FIGURE 22-1. Stage II pressure ulcer on an infant’s occiput due to immobility.
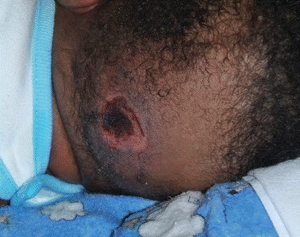
FIGURE 22-2. Unstageable pressure ulcer on an infant’s occiput due to immobility.
 Practice Point
Practice Point
The occiput is the most common site for immobility-related pressure ulcers in children from birth to age 3.
Risk Factors
Pressure ulcer development has traditionally been viewed as uncommon among neonatal and pediatric populations given the presumed relative ease of repositioning and frequency of movement.28,29 However, as survival rates among critically and chronically ill premature neonates and children increase through technological advances, so too does the risk for pressure ulcer formation.30 Infant skin has a higher absorption rate as compared with an adult’s skin. This difference in the absorption rates also predisposes infants to a dry, flaky, and impaired skin barrier.31 Premature neonates also have a higher ratio of skin surface area to weight with decreased or absent subcutaneous fat development based upon gestational age. Analogous to adults, critically ill and injured neonates and children may experience massive edema secondary to fluid resuscitation and hemodynamic, respiratory, renal, and neurologic instabilities that increase the risk for an unavoidable PU.32 Their anatomically immature skin is further challenged by prolonged intensive care to manage respiratory inefficiency, inadequate tissue perfusion, fluid and electrolytes issues, poor nutrition, and thermal regulatory issues that further increases risk for negative physiological responses to repositioning and pressure ulcer development. Newborns may present with birth injuries such as a fractured clavicle, humerus, femur, skull, or rib(s). They also may be born with musculoskeletal abnormalities such as torticollis (wry neck) and congenital dislocation of the hips that impedes repositioning. Consequently, prolonged periods of immobilization may be maintained, especially among those on extracorporeal membrane oxygenation (ECMO) and high-frequency oscillatory ventilation (HFOV).33–35
Furthermore, pediatrics is the branch of medicine caring not only for infants and children but for adolescents as well. Among pediatric hospitals, there exist variable age-limit policies; therefore, a wide variety of patients are cared for, from neonates to middle-aged patients. This poses additional challenges for clinicians to maintain clinical expertise in a diversity of support surfaces and products to manage neonates to bariatric patients seen in their facilities.
Medical Equipment or Devices
Increasing numbers of critical access and monitoring lines/catheters and other forms of medical equipment often limit safe turning and repositioning options and increase the risk for unavoidable PU.36,37 In critical care environments, positioning of the head is often limited by internal and external jugular catheters to avoid inadvertent catheter dislodgement, head and neck edema, and air leakage around endotracheal tubes with movement. Medical devices and monitoring equipment secured to the skin or mucosal membranes and compressing tissue are now widely recognized as a risk factor for pressure ulcer development. This is particularly a concern in pediatrics as young active patients or those with developmental impairment do not cognitively understand not to touch or play with equipment; therefore, clinicians tend to very securely adhere devices to the skin to avoid inadvertent dislodgement. In fact, Willock and colleagues26 found that 50% of neonatal and pediatric pressure ulcers were directly associated with equipment pressing on the skin. Boesch et al.38 found, while medical devices are a known risk factor in adult populations, up to 75% of hospital acquired pressure ulcers (HAPUs) have been associated with medical device use among infants and children (Table 22-1). Critically ill patients typically require a multitude of invasive and noninvasive equipment anchored securely to their skin placing them at particularly high risk. Specific devices that may result in pressure ulcers are respiratory devices (endotracheal/oral tracheal tubes, tracheostomy tube, noninvasive ventilation mask, and headgear), casts and orthotics, cervical spine immobilizers, pulse oximetry probes, and EEG leads to name a few (Figs. 22-3 to 22-8). CPAP devices place vulnerable neonates and children at risk for pressure ulcers. Robertson et al.39 first reported a rate of 20% for nasal deformities secondary to nCPAP (nasal CPAP) in very low birth weight infants (seven cases). The high risk for ala (lateral surface of external nose that forms outer wall of each nostril), caudal septal, columnar, and nasal bridge pressure ulceration, which can occur secondary to nasal prongs and/or CPAP mask and headgear, is of concern in all NICUs.40–42 Fischer et al. reported a 42.5% incidence of nasal trauma rate on 989 patients who were treated with nCPAP in NICU with 90% occurring in first 6 days of nCPAP.43
Table 22-1 Common Medical Devices That May Cause Pressure Ulcers in Neonates and Children
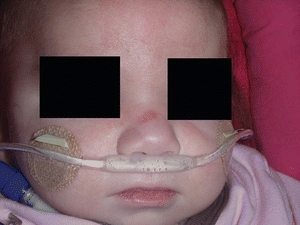
FIGURE 22-3. Stage 1 pressure ulcer on an infant’s nose caused by a medical device from BiPAP.
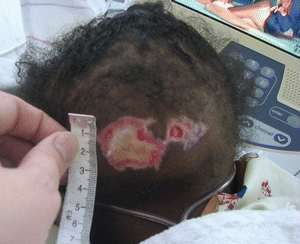
FIGURE 22-4. Unstageable pressure ulcer on an infant’s occiput caused by a medical device from oxygen tubing.
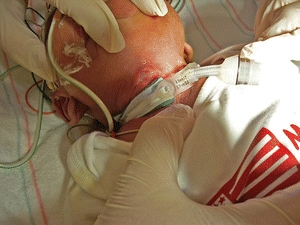
FIGURE 22-5. Unstageable pressure ulcer on an infant’s neck caused by a medical device from a trach flange.
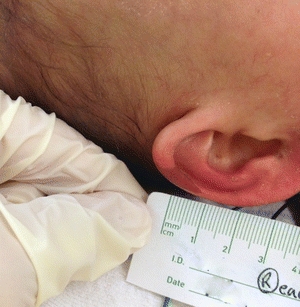
FIGURE 22-6. Suspected deep tissue injury right ear caused by a medical device from oxygen tubing.
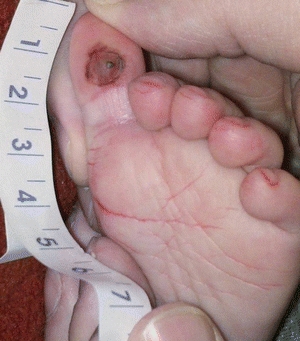
FIGURE 22-7. Suspected deep tissue injury right great toe of an infant caused by a medical device from an oxygen sat probe.

FIGURE 22-8. Unstageable pressure ulcer on a child’s left nasal nares caused by a medical device from a nasogastric device.
Preventive measures, such as hourly prong or mask repositioning with skin assessments and the use of protective hydrocolloid or silicone dressings, often prevent the occurrence of these devastating ulcers.40,42 Murray and colleagues44 recommend changing oxygen saturation probe locations every 2 to 3 hours, removing orthotics two to three times daily and as needed, and changing dressings under tracheostomy and enteral tube sites one to two times daily to ensure proper fit and a moisture-free environment, as well as to relieve the pressure. If these actions are medically or clinically contraindicated, this should be documented.
In a case-controlled study of 118 PICU patients by McCord and colleagues,45 edema, PICU length of stay longer than 96 hours, increased positive end-expiratory pressure (PEEP), weight loss, and not turning the patient or using a specialty bed in turn mode were all identified as risk factors for pressure ulcer development.
Paralysis
Paralysis and insensate skin related to spinal cord injury or myelomeningocele (“spina bifida”) have been shown to be risk factors for pressure ulcer development in pediatric patient populations.46,47 Ekmark46 reported that shear injuries can occur during wheelchair transfers or transferring out of a car on warm days. Ekmark46 suggests that new orthotic braces should be checked regularly, particularly 2 and 12 months, and also, whenever children gain or lose weight, wheelchair equipment must be modified. Schottler and colleagues47 completed a 27-year retrospective chart review of children who had spinal cord injuries at 5 years of age and younger and reported 41% had a history of a pressure ulcer with over 70% located in the foot and ankle. In a retrospective, exploratory study by Samaniego of 69 pediatric outpatients with a primary diagnosis of myelodysplasia, paralysis, insensate areas, high activity, and immobility were all identified as pressure ulcer risk factors.48
Risk Assessment Tools
When considering the broad spectrum of skin problems in infants and children, one may regard the phenomenon of pressure ulcers as irrelevant,49 although over the past decade increasing attention is emerging regarding pressure ulcer risk assessment and prevention in the acutely ill pediatric patient population. To utilize objective criteria to determine patient risk, quantify the severity of risk, and guide clinicians to institute interventions that prevent skin compromise, the use of a pressure ulcer risk assessment scale is indicated. Researchers have emphasized the importance of clinicians recognizing that risk assessment, prevention, and management of immobility-related and medical device–related pressure ulcers are different.50 It is critically important to differentiate the two phenomena.44 There are multiple published neonatal/pediatric pressure ulcer risk assessment scales.20,51–59 Schumacher and colleagues60 determined that available tools were too lengthy for clinical use and developed a short trigger tool to identify infants at risk for pressure ulcer development and performed it on 15 NICU patients and determined that although it did not quantify risk, it was an efficient initial screening tool. However, the only pediatric pressure ulcer risk assessment scales for which there are published sensitivity and specificity data are the Braden Q Scale,51 Neonatal Skin Risk Assessment Scale (NSRAS),20 and Glamorgan Scale.57 The three skin assessment tools that have been largely tested and validated are the Braden Q Scale, Glamorgan Q Scale, and the Neonatal Skin Risk Assessment Scale (NSRAS).61 Both the Braden Q20,51 and NSRAS were modeled after the adult Braden Scale for predicting pressure ulcer risk.
The NSRAS was created in 1997 for neonates and modeled after the Braden Scale for predicting pressure ulcer risk in adults, although the original Braden Scale has never been psychometrically tested with any age group in the pediatric population.62 NSRAS measures six subscales pertinent to neonates.20 The subscales are based on validity testing among a small sample size of 32 NICU patients and demonstrated a sensitivity of 83% and a specificity of 81%.20 The field of neonatology has changed over the last decade, necessitating a revision of the instrument to reflect evidence-based practice63; therefore, recently, the original NSRAS was updated to incorporate the specialized humidification incubator standardly used today to manage insensible water losses of micropreemies with a pilot study to be conducted of the revised tool.
The Braden Q Scale, developed in the early 1990s for predicting pediatric pressure ulcer risk, is a widely used, valid, and reliable pediatric-specific pressure ulcer risk assessment tool that is currently available in nine languages.58 In developing the Braden Q Scale, Quigley and Curley adapted the six subscale descriptions for the pediatric population and added a tissue perfusion and oxygenation subscale.36,51 In a multisite prospective study of 322 PICU patients, which excluded children with intracardiac shunting or unrepaired congenital heart disease, the Braden Q Scale was found to be 88% sensitive and 58% specific at a cutoff score of 16.36 When the Braden Q Scale was first published in 1996, Quigley and Curley used a cutoff of 8 years of age because at the time, the American Heart Association used that cutoff age for pediatric versus adult resuscitation standards. The Braden Q contains all the dimensions of the original Braden Scale and it is widely used in neonates through adulthood. The Braden Q incorporates the developmental needs of pediatric patients, the prevalence of gastric/transpyloric tube feedings, use of available blood studies, and noninvasive technology. The Braden Q scale51 has a “tissue perfusion and oxygenation” subscale to quantify risk associated with compromised tissue perfusion. The original study excluded those with complex congenital heart disease so as not to confound the tissue perfusion and oxygenation scale with acutely ill patients who have compromised baseline oxygen saturation levels. Tume et al.64 conducted a retrospective cohort study on 891 critically ill children to examine how the lowest Braden Q score recorded in first 24 hours of PICU admission related to risk of pressure ulcer development. Tume et al.64 found that the Braden Q score was found to perform well in children aged 3 weeks to 8 years without congenital heart disease. At a cutoff score of ≤16, it yielded a sensitivity of 100% and specificity of 73.1%.64 Quigley and Curley have never given permission for the Braden Q Scale to be adapted or modified in any way and discourage use of unvalidated versions such as the Neonatal/Infant Braden Q and Modified Braden Q. Of note, the Braden Q was also never designed to predict medical device–related injuries. The Braden Q, as designed, should not be used as a comparator in predicting device-related pressure ulcers.44,50 Quigley, Curley, and Noonan-Caillouette are conducting a multicenter study to re-establish the reliability and validity of the Braden Q for the development of immobility-related pressure ulcers and a new subscale dimension assessing the risk associated with medical devices. The new risk scale is called the Braden Q+D Scale, whereas the letter “D” is for device. Pediatric patients ranging from neonates to 21 years of age, including those born with complex congenital heart disease, are included in the study. They anticipate the study to conclude December 2014 with dissemination of study findings in 2015.
The Glamorgan Scale was developed by identifying risk factors through literature reviews and using the opinions of clinical experts and is based on factors thought to result in the development of pressure ulcers in pediatric patients. Scoring is in two categories mobility and equipment with a weighted risk value score of 15 if there is any equipment pressing on skin. Any patient who scores a 15 or higher is determined to be at “high risk.” When used in the assessment of pressure ulcer risk in 336 patients (1 day to 18 years of age), the Glamorgan Scale was found to be 98.4% sensitive and 67.4% specific at a cutoff score of 15.56,57,65 The higher the Glamorgan total score, the greater the pressure ulcer risk: ≥10 = at risk, ≥15 = high risk, and ≥20 = very high risk.56,57,65 In an observational validation study in a pediatric cardiac unit by Kottner and colleagues,66 they found the Glamorgan scale was unable to make clear distinctions in a low-risk setting; therefore, it is unlikely the tools in this setting provide additional information for clinical decision making.
A further challenge for clinicians is capturing the pressure ulcer risk to patients during procedures such as the operating room, radiology suite, or cardiac catheterization lab. It is imperative for nurses, surgeons, and anesthesiology colleagues to collaborate and assess risk for pressure ulcer development, specific to the positioning needs related to surgical procedures as well. Positioning will vary based upon specific surgical procedures, such as prone position for posterior spinal fusion, and require skin assessment on the frontal surface, which was gravity dependent during surgery. The incidence and prevalence of pressure ulcers for inpatients have been reported, but the effect of surgical procedures on pressure ulcer prevalence and development during admission has not been recognized. Current pressure ulcer risk tools do not address the patient who comes in for surgery, is admitted to the hospital after surgery, and then develops a pressure ulcer.36,59 Galvin and Curley conducted a comprehensive review of pressure-related skin injuries reported in 24 operative suites and then did a literature review to identify risk assessment scales and intraoperative pressure ulcer prevention strategies for pediatric patients. Consistent with the Braden Q Scale, Galvin and Curley used Braden and Bergstrom’s conceptual framework on the etiology of pressure ulcers to organize a pressure ulcer prevention bundle and developed the Braden Q + P Pressure Ulcer Risk Assessment Tool with a yes/no scoring system for each subscale.59
 Practice Point
Practice Point
Use a pressure ulcer risk assessment scale designed for pediatric patients.
Pressure Redistribution Products
Historically, infants and children were placed on support surfaces (mattresses and overlays) originally designed for adults, the clinical efficacy of many products with patients of lower weights and smaller stature is unclear. Also, variability of number of layers of linen and continence pads placed by caregivers under patients poses an additional challenge. Many support surface manufactures will disclose upper body weight capacity but not minimal weight requirements. Milbrath and colleagues67 reported that comparative data demonstrating product effectiveness with persons weighing less than 70 pounds, pressure distribution qualities of construction materials, support for positioning, impact on bed motility, and floatation under load at pressure points were missing. They further reported that patients and their families value comfort and quiet operation and expressed dissatisfaction with powered specialty mattresses due to the operating cyclic on-and-off noise.67 Premature neonates do not have the muscle tone or strength that full-term infants have and, when placed on their backs, may lie in an extended position causing atypical neuromotor development. To promote flexion patterns of movement, caregivers need to routinely reposition patients using external support aides such as gel or fluidized positioners that facilitate the curled, flexed intrauterine position. Integral to pediatric education is the recognition of each child’s uniqueness, the developmental characteristics of each age group, and the psychological and psychosocial factors they face.26,36
Today, clinicians have a wide variety of Isolette, crib, incubator, pediatric foam, and foam-/gel-topped mattresses available to choose from as compared to a decade ago. Because children are not “small adults,” they should not be placed on adult support surfaces but instead on pressure redistribution products specifically designed for their specialized loading needs. Low–air loss beds designed for adults do not have the numerical option to accommodate for the height and weight of an infant or small child.14 Children and infants often sink in and between cushions.14 When adult specialty beds are placed in the turn mode, the occiput of small children pivots on the same pressure point, potentially increasing shear and friction and not redistributing pressure.45 If a low–air loss bed or alternating overlay is clinically indicated, only those that are age appropriate, clinically efficacious, and safe should be used in accordance with manufacturer’s recommendations.
A pilot study of 54 children at Texas Children’s Hospital by McLane et al. found the highest interface readings were on the occiput from infancy to age 6 and on the occiput, coccyx, and heels from ages 6 to 18.68 For children younger than age 2, use of the Delta foam overlay (Span America, Greenville, SC) resulted in the lowest interface occiput pressures.68 For children over age 2, use of the Delta foam overlay with a Gel-E Donut head support and positioning aid (Philips Healthcare, Andover, MA) significantly lowered occipital pressure and provided generalized pressure redistribution.68 Another nonpowered fluidized positioner (Z-Flo, Sundance, White Plains, NY) is designed to function like a fluid but not flow in response to gravity and contour to body surface and redistribute pressure and is widely used in NICUs and in a variety of pediatric environments. More recently, a complete mattress replacement system designed for the pediatric population (Pediatric Pulse, Sizewise) is available for patients weighing between 13 and 55 pounds. It offers a three-zoned support surface and has vented air cells that provide the option of either static low air loss or pulsating low–air loss therapy. A product that simulates the effects of a body floating in a fluid and reduces vertical shear is the Dolphin System (Joerns, Arlington, TX), which applies Fluid Immersion Simulation technology originally developed to transport US Navy dolphins over long distances in a dry environment. Early clinical use was in the perioperative environment as a surgical overlay system for the operating room table, the Dolphin Pad to prevent pressure ulcers from lengthy surgical procedures. Other product lines are now available to replace a standard hospital surface, Dolphin Bed for use with a patient with a minimal weight of 21 pounds that is also available in bariatric sizes, as well as in stretcher and wheelchair pads.
 Practice Point
Practice Point
Clinicians must also read all manufacturer disclosures to ensure that product materials, particularly foam cellular pads and mattresses, meet their state- or city-approved flame-retardant fire regulation compliance standards.
Medical Devices
Considering that pressure ulcers occur under a plethora of medical devices, it is imperative that frequent, comprehensive skin inspection occur under all removable devices unless medically contraindicated. To complicate the situation further, materials that might be used to secure medical devices (e.g., tape, straps, dressings) may prevent assessment of adjacent skin and increase the risk for pressure ulcer development.44 It is particularly important to assess skin under devices when a patient is developmentally unable to communicate discomfort and has neurosensory impairment such as those with chemical paralysis or spinal cord injury, as well as those with fluid resuscitation that results in localized or systemic edema, which may affect proper fit of medical device. Given that upwards of 50% of pressure ulcers are related to pressure from equipment and devices,26 it’s important to perform frequent skin assessments, rotate blood pressure cuffs and transcutaneous oxygen tension (tcPO2) probes, and provide sufficient padding under tracheostomy plates, nasal prongs, CPAP and BiPAP masks, arm boards, orthotics/casts, and traction boots. All clinicians are responsible for monitoring skin and mucous membranes for alterations in skin integrity. Providers of care including nurses, clinical assistants, physicians, and therapists play an essential role in assessing, preventing, and managing pressure ulcers.69,70 Collaboration between nursing and respiratory therapy (RT) to move endotracheal tubes and noninvasive ventilation (NIV) bilevel or continuous positive airway pressure (BPAP/CPAP) devices to assess for pressure ulcers of the nose, lips, cheeks, or mucosal injuries of the tongue and buccal mucosa would help prevent or identify concerns early.44 Manufacturers of NIV equipment today offer variety of masks, headgear, pillows, and chin straps giving clinicians alternative interfaces to avoid skin breakdown in infants and children. Implementing prevention strategies to ensure proper fitting of orthotics, transabdominal feeding tube flanges, wheelchairs, and wheelchair cushions as children grow must be integrated into all clinician practices. Applying interface layers such as foam and gel dressings/products between devices and skin to protect the skin and redistribute pressure should routinely be incorporated into bedside practice.
Beds and cribs should be inspected to ensure that no tubing, cables, leads, hard toys, or syringe caps are inadvertently left under the patient’s skin.26 Tapes securing nasogastric and orogastric tubes, head dressings, and hats should be gently removed and the underlying skin assessed for pressure injuries. Routinely scheduled removal of EEG monitoring pads to assess skin on scalp and forehead should be coordinated with EEG technologist and bedside nursing.
 Practice Point
Practice Point
Best Practices for Prevention of Medical Device–Related Pressure Ulcers in Pediatric Populations Poster is available for free download at http://www.npuap.org/wp-content/uploads/2013/04/BestPractices-Pediattric1.pdf.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


