Penicillins and Cephalosporins
Objectives
• Explain the mechanisms of action of antibacterial drugs.
• Summarize the three general adverse effects associated with antibacterial drugs.
• Differentiate between narrow-spectrum and broad-spectrum antibiotics.
• Contrast the effects of first-, second-, third-, and fourth-generation cephalosporins.
• Apply the nursing process for patients receiving penicillins and cephalosporins.
Key Terms
acquired resistance, p. 402
antibacterials, p. 401
antimicrobials, p. 401
bactericidal, p. 401
bacteriostatic, p. 401
broad-spectrum antibiotics, p. 403
cross-resistance, p. 403
immunoglobulins, p. 402
inherent resistance, p. 402
microorganisms, p. 401
narrow-spectrum antibiotics, p. 403
nephrotoxicity, p. 409
nosocomial infections, p. 402
superinfection, p. 407
This chapter discusses the antibacterials and their effects, which include mechanisms of antibacterial action, body defenses, resistance to antibacterials, use of antibacterial combinations, general adverse reactions to antibacterials, and narrow- and broad-spectrum antibiotics. This chapter also discusses two antibacterials in detail: penicillin and cephalosporin.
Pathophysiology
Bacteria, known as prokaryotes, are single-celled organisms lacking a true nucleus and nuclear membrane. Most bacteria have a rigid cell wall, and the structure of the cell wall determines the shape of the bacteria. One classification of bacteria involves the appearance or shape under a microscope. A bacillus is a rod-shaped organism. Cocci are spherical. When cocci appear in clusters, they are called staphylococci; when cocci are arranged in chains, they are called streptococci. Bacteria reproduce by cell division about every 20 minutes.
Another classification of bacteria involves staining properties of the cell. The Gram staining method was devised in 1882 by Hans Christian Gram, a Danish bacteriologist. Gram staining is determined by the ability of the bacterial cell wall to retain a purple stain by a basic dye. Crystal violet is normally used in the staining process but may be substituted with methylene blue. If bacteria retain a purple stain, they are classified as gram-positive microorganisms. Those bacteria not stained are known as gram-negative microorganisms. Examples of gram-positive bacteria include Staphylococcus aureus, Streptococcus pneumoniae, Group B streptococcus, and Clostridium perfringens. Examples of gram-negative bacteria include Neisseria meningitides, Escherichia coli, and Haemophilus influenzae.
Bacteria produce toxins that cause cell lysis (cell death). Many bacteria produce the enzyme beta-lactamase, which destroys beta-lactam antibiotics such as penicillins and cephalosporins.
Antibacterial Drugs
Antibacterials/Antibiotics
Although the terms antibacterial, antimicrobial, and antibiotic are frequently used interchangeably, there are some subtle differences in meaning. Antibacterials and antimicrobials are substances that inhibit bacterial growth or kill bacteria and other microorganisms (microscopic organisms including viruses, fungi, protozoa, and rickettsiae). Technically, the term antibiotic refers to chemicals produced by one kind of microorganism that inhibits the growth of or kills another. For practical purposes, however, these terms may be used interchangeably. Several drugs, including antiinfective and chemotherapeutic agents, have actions similar to those of antibacterial and antimicrobial agents. Antibacterial drugs do not act alone in destroying bacteria. Natural body defenses, surgical procedures to excise infected tissues, and dressing changes may be needed along with antibacterial drugs to eliminate the infecting bacteria.
Antibacterial drugs are either obtained from natural sources or manufactured. The use of moldy bread on wounds to fight infection dates back 3500 years. In 1928, a British bacteriologist named Alexander Fleming noted that “mold” was contaminating his bacterial cultures and inhibiting bacterial growth. The mold was called Penicillium notatum; thus Fleming called the substance penicillin. In 1939 Howard Florey expanded on Fleming’s findings and purified the penicillin so it could be used commercially. Penicillin was used during World War II and marketed in 1945. Sulfonamide, a synthetic antibacterial, was introduced in 1935. Sulfonamides are discussed in greater detail in Chapter 31.
Bacteriostatic drugs inhibit the growth of bacteria, whereas bactericidal drugs kill bacteria. Some antibacterial drugs (tetracycline and sulfonamides) have a bacteriostatic effect, whereas other antibacterials (penicillins and cephalosporins) have a bactericidal effect. Depending on the drug dose and serum level, certain drugs can have both bacteriostatic and bactericidal effects.
For drugs with a narrow therapeutic index (e.g., aminoglycosides), peaks and troughs of serum antibiotic levels are monitored to determine if the drug is within the therapeutic range for its desired effect. If the serum peak level is too high, drug toxicity could occur. If the serum trough level (drawn minutes before administration of the next drug dose) is below the therapeutic range, the patient is not receiving an adequate antibiotic dose to kill the targeted microorganism.
Mechanisms of Antibacterial Action
Five mechanisms of antibacterial action are responsible for the inhibition of growth or destruction of microorganisms: (1) inhibition of bacterial cell-wall synthesis, (2) alteration of membrane permeability, (3) inhibition of protein synthesis, (4) inhibition of the synthesis of bacterial ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), and (5) interference with metabolism within the cell (Table 29-1).
TABLE 29-1
MECHANISMS OF ACTIONS OF ANTIBACTERIAL DRUGS
| ACTION | EFFECT | DRUGS |
| Inhibition of cell wall synthesis | Bactericidal effect Enzyme breakdown of cell wall Inhibition of enzyme in synthesis of cell wall. | penicillin cephalosporins bacitracin vancomycin |
| Alteration in membrane permeability | Bacteriostatic or bactericidal effect Increases membrane permeability. Loss of cellular substances causes lysis of the cell. | amphotericin B nystatin polymyxin colistin |
| Inhibition of protein synthesis | Bacteriostatic or bactericidal effect Interferes with protein synthesis without affecting normal cell. Inhibits steps of protein synthesis. | aminoglycosides tetracyclines erythromycin lincomycin |
| Inhibition of synthesis of bacterial RNA and DNA | Inhibits synthesis of RNA and DNA in bacteria. Binds to nucleic acid and enzymes needed for nucleic acid synthesis. | fluoroquinolones |
| Interference with cellular metabolism | Bacteriostatic effect Interferes with steps of metabolism within cells. | sulfonamides trimethoprim isoniazid (INH) nalidixic acid rifampin |
Pharmacokinetics
Antibacterial drugs must not only penetrate the bacterial cell wall in sufficient concentration, but also must have an affinity (attraction) to the binding sites on the bacterial cell. The time the drug remains at the binding sites increases the effect of the antibacterial action. This time factor is controlled by the pharmacokinetics (distribution, half-life, and elimination) of the drug.
Antibacterials that have a longer half-life usually maintain a greater concentration at the binding site; therefore, frequent dosing is not required. Most antibacterials are not highly protein-bound, with a few exceptions (e.g., oxacillin, ceftriaxone, cefoperazone, cefprozil, cloxacillin, nafcillin, clindamycin). Protein binding does not have a major influence on the effectiveness of most antibacterial drugs. The steady state of the antibacterial drug occurs after the fourth to fifth half-lives, and the drug is eliminated from the body, mainly through urine, after the seventh half-life.
Pharmacodynamics
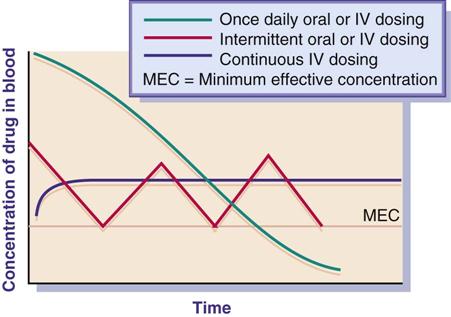
The drug concentration at the site or the exposure time for the drug plays an important role in bacterial eradication. Antibacterial drugs are used to achieve the minimum effective concentration (MEC) necessary to halt the growth of a microorganism. Many antibacterials have a bactericidal effect against the pathogen when the drug concentration remains constantly above the MEC during the dosing interval. Duration of time for use of the antibacterial varies according to the type of pathogen, site of infection, and immunocompetence of the host. With some severe infections, a continuous infusion regimen is more effective than intermittent dosing because of constant drug concentration and time exposure. Once-daily antibacterial dosing (e.g., aminoglycosides, macrolides, fluoroquinolones) has been effective in eradicating pathogens and has not caused severe adverse reactions (ototoxicity, nephrotoxicity) in most cases. The ease of compliance with once- or twice-daily drug dosing also increases the patient’s adherence to the drug regimen.
Figure 29-1 illustrates the effect of three methods of drug dosing. The drug dose is effective when it remains above the MEC.
Body Defenses
Body defenses and antibacterial drugs work together to stop the infectious process. The effect that antibacterial drugs have on an infection depends not only on the drug but also on the host’s defense mechanisms. Factors such as age, nutrition, immunoglobulins, white blood cells (WBCs), organ function, and circulation influence the body’s ability to fight infection. Older adults and undernourished individuals have less resistance to infection than younger, well-nourished populations. If the host’s natural body defense mechanisms are inadequate, drug therapy might not be as effective. As a result, drug therapy may need to be closely monitored or revised. When circulation is impeded, an antibacterial drug may not be distributed properly to the infected area. In addition, immunoglobulins (antibody proteins such as IgG and IgM) and other elements of the immune response system (WBCs) needed to combat infections may be depleted in individuals with poor nutritional status.
Resistance to Antibacterials
Bacteria may be sensitive or resistant to certain antibacterials. When bacteria are sensitive to a drug, the pathogen is inhibited or destroyed. If bacteria are resistant to an antibacterial, the pathogen continues to grow, despite administration of that antibacterial drug.
Bacterial resistance may result naturally (inherent resistance) or it may be acquired. A natural, or inherent, resistance occurs without previous exposure to the antibacterial drug. For example, the gram-negative (non–gram-staining) bacterium Pseudomonas aeruginosa is inherently resistant to penicillin G. An acquired resistance is caused by prior exposure to the antibacterial. Although Staphylococcus aureus was once sensitive to penicillin G, repeated exposures have caused this organism to evolve and become resistant to it. Penicillinase, an enzyme produced by the microorganism, is responsible for causing its penicillin resistance. Penicillinase metabolizes penicillin G, causing the drug to be ineffective. Penicillinase-resistant penicillins that are effective against S. aureus are available.
Antibiotic resistance is a major problem. In the early 1980s, pharmaceutical companies thought that enough antibiotics were on the market, so these companies concentrated on developing antiviral and antifungal drugs. As a result, fewer new antibiotics were developed during the 1980s. Now pharmaceutical companies have developed many new antibiotics, but antibiotic resistance continues to develop, especially when antibiotics are used frequently. As bacteria reproduce, some mutation occurs, and eventually the mutant bacteria survive the effects of the drug. One explanation is that the mutant bacteria strain may have grown a thicker cell wall.
In large health care institutions, there is a tendency toward drug resistance in bacteria. Mutant strains of organisms have developed, thus increasing their resistance to antibiotics that were once effective against them. Infections acquired while patients are hospitalized are called nosocomial infections. Many of these infections are caused by drug-resistant bacteria and can prolong hospitalization, which is costly to both the patient and third-party health care insurers.
Another problem related to antibiotic resistance is that bacteria can transfer their genetic instruction to another bacterial species. The other bacterial species becomes resistant to that antibiotic as well. Bacteria can pass along high resistance to a more virulent and aggressive bacterium (e.g., S. aureus, enterococci).
Methicillin (Staphcillin) was the first penicillinase-resistant penicillin developed in 1959 in response to a resistance of S. aureus. In 1968, strains of S. aureus were beginning to become resistant to methicillin. Highly resistant bacteria, known as methicillin-resistant Staphylococcus aureus (MRSA), became resistant not only to methicillin, but to all penicillins and cephalosporins as well. Resistance that was once found only in hospitals began to emerge in 1981 in the community as well. Methicillin is now off the market. The treatment of choice for MRSA is vancomycin (Vancocin). Other effective drugs used to treat MRSA include linezolid (Zyvox), daptomycin (Cubicin), trimethoprim/sulfamethoxazole (Bactrim), doxycycline (Vibramycin), and clindamycin (Cleocin). Telavancin (Vibativ), a glycopeptides antiinfective, was approved in September 2009 to treat gram-negative bacteria, including MRSA.
Many enterococcal strains are resistant to penicillin, ampicillin, gentamicin, streptomycin, and vancomycin. Another big resistance problem is vancomycin-resistant Enterococcus faecium (VREF), which can cause death in many persons with weakened immune systems. The incidence of VREF in hospitals has increased. A strain of MRSA has been reported to be resistant to vancomycin (vancomycin-resistant Staphylococcus aureus, or VRSA). Major medical problems result. One antibiotic after another is ineffective against new resistant strains of bacteria. As new drugs are developed, drug resistance will probably develop as well. Pharmaceutic companies and biotechnical firms are working on new classes of drugs to overcome the problem of bacterial resistance to antibiotics. A class of antibiotics, oxazolidinones, was discovered by a pharmaceutical company in 1988, but the company could not overcome toxicity problems in this class of drug. Another pharmaceutical company has taken the compound and made it less toxic. This antibiotic, linezolid, is effective against MRSA, VREF, and penicillin-resistant Streptococci. Quinupristin/dalfopristin (Synercid), which consists of two streptogramin antibacterials, is marketed in a combination of 30 : 70 for intravenous (IV) use against life-threatening infection caused by VREF and for treatment of bacteremia, S. aureus, and Streptococcus pyogenes.
Another way to attack antimicrobial resistance is to develop drugs that disable the antibiotic-resistant mechanism in the bacteria. Patients would take the antibiotic-resistance disabler along with the antibiotic already on the market, making the drug effective again. Developing a bacterial vaccine is another way to combat bacteria and lessen the need for antibiotics. The bacterial vaccine against pneumococcus has been effective in decreasing the occurrence of pneumonia and meningitis among various age groups.
Antibiotic misuse, a major problem today, increases antibiotic resistance. Studies reveal that 23% to 37.8% of patients in hospitals receive antibiotics and 50% of this population is receiving antibiotics inappropriately. When antibiotics are taken unnecessarily (e.g., for viral infections, when no infection is present) or incorrectly (e.g., skipping doses, not taking the full antibiotic regimen), one may develop resistance to antibacterials. Consumer education is important because many patients “demand” antibiotics for viral conditions. Antibiotics are ineffective against viruses. However, viral infections that persist could decrease the body’s immune system, thus promoting a bacterial infection. The nurse should teach patients about the proper use of antibiotics to prevent situations that promote drug resistance to bacteria.
Cross-resistance can also occur between antibacterial drugs that have similar actions, such as the penicillins and cephalosporins. To ascertain the effect antibacterial drugs have on a specific microorganism, culture and sensitivity or antibiotic susceptibility laboratory testing is performed. A culture and sensitivity test (C&S) can detect the infective microorganism present in a sample (e.g., blood, sputum, swab) and what drug can kill it. The organism causing the infection is determined by culture, and the antibiotics the organism is sensitive to are determined by sensitivity. The susceptibility or resistance of one microorganism to several antibacterials can be determined by this method. Multiantibiotic therapy (daily use of several antibacterials) delays the development of microorganism resistance.
Use of Antibiotic Combinations
Combination antibiotics should not be routinely prescribed or administered except for specific uncontrollable infections. Usually a single antibiotic will successfully treat a bacterial infection. When there is a severe infection that persists and is of unknown origin or has been unsuccessfully treated with several single antibiotics, a combination of two or three antibiotics may be suggested. Before beginning antibiotic therapy, a culture or cultures should be taken to identify the bacteria.
When two antibiotics are combined, the result is additive, potentiative, or antagonistic. The additive effect is equal to the sum of the effects of two antibiotics. The potentiative effect occurs when one antibiotic potentiates the effect of the second antibiotic, increasing their effectiveness. The antagonistic result is a combination of a drug that is bactericidal, such as penicillin, and a drug that is bacteriostatic, such as tetracycline. When these two drugs are used together, the desired effect may be greatly reduced.
General Adverse Reactions to Antibacterials
Three major adverse reactions associated with the administration of antibacterial drugs are allergic (hypersensitivity) reactions, superinfection, and organ toxicity. Table 29-2 describes these adverse reactions, all of which require close monitoring of the patient.
TABLE 29-2
GENERAL ADVERSE REACTIONS TO ANTIBACTERIAL DRUGS
| TYPE | CONSIDERATIONS |
| Allergy or hypersensitivity | Allergic reactions to drugs may be mild or severe. Examples of mild reactions are rash, pruritus, and hives. An example of a severe response is anaphylactic shock. Anaphylaxis results in vascular collapse, laryngeal edema, bronchospasm, and cardiac arrest. Shortness of breath is frequently the first symptom of anaphylaxis. Severe allergic reaction generally occurs within 20 minutes. Mild allergic reaction is treated with an antihistamine; anaphylaxis requires treatment with epinephrine, bronchodilators, and antihistamines. |
| Superinfection | Superinfection is a secondary infection that occurs when the normal microbial flora of the body are disturbed during antibiotic therapy. Superinfections can occur in the mouth, respiratory tract, intestine, genitourinary tract, or skin. Fungal infections frequently result in superinfections, although bacterial organisms (e.g., Proteus, Pseudomonas, staphylococci) may be the offending microorganisms. Superinfections rarely develop when the drug is administered for less than 1 week. They occur more commonly with the use of broad-spectrum antibiotics. For fungal infection of the mouth, nystatin is frequently used. |
| Organ toxicity | The liver and kidneys are involved in drug metabolism and excretion. Antibacterials may result in damage to these organs. For example, aminoglycosides can be nephrotoxic (as well as ototoxic). |
Narrow-Spectrum and Broad-Spectrum Antibiotics
Antibacterial drugs are either narrow spectrum or broad spectrum. The narrow-spectrum antibiotics are primarily effective against one type of organism. For example, penicillin and erythromycin are used to treat infections caused by gram-positive bacteria. Certain broad-spectrum antibiotics (tetracycline and cephalosporins) can be effective against both gram-positive and gram-negative organisms. Because narrow-spectrum antibiotics are selective, they are more active against those single organisms than the broad-spectrum antibiotics. Broad-spectrum antibiotics are frequently used to treat infections when the offending microorganism has not been identified by C&S.
Penicillins and Cephalosporins
Penicillins
Penicillin, a natural antibacterial agent obtained from the mold genus Penicillium, was introduced to the military during World War II and is considered to have saved many soldiers’ lives. It became widely used in 1945 and was labeled a “miracle” drug. With the advent of penicillin, many patients survived who would have normally died from wound and severe respiratory infections.
Penicillin’s beta-lactam structure (beta-lactam ring) interferes with bacterial cell-wall synthesis by inhibiting the bacterial enzyme that is necessary for cell division and cellular synthesis. The bacteria die of cell lysis (cell breakdown). The penicillins can be both bacteriostatic and bactericidal, depending on the drug and dosage. Penicillin G is primarily bactericidal.
Penicillins are mainly referred to as beta-lactam antibiotics. Bacteria can produce a variety of enzymes, such as beta-lactamases, that can inactivate penicillin and other beta-lactam antibiotics such as the cephalosporins. The beta-lactamases, which attack penicillins, are called penicillinases.
Penicillin G was the first penicillin administered orally and by injection. With oral administration, only about one third of the dose is absorbed. Because of its poor absorption, penicillin G given by injection (intramuscular [IM] and intravenous [IV]) is more effective in achieving a therapeutic serum penicillin level. Aqueous penicillin G has a short duration of action, and the IM injection is very painful, because it is an aqueous drug solution. As a result, a longer-acting form of penicillin, procaine penicillin (milky color), was produced to extend the activity of the drug. Procaine in the penicillin decreases the pain related to injection.
Penicillin V was the next type of penicillin produced. Although two thirds of the oral dose is absorbed by the gastrointestinal (GI) tract, it is a less potent antibacterial drug than penicillin G. Penicillin V is effective against mild to moderate infections, including anthrax as a biologic weapon of bioterrorism.
Initially penicillin was overused. It was first introduced for the treatment of staphylococcal infections, but after a few years mutant strains of Staphylococcus developed that were resistant to penicillins G and V because of the bacterial enzyme penicillinase, which destroys penicillin. This led to the development of new broad-spectrum antibiotics with structures similar to penicillin to combat infections resistant to penicillins G and V.
Food in the stomach does not significantly alter absorption of penicillin V, so it should be taken after meals. Amoxicillins are penicillins that are unaffected by food.
Broad-Spectrum Penicillins (Aminopenicillins)
Broad-spectrum penicillins are used to treat both gram-positive and gram-negative bacteria. They are not, however, as “broadly” effective against all microorganisms as they were once considered to be. This group of drugs is costlier than penicillin and therefore should not be used when ordinary penicillins, such as penicillin G, are effective. The broad-spectrum penicillins are effective against some gram-negative organisms: Escherichia coli, Haemophilus influenzae, Shigella dysenteriae, Proteus mirabilis, and Salmonella. However, these drugs are not penicillinase resistant. They are readily inactivated by beta-lactamases, thus ineffective against S. aureus. Examples of this group are ampicillin (Omnipen), and amoxicillin (Amoxil) (Table 29-3). Amoxicillin is the most prescribed penicillin derivative for adults and children.
TABLE 29-3
| GENERIC (BRAND) | ROUTE AND DOSAGE | USES AND CONSIDERATIONS |
| Basic Penicillins | ||
| penicillin G procaine (Crysticillin, Wycillin) | A: IM: 600,000-1 million units/d in 1-2 divided doses C: IM: 300,000 units/d in 1-2 divided doses | For moderately serious infections. Slow IM absorption with prolonged action. Solution is milky. Pregnancy category: B; PB: 60%; 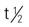 : 0.5-1 h : 0.5-1 h |
| penicillin G benzathine (Bicillin L-A) | A: IM: 1.2 million units as a single dose C >27 kg: IM: 900,000 units/dose C <27 kg: IM: 300,000-600,000 units/dose | Long-acting penicillin when given by injection. Used as prophylaxis for rheumatic fever. Pregnancy category: B; PB: 60%; 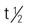 : 0.5-1 h : 0.5-1 h |
| penicillin G sodium/potassium (Pfizerpen) | A: IM/IV: 5-24 million units/d in divided doses q4-6 h; max: 80 million units/d C: IM/IV: 150,000-300,000 units/d in divided doses q4-6h; max: 24 million units/d Neonates: IM/IV: 50,000- 75,000 units/kg/d in divided doses q6-12h; max: 100,000/kg/d | Treatment of lower respiratory infections. Monitor electrolyte levels with high doses. Injectable solution is clear. Pregnancy category: B; PB: 45%-68%; 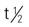 : 20-30 min : 20-30 min |
| penicillin V potassium (Veetids) | A/C >12 y: PO: 125-500 mg q6h | Treatment of upper respiratory infections. Acid-stable and less active than penicillin G against some bacteria. Not recommended in renal failure. Take drug after meals. Pregnancy category: B; PB: 75%-89%; 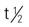 : 0.5 h : 0.5 h |
| Broad-Spectrum Penicillins | ||
| amoxicillin (Amoxil) | See Prototype Drug Chart 29-1. | |
| amoxicillin-clavulanate (Augmentin) | A/C >40 kg: PO: 250-500/125 mg q8-12h for 10 d | Treatment of lower respiratory infections, otitis media, sinusitis, skin infections, and UTIs. Pregnancy category: B; PB: 17%-30%; 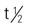 : 1-1.5 h : 1-1.5 h |
| ampicillin (Principen) | A: PO/IM/IV: 250-500 mg q6h C: PO/IM/IV: 25-50 mg/kg/d in 4 divided doses | Treatment of lower respiratory tract infections. First broad-spectrum penicillin. Effective against gram-negative and gram-positive bacteria. Individuals with penicillin allergies may also be allergic to ampicillin. Pregnancy category: B; PB: 15%-28%; 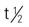 : 1-1.5 h : 1-1.5 h |
| ampicillin-sulbactam (Unasyn) | A: IM/IV: 1.5-3 g q6h; max: 4 g sulbactam/d C: IV: 100-300 mg/kg/d, in divided doses q6h | Same as ampicillin. Treatment of streptococcus pneumonia, nosocomial pneumonia, skin infections, and Neisseria gonorrhoeae. Sulbactam inhibits beta-lactamase thus extending the spectrum. Pregnancy category: B; PB; 28%-38%; 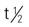 : 1-2 h : 1-2 h |
| Penicillinase-Resistant Penicillins | ||
| dicloxacillin sodium (Dynapen) | See Prototype Drug Chart 29-1. | |
| nafcillin (Nallpen) | A: IM/IV: 500 mg-2 g q4-6h; max: 12 g/d | Highly effective against penicillin G–resistant Staphylococcus aureus. Not recommended orally due to instability in gastric juices. Pregnancy category: B; PB: 70%-90%; 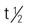 : 0.5-1.5 h : 0.5-1.5 h |
| oxacillin sodium | A/C >40 kg: IM/IV: 250 mg-1 g q4-6h; max: 6 g C <40 kg: IM/IV: 50-100 mg/kg/d in divided doses q4-6h; max: 6 g/d Neonates: IM/IV: 25 mg/kg/d in divided doses q6h | For penicillin-resistant staphylococci in bone, joint, and skin infections. Pregnancy category: B; PB: 89%-94%; 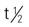 : 0.5 h : 0.5 h |
| Extended-Spectrum Penicillins | ||
| carbenicillin indanyl (Geocillin) | A: PO: 382-764 mg q6h | The first penicillin-like drug developed to treat infections caused by Pseudomonas aeruginosa and Proteus spp. Contains large amounts of sodium. Use with caution when administering to patients with hypertension or heart failure. Pregnancy category: B; PB: 30%-60%; 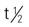 : 1 h : 1 h |
| piperacillin-tazobactam (Zosyn) | A: IV: 3.375 g, q6h over 30 min, 7-10 d | To treat severe appendicitis, skin infections, pneumonia, and beta-lactamase–producing bacteria. Tazobactam is a beta-lactamase inhibitor. Pregnancy category: B; PB: 30%; 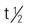 : 0.7-1.5 h : 0.7-1.5 h |
| ticarcillin-clavulanate (Timentin) | A: IV: 3.1 g q6h C >3 mo: IV: 50 mg/kg/d in 4-6 divided doses | Clavulanic acid protects ticarcillin from degradation by beta-lactamase enzymes. Effective for treating septicemia and lower respiratory tract, urinary tract, skin, bone, and joint infections. Pregnancy category: B; PB: 45%-65%;  : 1 h : 1 h |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


