Patient scenario
Femi is a 21-year-old female admitted to the emergency department in a collapsed state following recreational ingestion of 2 ‘ecstasy’ tablets over a 5-hour period. History from the ambulance crew revealed that she was brought from a nightclub situated underground with high ambient temperatures and extremely poor ventilation. Friends at the scene reported that though witnessed to drink plenty of water, Femi had been dancing continuously prior to complaining of feeling unwell, vomiting and appearing disorientated. On arrival, she was self-ventilating, but drowsy and incoherent. Initial management included oxygen, cannulation, blood screen, urinalysis and fluid therapy. No antidote was given and initial treatment was based on symptom reduction.
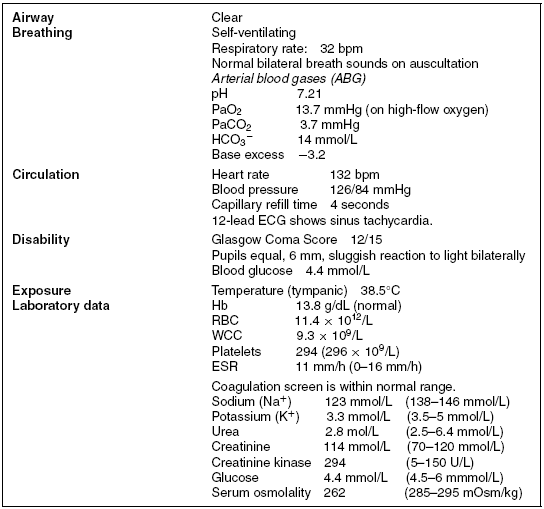
Femi is fast-tracked to the high dependency unit (HDU) and, on arrival, is receiving 10 L of oxygen via a non-rebreathe mask. Peripheral access has been obtained and a 0.9% saline infusion is running. A urinary catheter has been inserted. Results from the initial assessment can be seen in Table 15.1.
Table 15.1 Initial assessment results.
Reader activities
Having read this scenario, consider the following:
- What are the physiological and toxicological effects of ‘ecstasy’/MDMA?
- Why is Femi hyperthermic?
- What are the normal ranges of serum sodium and why might these be altered in the MDMA toxic patient?
- What are the priorities in the management of Femi?
Related pathophysiology
Ingesting MDMA would have resulted in the release of high levels of serotonin in Femi, stimulating serotonin, adrenergic and dopaminergic receptors. Onset of effects occurs within 30-60 minutes and users commonly report feeling euphoria, loss of inhibition, a distorted sense of time, mild visual distortions, reduced anxiety, greater insight, communicativeness and empathy, and increased sensuality. Femi might also have performed ‘stacking’, the intentional use of multiple staggered doses in order to enhance and prolong the effects of MDMA. ‘Stacking’ is a frequent behaviour amongst regular users (Ben-Abraham et al. 2003; Emde 2003; Galhinger 2004).
Femi may have also experienced other less-desired effects associated with the use of the drug, including diaphoresis (sweating), bruxism (teeth grinding), jaw clenching, dry mouth and blurred vision. MDMA also causes an enlargement in pupil size with the maximal change in pupil diameter usually occurring one to two hours post-ingestion. Larger doses can also produce significant oesophoria (tendency for the eyes to turn inwards) (de la Torre 2000; Hahn and Yew 2009).
Research into the cognitive performance of those under the effects of MDMA has further demonstrated memory deficits and a reduced ability to both concentrate and to process cognitive information (Parrott and Lasky 1998). Individuals such as Femi, who have taken the drug, may experience agitation, anxiety and toxic psychosis with symptoms of paranoia, as well as auditory and visual hallucinations. Related problems such as hyponatraemia can also cause confusion and disorientation.
Readily absorbed from the intestinal tract, MDMA is metabolised mainly in the liver by the enzyme cytochrome 450 oxidase – CYP2D6. Femi could be genetically deficient for this enzyme and thus more likely to develop liver damage and suffer exaggerated effects. Liver damage can also occur due to ischaemia resulting from both MDMA-induced hypotension and secondary to the hyperthermia. Thromboplastin released from injured myocytes can also cause clotting abnormalities.
Mechanisms of hyperthermia
The normal regulation of body temperature depends on both heat production and heat exchange with the surrounding environment. The relative magnitude of temperature elevation cannot be used to distinguish between the causes of hyperthermia, so consideration of event history, environmental factors and the drug behaviour of the individual are crucial to diagnosis (Bodenham and Mallick 1996). Following MDMA, intoxication hyperthermia is thought to be triggered by a failure of the thermoregulatory system due to interference with the serotonin pathway. This is in contrast to the controlled mechanism of fever induced by inflammation (see Chapter 3 for more information on fever).
Femi’s hyperthermia due to the MDMA toxicity may be caused by a number of co-related factors (see Box 15.1). Excessive diaphoresis due to the exertion of prolonged dancing will have resulted in a loss of water and electrolytes. This may have been compounded by a decreased sensorium, which in turn may have lessened any sensation of thirst or tiredness, leading to dehydration, electrolyte imbalance and additional physical exhaustion (Libiseller et al. 2005). It is likely that the high ambient temperatures and humidity at the nightclub, together with the vasoconstricting effects of MDMA, may have impaired Femi’s normal heat loss by conduction and evaporation, thus limiting effective dissipation. Prolonged muscular contractions related to dancing will have added a further thermal burden leading to an increase in her core temperature.
Box 15.1 Causes of hyperthermia associated with MDMA.
Prolonged physical exertion
Warm environment
Amphetamine effects
Promotion of repetitive activity
Loss of sensation of thirst and tiredness
Mood-enhancing effects
Euphoria
Increased energy
Serotonin effects
Increased muscle tone
Heat production
Secondary effects of hyperthermia
Increased muscle tone
Further heat production
Source: Adapted from Hall and Henry (2006).
Severe hyperthermia can result in neurological dysfunction. Delirium, seizures or coma associated with MDMA toxicity are reported as the most prevalent life-threatening complications (Kalant 2001). Susceptibility does not, however, appear to be related to ingested MDMA dose. Further potential complications of hyperthermia following MDMA ingestion include acute hepatic failure, rhabdomyolysis, disseminated intravascular coagulation (DIC) and non-cardiogenic pulmonary oedema.
Electrolyte disturbance
Femi is likely to have a range of electrolyte abnormalities including hyponatraemia, hyperkalaemia and hypoglycaemia.
Acute hyponatraemia is a serious complication of MDMA toxicity, linked to excessive sweating as a result of physical exertion, increased water intake and the abnormal release of vasopressin leading to excess levels of anti-diuretic hormone (ADH) secretion (Hartung et al. 2002; Devlin and Henry 2008).
Normal regulation of sodium levels in extracellular fluid is achieved through the actions of aldosterone and ADH. Plasma sodium and water concentration levels are continuously monitored by osmoreceptors situated in the hypothalamus. Any increase in the plasma concentration of sodium results in a corresponding increase in plasma osmolality. This triggers release of ADH from the posterior pituitary gland, resulting in greater permeability of renal tubular cells to water, increased water re-uptake, and subsequent expansion of plasma volume, reducing serum osmolality and concentrating the urine (Marieb 2006).
It is likely that Femi lost large amounts of sodium through profuse sweating in the hot and humid conditions of the nightclub. It is also probable that she drank excessive amounts of water (to avoid dehydration and over-heating), resulting in over-hydration. Alongside this, MDMA intoxication causes excess ADH production leading to a reduced renal response to water loading and subsequent dilutional hyponatraemia (Devlin and Henry 2008).
The consequences of hyponatraemia and increased water re-absorption are a fall in extracellular osmotic pressure, resulting in the passage of water from the blood into cells. Untreated, this leads ultimately to cerebral oedema causing obtundation, seizures and brainstem compression (Kalant 2001). Hyponatraemia should therefore be considered in all patients like Femi who present with suspected MDMA ingestion and neurological deficit, nausea and vomiting, headache, muscle cramps and weakness.
Rhabdomyolysis is a potentially fatal syndrome caused by the breakdown of skeletal muscle fibres. Characterized by the presence of myoglobin in the urine and raised creatinine kinase (CK) levels in excess of 3–5 times normal values (33–145 IU/L in women), rhabdomyolysis is associated with acute kidney injury and is a serious complication following MDMA ingestion.
Hyperkalaemia following ecstasy consumption (>5.0 mEq/L) has the potential to cause fatal cardiac arrhythmias. If rhabdomyolysis develops, raised levels of potassium ions are released from the cell into the extracellular fluid as a direct result of muscle cell degradation causing hyperkalaemia. This in turn causes hypoglycaemia due to the exchange of ions across the cell membrane.
Tests and investigations
Temperature assessment
Close monitoring of Femi’s temperature is required during her critical care stay. A device which reflects core temperature should be used as peripheral temperature devices can be affected by a variety of other factors, which reduce reliability and validity. (see Chapter 3 for a critical review of the efficacy of different modes of temperature assessment).
Drug screening
As 50–70% of MDMA is excreted unchanged in the urine (Teter and Guthrie 2001), a spot urinalysis is the most feasible and appropriate method of screening for drugs of abuse. Tests available may simply identify amphetamines or specify MDMA. Femi’s urine tested positive for amphetamine derivatives in the emergency department; however, this is of limited clinical value as there is little correlation between ingested dose and the subsequent severity of clinical symptoms (Dar and McBrien 1996). Sweat and saliva are also useful for the detection and analysis of recreational drugs, but use will be dependent on tests available in the department.
Blood screen
In order to identify fluid and electrolyte disturbances, Femi will require assessment of serum sodium, potassium and glucose concentration, and serum and urine osmolality. In addition, Femi’s blood toxicity screen should include a full blood count, assessment of kidney and liver function, cardiac enzymes, CK, and clotting.
To monitor for rhabdomyolysis, serial monitoring of CK levels should be performed. The first 24 hours may reveal an initial increase in total CK until a peak level is determined (Cunningham 1997). Close monitoring of blood urea nitrogen and creatinine levels enables early detection of reduced kidney function. (see Chapter 9 for more information on acute kidney injury). Prothrombin time, activated partial thromboplastin time, and platelet count should be routinely tested in all patients with rhabdomyolysis.
If liver damage is evident, Femi may have jaundice, an enlarged liver, prolonged clotting times and raised plasma levels of liver enzymes (Kalant 2001; Brotto and Lee 2007).
Urinalysis
Femi’s urinalysis is unremarkable except for a specific gravity of less than 1.005 (1.008–1.035) and osmolality of 253 mOsm/kg H2 O (275–295). The pH is 5. The rate of excretion of MDMA is thought to be linked to the pH of the urine; the greater the acidity, the faster the rate of excretion. As MDMA is highly toxic to the foetus in pregnancy, Femi’s urine should also be tested for human chorionic gonadotropin (hCG).
Femi’s high-urine osmolality despite a decreased serum osmolality and hyponatraemia is suggestive of syndrome of inappropriate anti-diuretic hormone (SIADH) found in MDMA toxicity. Laboratory tests determining raised levels of excreted sodium levels can confirm the diagnosis.
Analysis of Femi’s urine must also include screening for haemoglobin and/or myoglobin in order to detect rhabdomyolysis.
12-lead ECG
Autonomic hyperactivity is commonly found in patients presenting with MDMA toxicity due to the amphetamine-induced surge of catecholamines causing tachycardias and dysrhythmias, which will be evident on a 12-lead ECG. The 12-lead ECG performed on Femi should also be examined for long QT syndrome and non-specific ST-T changes (Ben-Abraham et al. 2003) as individuals with pre-existing cardiac conditions are at an increased risk of fatal arrhythmias and cardiac collapse.
Acid–base balance
Metabolic acidosis in MDMA toxicity is thought to occur as a complication of hyperthermia, rhabdomyolysis and seizures. Prolonged physical activity leads to increased lactic acid production, and in the presence of muscle cell breakdown, the resulting extracellular increase of potassium further acidifies the blood. Metabolic acidosis is linked to increased incidence of ventricular arrhythmias, and could predispose Femi to kidney injury if due to rhabdomyolysis. Regular arterial blood gases should therefore be undertaken in order to monitor Femi’s acid–base status.
Developing scenario
One hour after admission, Femi suffers a tonic–clonic seizure lasting one minute during which intravenous (IV) diazepam (0.3 mg/kg) is administered. You reassess her and find the following: spontaneous ventilation, respiratory rate 10 breaths per minute, SpO2 98% (on 10 L O2 via a facemask), heart rate 114 beats per minute, blood pressure 98/75 mmHg, Glasgow Coma Score 11/15 and blood sugar 5.2 mmol/L.
You suspect either hyperthermia or hyponatraemia in the presence of MDMA as a possible cause for the seizure. Management of Femi’s hyperthermia is ongoing with a cooling blanket in situ and reassessment reveals Femi’s core temperature via a rectal probe is now 39.2°C. Hourly urine output is 20 mL and the urine appears concentrated. The decrease of urine output is significant and may be due to abnormally excessive production of ADH. Repeat blood tests reveal a serum sodium level of 121 mmol/L and serum osmolality of 259 mOsm/kg. Arterial blood gases show mild metabolic acidosis (see Box 15.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


