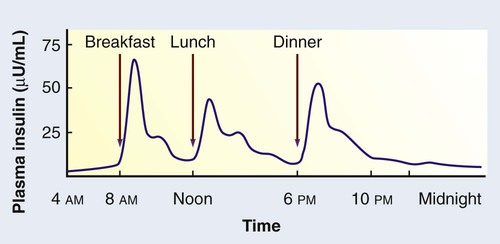
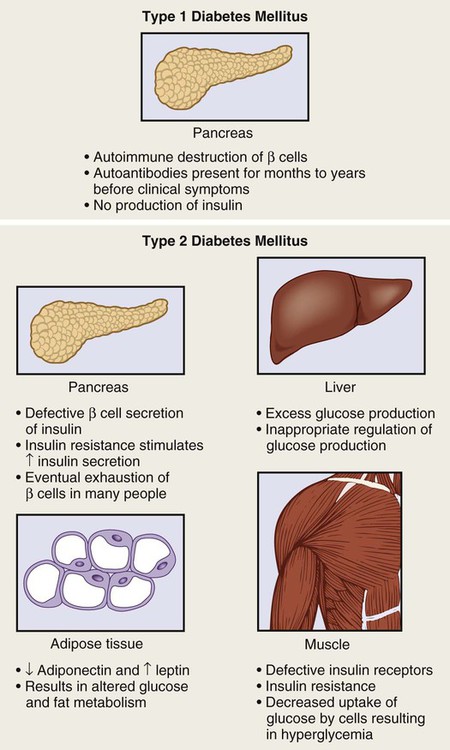
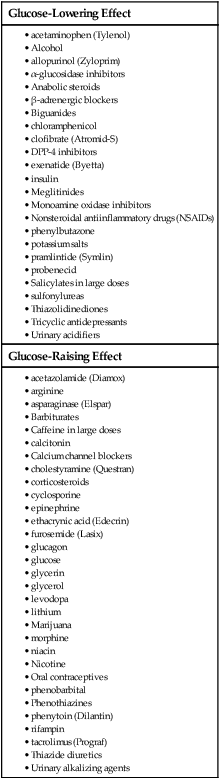
1. Describe the pathophysiology and clinical manifestations of diabetes mellitus. 2. Differentiate between type 1 and type 2 diabetes mellitus. 3. Describe the collaborative care of the patient with diabetes mellitus. 4. Describe the role of nutrition and exercise in the management of diabetes mellitus. 5. Discuss the nursing management of a patient with newly diagnosed diabetes mellitus. 6. Describe the nursing management of the patient with diabetes mellitus in the ambulatory and home care settings. 7. Relate the pathophysiology of acute and chronic complications of diabetes mellitus to the clinical manifestations. 8. Explain the collaborative care and nursing management of the patient with acute and chronic complications of diabetes mellitus. Diabetes mellitus (DM) is a chronic multisystem disease related to abnormal insulin production, impaired insulin utilization, or both. Diabetes mellitus is a serious health problem throughout the world, and its prevalence is rapidly increasing. Currently in the United States an estimated 25.8 million people, or 8.3% of the population, have diabetes mellitus, and 79 million more people have prediabetes.1 Approximately 7 million people with diabetes mellitus have not been diagnosed and are unaware that they have the disease. Diabetes mellitus is the seventh leading cause of death in the United States, but it is likely to be underreported. The annual cost of diabetes exceeds $174 billion, with $116 billion in direct medical costs.2 eTABLE 49-1 EFFECT OF DRUGS ON BLOOD GLUCOSE LEVELS The long-term complications of diabetes make it a devastating disease. Diabetes is the leading cause of adult blindness, end-stage kidney disease, and nontraumatic lower limb amputations. It is also a major contributing factor to heart disease and stroke. Adults with diabetes have heart disease death rates two to four times higher than adults without diabetes. The risk for stroke is also two to four times higher among people with diabetes. In addition, it is estimated that 67% of adults with diabetes have hypertension.1 The American Diabetes Association (ADA) recognizes four different classes of diabetes. The two most common are type 1 and type 2 diabetes mellitus (Table 49-1). The two other classes are gestational diabetes and other specific types of diabetes with various causes. TABLE 49-1 COMPARISON OF TYPE 1 AND TYPE 2 DIABETES MELLITUS Insulin is a hormone produced by the β cells in the islets of Langerhans of the pancreas. Under normal conditions, insulin is continuously released into the bloodstream in small pulsatile increments, with increased release when food is ingested (Fig. 49-1). Insulin lowers blood glucose and facilitates a stable, normal glucose range of approximately 70 to 120 mg/dL (3.9 to 6.66 mmol/L). The average amount of insulin secreted daily by an adult is approximately 40 to 50 U, or 0.6 U/kg of body weight. Type 1 diabetes mellitus, formerly known as juvenile-onset diabetes or insulin-dependent diabetes, accounts for approximately 5% of all people with diabetes. Type 1 diabetes generally affects people under 40 years of age, and 40% develop it before 20 years of age. The incidence of type 1 diabetes has increased 3% to 5% over recent decades, and for unknown reasons it is occurring more frequently in younger children.3 Type 1 diabetes is an immune-mediated disease, caused by autoimmune destruction of the pancreatic β cells, the site of insulin production. This eventually results in a total absence of insulin production. Autoantibodies to the islet cells cause a reduction of 80% to 90% of normal function before hyperglycemia and other manifestations occur (Fig. 49-2). A genetic predisposition and exposure to a virus are factors that may contribute to the pathogenesis of immune-related type 1 diabetes. Predisposition to type 1 diabetes is related to human leukocyte antigens (HLAs). (See Chapter 14 for a discussion of HLAs and disease associations.) Theoretically, when an individual with certain HLA types is exposed to a viral infection, the β cells of the pancreas are destroyed, either directly or through an autoimmune process. The HLA types associated with an increased risk for type 1 diabetes include HLA-DR3 and HLA-DR4 (see Genetics in Clinical Practice box). Idiopathic diabetes is a form of type 1 diabetes that is strongly inherited and not related to autoimmunity. It occurs only in a small number of people with type 1 diabetes, most often of Hispanic, African, or Asian ancestry.3 Latent autoimmune diabetes in adults (LADA), a slowly progressing autoimmune form of type 1 diabetes, usually occurs in people who are over the age of 35 who are not obese.4 Type 2 diabetes mellitus was formerly known as adult-onset diabetes (AODM) or non–insulin-dependent diabetes (NIDDM). Type 2 diabetes mellitus is, by far, the most prevalent type of diabetes, accounting for approximately 90% to 95% of patients with diabetes.2 Risk factors for developing type 2 diabetes include being overweight or obese, being older, and having a family history of type 2 diabetes. Although the disease is seen less frequently in children, the incidence is increasing due to the increasing prevalence of childhood obesity. Type 2 diabetes is more prevalent in some ethnic populations. African Americans, Asian Americans, Hispanics, Native Hawaiians or other Pacific Islanders, and Native Americans have a higher rate of type 2 diabetes than whites.4 Four major metabolic abnormalities have a role in the development of type 2 diabetes (see Fig. 49-2). The first factor is insulin resistance, a condition in which body tissues do not respond to the action of insulin because insulin receptors are unresponsive, are insufficient in number, or both. Most insulin receptors are located on skeletal muscle, fat, and liver cells. When insulin is not properly used, the entry of glucose into the cell is impeded, resulting in hyperglycemia. In the early stages of insulin resistance, the pancreas responds to high blood glucose by producing greater amounts of insulin (if β-cell function is normal). This creates a temporary state of hyperinsulinemia that coexists with hyperglycemia. A fourth factor is altered production of hormones and cytokines by adipose tissue (adipokines). Adipokines secreted by adipose tissue appear to play a role in glucose and fat metabolism and are likely to contribute to the pathophysiology of type 2 diabetes.5 Adipokines are thought to cause chronic inflammation, a factor involved in insulin resistance, type 2 diabetes, and cardiovascular disease (CVD). The two main adipokines believed to affect insulin sensitivity are adiponectin and leptin. Individuals with metabolic syndrome are at an increased risk for the development of type 2 diabetes. Metabolic syndrome has five components: elevated glucose levels, abdominal obesity, elevated blood pressure (BP), high levels of triglycerides, and decreased levels of high-density lipoproteins (HDLs) (see Table 41-10). An individual with three of the five components is considered to have metabolic syndrome.6 Overweight individuals with metabolic syndrome can prevent or delay the onset of diabetes through a program of weight loss and regular physical activity. (See Chapter 41 for a discussion of metabolic syndrome.) Individuals diagnosed with prediabetes are at increased risk for the development of type 2 diabetes. Prediabetes is defined as impaired glucose tolerance (IGT), impaired fasting glucose (IFG), or both. It is an intermediate stage between normal glucose homeostasis and diabetes where the blood glucose levels are elevated, but not high enough to meet the diagnostic criteria for diabetes. A diagnosis of IGT is made if the 2-hour oral glucose tolerance test (OGTT) values are 140 to 199 mg/dL (7.8 to 11.0 mmol/L).3 IFG is diagnosed when fasting blood glucose levels are 100 to 125 mg/dL (5.56 to 6.9 mmol/L). Gestational diabetes develops during pregnancy and occurs in about 2% to 10% of pregnancies in the United States.7 Women with gestational diabetes have a higher risk for cesarean delivery, and their babies have increased risk for perinatal death, birth injury, and neonatal complications. Women who are at high risk for gestational diabetes should be screened at the first prenatal visit.8 Those at high risk include women who are obese, are of advanced maternal age, or have a family history of diabetes. Women with an average risk for gestational diabetes are screened using an OGTT at 24 to 28 weeks of gestation. Most women with gestational diabetes have normal glucose levels within 6 weeks postpartum. Be aware that women with a history of gestational diabetes have a 35% to 60% chance of developing type 2 diabetes within 10 years.7 Gestational diabetes and management of the pregnant patient with diabetes are specialized areas not covered in detail in this chapter. Consult an obstetric text for more information.
Nursing Management
Diabetes Mellitus
Diabetes Mellitus
Glucose-Lowering Effect
Glucose-Raising Effect
Etiology and Pathophysiology
Factor
Type 1 Diabetes Mellitus
Type 2 Diabetes Mellitus
Age at onset
More common in young people but can occur at any age.
Usually age 35 yr or older but can occur at any age.
Incidence is increasing in children.
Type of onset
Signs and symptoms usually abrupt, but disease process may be present for several years.
Insidious, may go undiagnosed for years.
Prevalence
Accounts for 5%-10% of all types of diabetes.
Accounts for 90%-95% of all types of diabetes.
Environmental factors
Virus, toxins.
Obesity, lack of exercise.
Primary defect
Absent or minimal insulin production.
Insulin resistance, decreased insulin production over time, and alterations in production of adipokines.
Islet cell antibodies
Often present at onset.
Absent.
Endogenous insulin
Absent.
Initially increased in response to insulin resistance. Secretion diminishes over time.
Nutritional status
Thin, normal, or obese.
Frequently overweight or obese. May be normal.
Symptoms
Polydipsia, polyuria, polyphagia, fatigue, weight loss.
Frequently none, fatigue, recurrent infections. May also experience polyuria, polydipsia, and polyphagia.
Ketosis
Prone at onset or during insulin deficiency.
Resistant except during infection or stress.
Nutritional therapy
Essential.
Essential.
Insulin
Required for all.
Required for some. Disease is progressive and insulin treatment may need to be added to treatment regimen.
Vascular and neurologic complications
Frequent.
Frequent.
Normal Insulin Metabolism.
Type 1 Diabetes Mellitus.
Etiology and Pathophysiology.
![]() Genetic Link
Genetic Link
Type 2 Diabetes Mellitus.
![]() Genetic Link
Genetic Link
Prediabetes.
Gestational Diabetes.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nursing Management: Diabetes Mellitus
Get Clinical Tree app for offline access