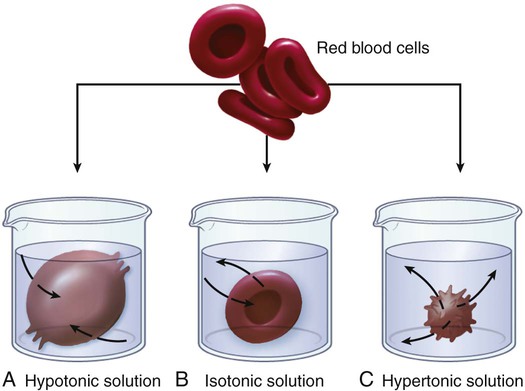
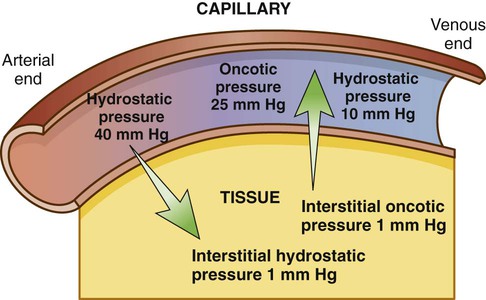
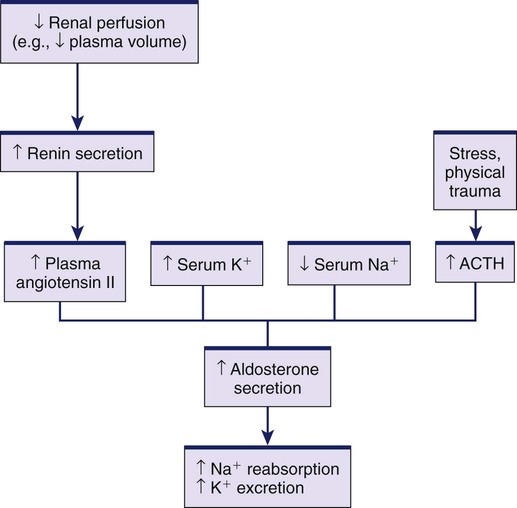
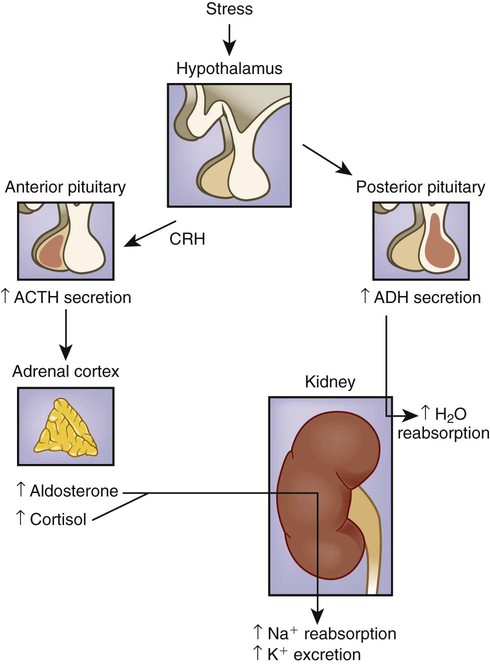
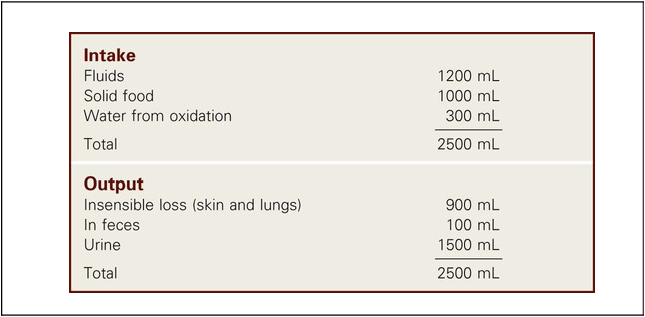
1. Describe the composition of the major body fluid compartments. 2. Define processes involved in the regulation of movement of water and electrolytes between the body fluid compartments. 3. Describe the etiology, laboratory diagnostic findings, clinical manifestations, and nursing and collaborative management of the following disorders: a. Extracellular fluid volume imbalances: fluid volume deficit and fluid volume excess b. Sodium imbalances: hypernatremia and hyponatremia c. Potassium imbalances: hyperkalemia and hypokalemia d. Magnesium imbalances: hypermagnesemia and hypomagnesemia e. Calcium imbalances: hypercalcemia and hypocalcemia f. Phosphate imbalances: hyperphosphatemia and hypophosphatemia 4. Identify the processes to maintain acid-base balance. 5. Discuss the etiology, laboratory diagnostic findings, clinical manifestations, and nursing and collaborative management of the following acid-base imbalances: metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis. 6. Describe the composition and indications of common IV fluid solutions. 7. Discuss the types and nursing management of commonly used central venous access devices. Body fluids and electrolytes play an important role in maintaining homeostasis, the stable internal environment of the body.1 Body fluids are in constant motion transporting nutrients, electrolytes, and oxygen to cells and carrying waste products away from cells. The body uses a number of adaptive responses associated with these activities to keep the composition and volume of body fluids and electrolytes within the narrow limits of normal to maintain homeostasis and promote health. Water is the primary component of the body, accounting for approximately 50% to 60% of body weight in the adult. The water content varies with gender, body mass, and age (Fig. 17-1). The percentage of body weight that is composed of water is generally greater in men than in women because men tend to have more lean body mass. The more fat present in the body, the less the total water content. Therefore obese individuals have a lower percentage of body water than slender people do. Older adults, with less muscle mass and more fat content, have less body water for this same reason. In the older adult, body water content averages 45% to 55% of body weight, leaving them at a higher risk for fluid-related problems than young adults. The two fluid compartments in the body are the intracellular space (inside the cells) and the extracellular space (outside the cells) (Fig. 17-2). Approximately two thirds of the body water is located within cells and is termed intracellular fluid (ICF); ICF constitutes approximately 40% of body weight of an adult. The body of a 70-kg young man would contain approximately 42 L of water, of which 28 L would be located within cells. Electrolyte composition varies between ECF and ICF. The overall concentration of electrolytes is approximately the same in the two compartments. However, concentrations of specific ions differ greatly (Fig. 17-3). In ECF the main cation is sodium, with small amounts of potassium, calcium, and magnesium. The primary ECF anion is chloride, with small amounts of bicarbonate, sulfate, and phosphate anions. In ICF the most prevalent cation is potassium, with small amounts of magnesium and sodium. The prevalent ICF anion is phosphate, with some protein and a small amount of bicarbonate. (Normal serum electrolyte values are presented in Table 17-1.) TABLE 17-1 NORMAL SERUM ELECTROLYTE VALUES *The majority of the phosphorus (P) in the body is found as phosphate (PO43−). The terms are used interchangeably in this text. Diffusion is the movement of molecules from an area of high concentration to one of low concentration (Fig. 17-4). It occurs in liquids, gases, and solids. Net movement of molecules across a membrane stops when the concentrations are equal in both areas. The membrane separating the two areas must be permeable to the diffusing substance for the process to occur. Simple diffusion requires no external energy. Active transport is a process in which molecules move against the concentration gradient. External energy is required for this process. An example is the sodium-potassium pump. The concentrations of sodium and potassium differ greatly intracellularly and extracellularly (see Fig. 17-3). To maintain this concentration difference, the cell uses active transport to move sodium out of the cell and potassium into the cell (Fig. 17-5). The energy source for this mechanism is adenosine triphosphate (ATP), produced in the cell’s mitochondria. Osmosis is the movement of water “down” a concentration gradient, that is, from a region of low solute concentration to one of high solute concentration, across a semipermeable membrane.2 Imagine a chamber with two compartments separated by a semipermeable membrane, one that allows only the movement of water (Fig. 17-6). Water will move from the less concentrated side (has more water) to the more concentrated side of the chamber water (has less water). Osmosis requires no outside energy sources and stops when the concentration differences disappear or when hydrostatic pressure builds and is sufficient to oppose any further movement of water. Whenever dissolved substances are contained in a space with a semipermeable membrane, they can pull water into the space by osmosis. The concentration of the solution determines the strength of the osmotic pull. The higher the concentration, the greater the solution’s pulling, or osmotic pressure. Osmotic pressure is measured in milliosmoles (mOsm) and may be expressed as either fluid osmolarity or fluid osmolality. Although the terms osmolarity and osmolality are often used interchangeably, they are different measurements. Osmolarity measures the total milliosmoles per liter of solution, or the concentration of molecules per volume of solution (mOsm/L). Osmolality measures the number of milliosmoles per kilogram of water, or the concentration of molecules per weight of water. Osmolality is the test typically performed to evaluate the concentration of plasma and urine.2 Changes in the osmolality of ECF alter the volume of cells. Solutions in which the solutes are less concentrated than in the cells are termed hypotonic (hypoosmolar). If a cell is surrounded by hypotonic fluid, water moves into the cell, causing it to swell and possibly to burst. Fluids with solutes more concentrated than in cells, or an increased osmolality, are termed hypertonic (hyperosmolar). If hypertonic fluid surrounds a cell, water leaves the cell to dilute ECF; the cell shrinks and may eventually die (Fig. 17-7). Capillary hydrostatic pressure and interstitial oncotic pressure move water out of the capillaries. Plasma oncotic pressure and interstitial hydrostatic pressure move fluid into the capillaries. At the arterial end of the capillary, capillary hydrostatic pressure exceeds plasma oncotic pressure, and fluid moves into the interstitial space. At the venous end of the capillary, the capillary hydrostatic pressure is lower than plasma oncotic pressure, drawing fluid back into the capillary by the oncotic pressure created by plasma proteins (Fig. 17-8). Fluid spacing is a term used to describe the distribution of body water. First spacing describes the normal distribution of fluid in ICF and ECF compartments. Second spacing refers to an abnormal accumulation of interstitial fluid (i.e., edema). Third spacing occurs when fluid accumulates in a portion of the body from which it is not easily exchanged with the rest of the ECF. Third-spaced fluid is trapped and unavailable for functional use. Examples of third spacing are ascites; sequestration of fluid in the abdominal cavity with peritonitis; and edema associated with burns, trauma, or sepsis.3 The kidneys are the primary organs for regulating fluid and electrolyte balance (see Chapter 45). The kidneys regulate water balance by adjusting urine volume and the urinary excretion of most electrolytes to maintain a balance between overall intake and output. The kidneys filter the total plasma volume many times each day. In the average adult the kidneys reabsorb 99% of this filtrate, producing approximately 1.5 L of urine per day. As the filtrate moves through the renal tubules, selective reabsorption of water and electrolytes and secretion of electrolytes result in the production of urine that is greatly different in composition and concentration from plasma. This process helps maintain normal plasma osmolality, electrolyte balance, blood volume, and acid-base balance. The renal tubules are the site for the actions of ADH and aldosterone. With severely impaired renal function, the kidneys cannot maintain fluid and electrolyte balance. This condition results in edema, potassium and phosphorus retention, acidosis, and other electrolyte imbalances (see Chapter 47). Glucocorticoids and mineralocorticoids secreted by the adrenal cortex help regulate both water and electrolytes. The glucocorticoids (e.g., cortisol) primarily have an antiinflammatory effect and increase serum glucose levels, whereas the mineralocorticoids (e.g., aldosterone) enhance sodium retention and potassium excretion (Fig. 17-9). When sodium is reabsorbed, water follows because of osmotic changes. Cortisol is the most abundant glucocorticoid. In large doses, cortisol has both glucocorticoid (glucose elevating and antiinflammatory) and mineralocorticoid (sodium-retention) effects. Normally cortisol secretion is in a diurnal or circadian pattern. Increased cortisol secretion occurs in response to physical and psychologic stress, affecting many body functions, including fluid and electrolyte balance (Fig. 17-10). Aldosterone is a mineralocorticoid with potent sodium-retaining and potassium-excreting capabilities. Decreased renal perfusion or decreased sodium delivery to the distal portion of the renal tubule activates the renin-angiotensin-aldosterone system (RAAS), which results in aldosterone secretion (see Fig. 45-4). In addition to the RAAS, increased plasma potassium, decreased plasma sodium, and adrenocorticotropic hormone (ACTH) from the anterior pituitary act directly on the adrenal cortex to stimulate the secretion of aldosterone (see Fig. 17-9). Daily water intake and output are normally between 2000 and 3000 mL (Table 17-2). Oral intake of fluids accounts for most of the water intake. Water intake also includes water from food metabolism and water present in solid foods. Lean meat is approximately 70% water, whereas the water content of many fruits and vegetables approaches 100%. The older adult experiences normal physiologic changes with aging that increase susceptibility to fluid and electrolyte imbalances. Structural changes to the kidneys and a decrease in the renal blood flow lead to decreased glomerular filtration rate, decreased creatinine clearance, and loss of the ability to concentrate urine and conserve water. Hormonal changes include a decrease in renin and aldosterone and an increase in ADH and ANP.4 Loss of subcutaneous tissue and thinning of the dermis lead to increased loss of moisture through the skin and an inability to respond to heat or cold quickly. Older adults experience a decrease in the thirst mechanism, resulting in decreased fluid intake despite increases in osmolality and serum sodium level. Older adults, especially if they are ill, are at increased risk of free-water loss and subsequent development of hypernatremia secondary to impairment of the thirst mechanism and barriers to obtaining fluids to drink.5 Fluid and electrolyte imbalances occur to some degree in most patients with a major illness or injury because illness disrupts the normal homeostatic mechanism. Some fluid and electrolyte imbalances are directly caused by illness or disease (e.g., burns, heart failure). At other times, therapeutic measures (e.g., IV fluid replacement, diuretics) cause or contribute to fluid and electrolyte imbalances. Perioperative patients are at risk for the development of fluid and electrolyte imbalances because of fluid restrictions, blood or fluid loss, and the stress of surgery.6 Fluid volume deficit can occur with abnormal loss of body fluids (e.g., diarrhea, fistula drainage, hemorrhage, polyuria), inadequate intake, or a shift of fluid from plasma into interstitial fluid. The term fluid volume deficit is not interchangeable with the term dehydration. Dehydration refers to loss of pure water alone without a corresponding loss of sodium. Causes and clinical manifestations of fluid volume deficit are listed in Table 17-3. TABLE 17-3 EXTRACELLULAR FLUID IMBALANCES: CAUSES AND CLINICAL MANIFESTATIONS Fluid volume excess may result from excessive intake of fluids, abnormal retention of fluids (e.g., heart failure, renal failure), or a shift of fluid from interstitial fluid into plasma fluid. Although fluid shifts between the interstitial space and plasma do not alter the overall volume of ECF, these shifts result in changes in the intravascular volume. Causes and clinical manifestations of fluid volume excess are listed in Table 17-3. • Deficient fluid volume related to excessive ECF losses or decreased fluid intake • Decreased cardiac output related to excessive ECF losses or decreased fluid intake • Risk for deficient fluid volume related to excessive ECF losses or decreased fluid intake • Excess fluid volume related to increased water and/or sodium retention • Impaired gas exchange related to water retention leading to pulmonary edema • Risk for impaired skin integrity related to edema • Activity intolerance related to increased water retention, fatigue, and weakness • Disturbed body image related to altered body appearance secondary to edema Detect clues to ECF volume deficit and excess by inspecting the skin. Examine the skin for turgor and mobility. Normally a fold of skin, when pinched, will readily move and, on release, rapidly return to its former position. Skin areas over the sternum, abdomen, and anterior forearm are the usual sites for evaluation of tissue turgor (Fig. 17-11). In older people, decreased skin turgor is less predictive of fluid deficit because of the loss of tissue elasticity.4 In ECF volume deficit, skin turgor is diminished, and there is a lag in the pinched skinfold’s return to its original state (referred to as tenting). Nurses in hospitals and long-term care facilities should encourage and help the older or debilitated patient to maintain adequate oral intake. Assess the patient’s ability to obtain adequate fluids independently, express thirst, and swallow effectively.4 Fluids should be easily accessible. Assist older adults with physical limitations, such as arthritis, to open and hold containers. A variety of types of fluids should be available, and assess for individual preferences. Serve fluids at the temperature preferred by the patient. Seventy percent to 80% of the daily intake of fluids should be with meals, with fluid supplements between meals. Older adults may choose to decrease or eliminate fluids 2 hours before bedtime to decrease nocturia or incontinence. The unconscious or cognitively impaired patient is at increased risk because of an inability to express thirst and act on it. In these patients, accurately document fluid intake and losses and carefully evaluate the adequacy of intake and output.4 Because sodium is the primary determinant of ECF osmolality, sodium imbalances are typically associated with parallel changes in osmolality. Serum sodium is measured in milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L). The serum sodium level reflects the ratio of sodium to water, not necessarily the loss or gain of sodium. Changes in the serum sodium level may reflect a primary water imbalance, a primary sodium imbalance, or a combination of the two. Sodium imbalances are typically associated with imbalances in ECF volume (Figs. 17-12 and 17-13). Several clinical states can produce hypernatremia from water loss (Table 17-4). A deficiency in the synthesis or release of ADH from the posterior pituitary gland (central diabetes insipidus) or a decrease in kidney responsiveness to ADH (nephrogenic diabetes insipidus) can result in profound diuresis, thus producing a water deficit and hypernatremia. Hyperosmolality with osmotic diuresis can result from administration of concentrated hyperosmolar tube feedings and hyperglycemia associated with uncontrolled diabetes mellitus. Other causes of hypernatremia include excessive sweating and increased sensible losses from high fever. TABLE 17-4 SODIUM IMBALANCES: CAUSES AND CLINICAL MANIFESTATIONS The manifestations of hypernatremia are primarily the result of water shifting out of cells into ECF with resultant dehydration and shrinkage of cells (see Table 17-4). Dehydration of brain cells results in neurologic manifestations such as intense thirst, agitation, and decreased alertness, ranging from sleepiness to coma.7 If there is any accompanying ECF volume deficit, manifestations such as postural hypotension, weakness, and decreased skin turgor occur. • Risk for injury related to altered sensorium and seizures • Risk for fluid volume deficit related to excessive intake of sodium and/or loss of water • Risk for electrolyte imbalance related to excessive intake of sodium and/or loss of water • Potential complication: seizures and coma leading to irreversible brain damage The primary goal of treatment of hypernatremia is to treat the underlying cause. In primary water deficit, fluid replacement is provided either orally or IV with isotonic or hypotonic fluids such as 5% dextrose in water or 0.45% sodium chloride saline solution.8 The goal of treatment for sodium excess is to dilute the sodium concentration with sodium-free IV fluids, such as 5% dextrose in water, and to promote excretion of the excess sodium by administering diuretics. (See Chapter 50 for specific treatment of diabetes insipidus.) Hyponatremia (low serum sodium) may result from a loss of sodium-containing fluids, water excess in relation to the amount of sodium (dilutional hyponatremia), or a combination of both (see Table 17-4). Common causes of hyponatremia from loss of sodium-rich body fluids include profuse diaphoresis, draining wounds, excessive diarrhea or vomiting, and trauma with significant blood loss. Hyponatremia causes hypoosmolality with a shift of water into the cells. A common cause of hyponatremia from water excess is inappropriate use of sodium-free or hypotonic IV fluids. This may occur in patients after surgery or major trauma or during administration of fluids in patients with renal failure. Patients with psychiatric disorders may have an excessive water intake. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) will result in dilutional hyponatremia caused by abnormal retention of water. (See Chapter 50 for a discussion of the causes of SIADH.) Manifestations of hyponatremia are due to cellular swelling and first manifested in the central nervous system (CNS) (see Table 17-4). The excess water lowers plasma osmolality, shifting fluid into brain cells, causing irritability, headache, confusion, seizures, and even coma. Severe acute hyponatremia, if untreated, can cause irreversible neurologic damage or death.7 • Risk for acute confusion related to electrolyte imbalance • Risk for injury related to altered sensorium and decreased level of consciousness • Risk for electrolyte imbalance related to excessive loss of sodium and/or excessive intake or retention of water The drugs conivaptan (Vaprisol) and tolvaptan (Samsca) are given to block the activity of ADH. Conivaptan results in increased urine output without loss of electrolytes such as sodium and potassium. It should not be used in patients with hyponatremia from excess water loss. Tolvaptan is used to treat hyponatremia associated with heart failure, liver cirrhosis, and SIADH.9 Treatment with these drugs is started in a hospital setting so the patient’s clinical status and serum sodium levels can be carefully monitored. Hyperkalemia (high serum potassium) may result from impaired renal excretion, a shift of potassium from ICF to ECF, a massive intake of potassium, or a combination of these factors (Table 17-5). The most common cause of hyperkalemia is renal failure. Adrenal insufficiency with a subsequent aldosterone deficiency leads to retention of potassium ions. Factors that cause potassium to move from ICF to ECF include acidosis, massive cell destruction (as in burn or crush injury, tumor lysis, severe infections), and exercise. In metabolic acidosis, potassium ions shift from ICF to ECF in exchange for hydrogen ions moving into the cell. TABLE 17-5 POTASSIUM IMBALANCES: CAUSES AND CLINICAL MANIFESTATIONS • Excessive or rapid parenteral administration • Potassium-containing drugs (e.g., potassium penicillin)
Fluid, Electrolyte, and Acid-Base Imbalances
Homeostasis
Water Content of the Body
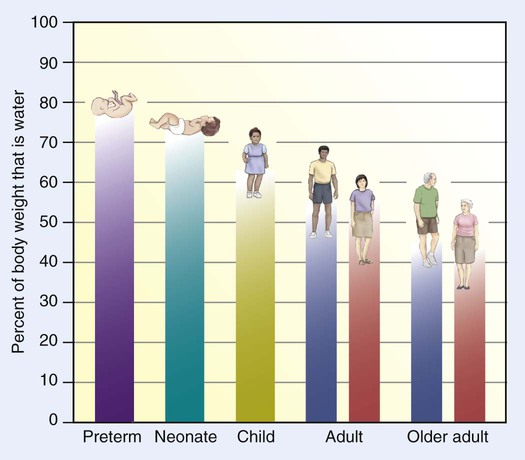
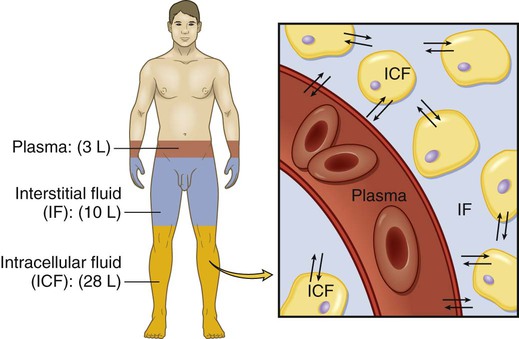
Body Fluid Compartments
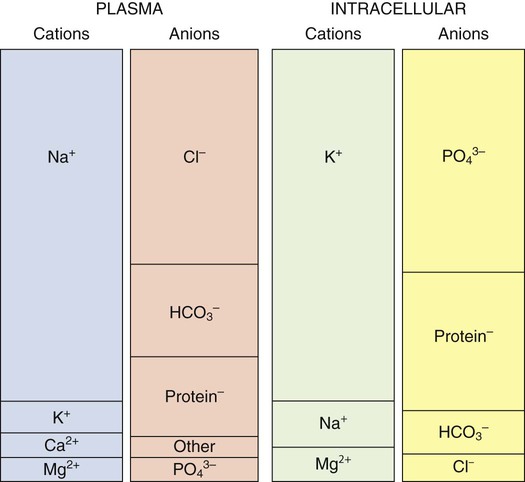
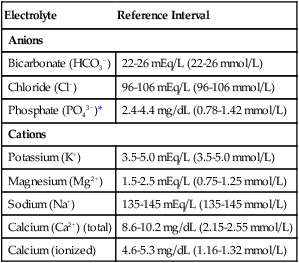
Electrolytes
Electrolyte Composition of Fluid Compartments
Electrolyte
Reference Interval
Anions
Bicarbonate (HCO3−)
22-26 mEq/L (22-26 mmol/L)
Chloride (Cl−)
96-106 mEq/L (96-106 mmol/L)
Phosphate (PO43−)*
2.4-4.4 mg/dL (0.78-1.42 mmol/L)
Cations
Potassium (K+)
3.5-5.0 mEq/L (3.5-5.0 mmol/L)
Magnesium (Mg2+)
1.5-2.5 mEq/L (0.75-1.25 mmol/L)
Sodium (Na+)
135-145 mEq/L (135-145 mmol/L)
Calcium (Ca2+) (total)
8.6-10.2 mg/dL (2.15-2.55 mmol/L)
Calcium (ionized)
4.6-5.3 mg/dL (1.16-1.32 mmol/L)
Mechanisms Controlling Fluid and Electrolyte Movement
Diffusion
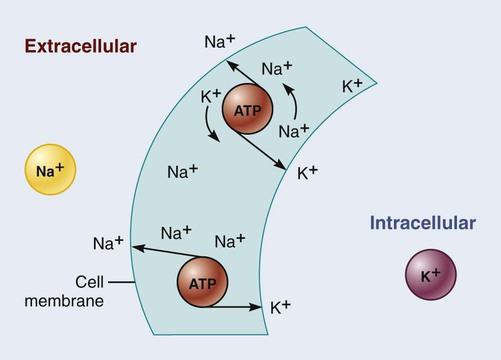
Active Transport
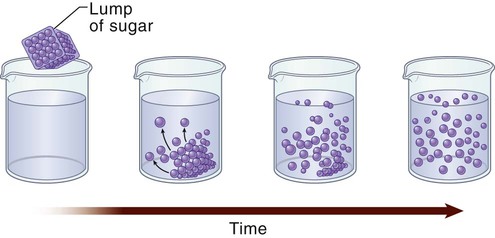
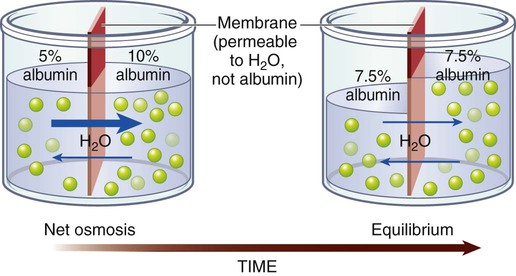
Osmosis
Osmotic Movement of Fluids.
Fluid Movement in Capillaries
Fluid Spacing
Regulation of Water Balance
Renal Regulation
Adrenal Cortical Regulation
Gastrointestinal Regulation
Gerontologic Considerations
Fluid and Electrolytes
Fluid and Electrolyte Imbalances
Extracellular Fluid Volume Imbalances
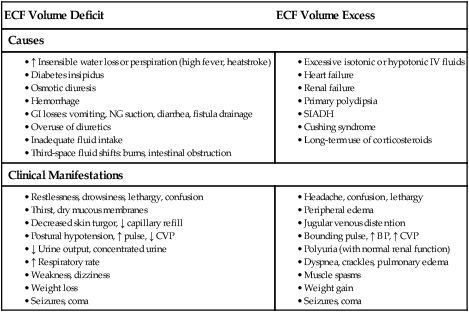
Fluid Volume Deficit
ECF Volume Deficit
ECF Volume Excess
Causes
Clinical Manifestations
Fluid Volume Excess
Nursing Management Extracellular Fluid Volume Imbalances
Nursing Diagnoses
Nursing Implementation
Intake and Output.
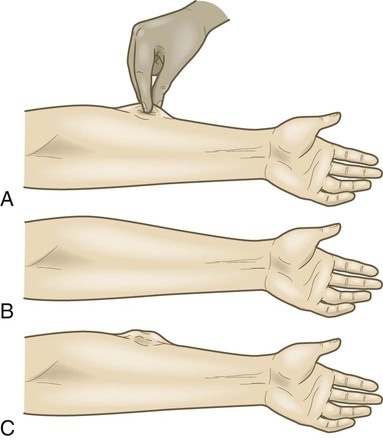
Skin Assessment and Care.
Other Nursing Measures.
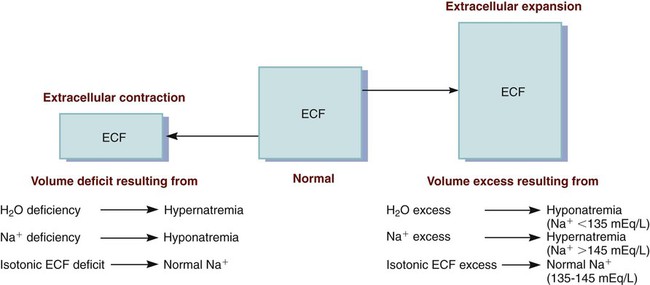
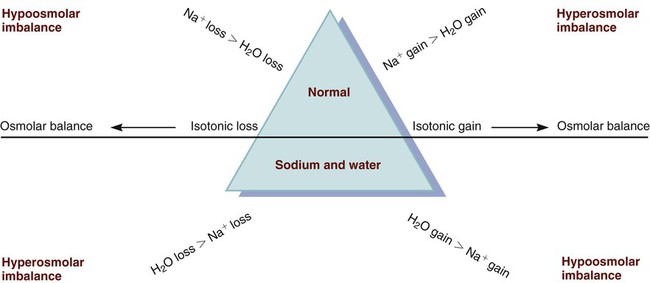
Sodium Imbalances
Hypernatremia
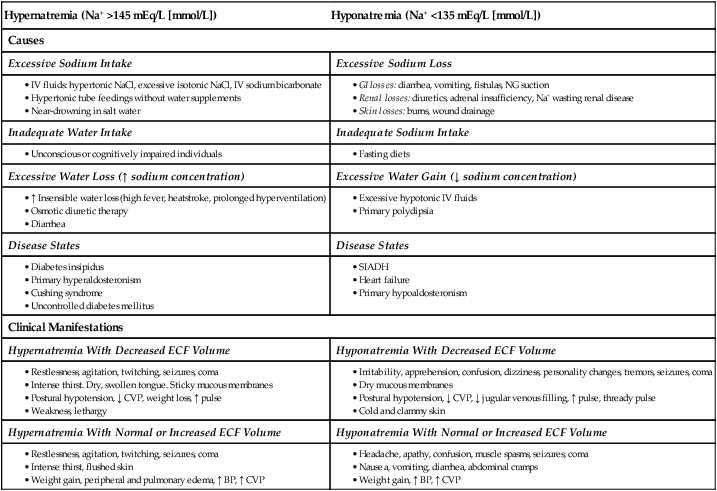
Hypernatremia (Na+ >145 mEq/L [mmol/L])
Hyponatremia (Na+ <135 mEq/L [mmol/L])
Causes
Excessive Sodium Intake
Excessive Sodium Loss
Inadequate Water Intake
Inadequate Sodium Intake
Excessive Water Loss (↑ sodium concentration)
Excessive Water Gain (↓ sodium concentration)
Disease States
Disease States
Clinical Manifestations
Hypernatremia With Decreased ECF Volume
Hyponatremia With Decreased ECF Volume
Hypernatremia With Normal or Increased ECF Volume
Hyponatremia With Normal or Increased ECF Volume
Clinical Manifestations.
Nursing and Collaborative Management Hypernatremia
Nursing Diagnoses
Nursing Implementation
Hyponatremia
Clinical Manifestations.
Nursing and Collaborative Management Hyponatremia
Nursing Diagnoses
Nursing Implementation
Potassium Imbalances
Hyperkalemia
Hyperkalemia (K+ >5.0 mEq/L [mmol/L])
Hypokalemia (K+ <3.5 mEq/L [mmol/L])
Causes
Excess Potassium Intake
Potassium Loss
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Fluid, Electrolyte, and Acid-Base Imbalances
Get Clinical Tree app for offline access