly for evidence of fever recurrence, as this would necessitate a review of his antibiotic therapy.
Disability of the central nervous system
During a convulsive seizure, the nurse needs to protect Simon from injury and must remain with him throughout the event (Hickey 2008). Eye glasses should be removed and restrictive clothing loosened, and if safe to do so, he should be placed on his side. It may also be possible to guide the movements of limbs to prevent injury but the nurse should not attempt to restrain Simon’s movements.
As Simon has had repeated seizure activity, a benzodiazepine drug would normally be administered to instigate prompt termination of electrical seizure activity and prevent progression into SE (Varelas and Spinaki 2006; Barker 2008). Lorazepam 2–4 mg administered by intravenous push over 2 minutes or diazepam 10–20 mg similarly administered are used (Pena 2003). Both drugs have been shown to be effective in producing seizure cessation, although lorazepam may be more effective in preventing further seizures with fewer doses needed (Cock and Schapira 2002) and is the agent currently recommended for first-line use in the UK (NICE 2004). Benzodiazepine administration should bring about a rapid termination of seizure activity through depression of neuronal activity from gamma aminobyturic acid (GABA)-induced neurotransmission. Repeated doses of lorazepam may be given up to a maximum of 8 mg, should Simon have another seizure. It is important, however to be aware that lorazepam can cause apnoea and profound hypotension in excess doses.
Simon has now experienced multiple seizure activity and current guidelines recommend that he should be administered further AEDs in addition to further investigation of the underlying cause (NICE 2004) (see Table 14.3). In the critical care setting, phenytoin is the most commonly used AED for rapid therapeutic control of repeated seizures. It is given intravenously at a dose of 15–20 mg/kg. It should not be administered more rapidly than 50 mg/min as it can induce cardiac disturbances and is very irritant to veins (Pena 2003). Phenytoin can only be administered in normal saline and is highly protein bound, so blood levels may be difficult to ascertain in low albumin states (Valeras and Spanaki 2006). Phenytoin is absorbed well from the GI tract and, following intravenous loading, is given orally at 100 mg three times per day.
Table 14.3 Drug treatment by seizure type.
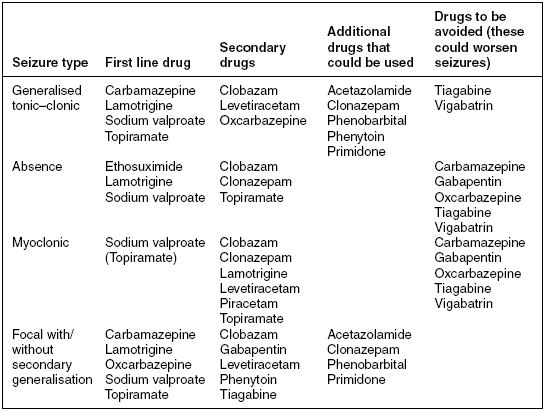
Fosphenytoin can be given as an alternative to phenytoin. It is a newer water- soluble phenytoin prodrug that is activated by the body’s metabolic processes (Pena 2003). This is prescribed at a dose of 15–20 mg/kg/PE (phenytoin equivalents) loading dose and intravenously 300–400 mg/day. Fosphenytoin cannot be given orally but can be administered in 5% dextrose solution and infused at a more rapid rate than phenytoin as it causes fewer cardiac side effects. The goal of either drug is to achieve a therapeutic serum level of 10–20 \umug/mL or free levels (not albumin bound) of 1–2 \umug/mL but avoiding toxic levels. Patients who are intolerant to phenytoin should be given alternative AEDs such as intravenous or oral sodium valproate 600–3000 mg/day or oral carbamazepine 600–1200 mg/day.
Patients are often confused and agitated following a seizure and may become combative and aggressive. As Simon emerges from the seizure, it is important to remain with him and attempt to re-orient him to his surroundings, informing him of your nursing activities to reduce his anxiety (Marthaler 2004). It is important that the medical team is informed of Simon’s recent seizures and detailed and accurate information documented concerning the specific nature and duration of the seizure activity (see Table 14.4). Simon should be investigated for possible contributing causes for this new onset of seizure activity. A blood glucose level should be obtained to rule out hypoglycaemia, as this can be a precipitating cause of seizure activity.
Table 14.4 Seizure documentation.
Source: Adapted from Hickey (2008).
| Information for documentation following a seizure |
| Document if patient remained conscious or had reduced or complete loss of consciousness. |
| Document the time seizure commenced and its duration. Seizures >5 minutes or repeated seizures require urgent review |
| Note time of cessation of ictal stage and beginning of post-ictal stage. |
| Document if the patient displayed any warning signs or auras (sense of doom, elation, déjà vu, smells, tastes or sounds). |
| Record abnormal movements and note which parts of the body were involved. Was the seizure simple partial (focal), complex partial or generalized? |
| Was there evidence of clonic or tonic–clonic activity? |
| Record any incontinence of urine or faeces. |
| Note any cognitive changes following the seizure. |
| Perform neurological observations note any pupil abnormalities, deviant gaze or motor weakness in any limbs and its duration. |
| Assess and document any injuries sustained during the seizure. Monitor the duration of sleep after the seizure. |
The post-ictal phase is the period following cessation of the seizure and lasts until the patient has recovered consciousness and orientation (or returned to his pre-seizure neurological status). During this time, Simon should be closely observed and, if necessary, GCS and neurological observations performed half- hourly (NICE 2004). It is important to distinguish alterations in Simon’s neurological function arising from the seizure itself and those that might be due to new or extended neurological damage or function. Simon should return to his previous neurological function at the end of the post-ictal stage and, during this time, should be able to localise to painful stimuli. Medical review of the patient should be undertaken and further investigations considered if there is no return to his former state or any deterioration in neurological performance from the nursing assessment is detected.
As Simon has had repeated seizure activity, the medical team may request further investigations to determine if his encephalitis is worsening or to establish if he has developed an epilepsy syndrome. Diagnostic tests currently recommended by the NICE (2004) would be a computed tomography (CT) scan or magnetic resonance imaging (MRI) and EEG (or video EEG).
An EEG would be performed if a diagnosis of epilepsy was suggested by the developing clinical picture (NICE 2004). A CT or MRI scan of Simon would be indicated if there was suspicion that his encephalitis had worsened or if his seizure activity might be related to a new, undiagnosed condition or if Simon fails to return to his pre-ictal neurological status.
Accurate diagnosis of seizure type and epilepsy syndrome is key to the development of an appropriate treatment strategy as the selection of AEDs would largely be determined by these results (Hickey 2008; NICE 2004). Simon’s longer term drug therapy would be reviewed by an epilepsy specialist prior to discharge as patient adherence to AED treatment is important in maintaining the patient seizure free. AED regimens should also be tailored according to seizure type, epilepsy syndrome, co-medication and the individual’s lifestyle and preferences (NICE 2004).
Exposure/environment
Maintaining a safe environment for Simon can be difficult as seizures are unpredictable and can result in falls, traumatic injuries or disconnections from lines or tubes (Pena 2003). Simon will require close observation at all times whilst at risk of seizures and it may be prudent to lower his bed as near to the ground as possible in case of falls (Hickey 2008). In addition, padded cot sides may be required to protect limbs during a seizure. The nurse should also ensure that lines are anchored securely to prevent disconnections and ensure that during a seizure Simon does not become entangled in wires or tubing.
Simon may be incontinent of urine and/or faeces during convulsive seizures, and if he is not catheterised, this has implications for assessment of fluid balance, protection of his dignity and maintenance of hygiene and skin integrity. Following a seizure, Simon should be assessed for evidence of incontinence and appropriate hygiene measures undertaken as soon as feasible. In addition, he should be assessed for any signs of physical injury and this should be reported and carefully documented (Pena 2003; Hickey 2008).
Simon will also require appropriate nutritional assessment and maintenance of an adequate fluid and dietary intake. He should also have standard thromboembolic prophylaxis.
Conclusion
Seizures are fairly frequent events in the critically ill and can occur in a variety of critically ill patients. They arise from a number of clinical causes and are not necessarily the result of an existing epilepsy syndrome. Indeed, many seizures witnessed in the critically ill are sub-clinical and cease following resolution of the initial condition. Critical care nurses should be aware of the risks of seizure activity in their patients and be able to accurately assess seizure type using recognized classification criteria. In addition, nurses should be able to provide prompt and appropriate nursing management to maintain patient safety, terminate seizure activity using pharmacological measures and be conversant with standard AED treatments.
Key learning points from the scenario
- Pathophysiology of seizures
- Assessment, diagnosis and classification of seizures
- Evidence based management of seizure activity
- Understanding of the rationale for anti-convulsant drug therapy
Critical appraisal of a research paper
There is a paucity of quality, evidence-based and nursing-focused literature on the management of patients with seizures in the critical care environment, and further publication in this area is sorely needed.
Drislane FW, Lopez MR, Blum AS, Schjomer DLl (2008) Detection and treatment of refractory status epilepticus in the intensive care unit. Journal of Clinical Neurophysiology 25(4), 181–186.
The purpose of this US-based article was to research the underlying causes, prevalence and difficulties in identifying and treating SE in patients in a medical ICU. Undiagnosed SE is a significant problem in the ICU population as sub-clinical seizures may be undetectable in patients who are sedated or sedated and on NMB and where an altered mental state might arise from any number of causes. Sub-clinical seizures may be non-convulsive and difficult to observe in this patient population. Undiagnosed seizure activity may lead to SE with serious or life-threatening complications. Earlier diagnosis and prompt instigation of AED therapy may assist in the prevention of refractory SE and decrease mortality.
Reader activities
1. Read the research article written by Drislane et al. (2008).
2. Using the critical appraisal framework in Appendix I, consider the methodological quality of the paper.
3. Reflect on this aspect of your own practice and the implications for future practice management that this paper arises.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


