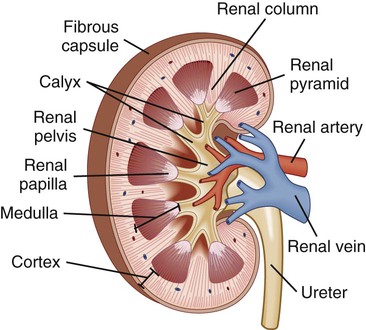
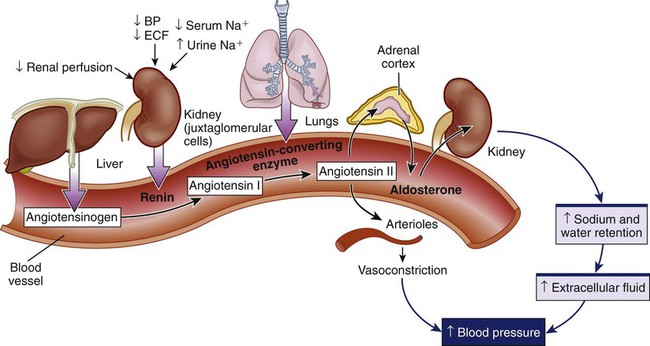
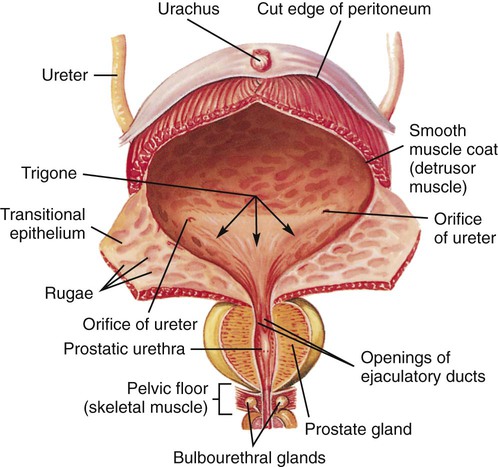
Chapter 45 1. Differentiate among the anatomic location and functions of the kidneys, ureters, bladder, and urethra. 2. Explain the physiologic events involved in the formation and passage of urine from glomerular filtration to voiding. 3. Select significant subjective and objective data related to the urinary system that should be obtained from a patient. 4. Link the age-related changes of the urinary system to the differences in assessment findings. 5. Select appropriate techniques to use in the physical assessment of the urinary system. 6. Differentiate normal from abnormal findings of a physical assessment of the urinary system. 7. Describe the purpose, significance of results, and nursing responsibilities related to diagnostic studies of the urinary system. 8. Differentiate the normal from the abnormal findings of a urinalysis. The upper urinary system consists of two kidneys and two ureters. The lower urinary system consists of a urinary bladder and a urethra (Fig. 45-1). Urine is formed in the kidneys, drains through the ureters to be stored in the bladder, and then passes from the body through the urethra. The parenchyma (actual tissue) of the kidney can be visualized on a longitudinal section of the kidney (Fig. 45-2). The outer layer is the cortex, and the inner layer is the medulla. The medulla consists of a number of pyramids. The apices (tops) of these pyramids are called papillae, through which urine passes to enter the calyces. The minor calyces widen and merge to form major calyces, which form a funnel-shaped sac called the renal pelvis. The minor and major calyces transport urine to the renal pelvis, from which it drains through the ureter to the bladder. The renal pelvis can store a small volume of urine (3 to 5 mL). The nephron is the functional unit of the kidney. Each kidney contains approximately 1 million nephrons. Each nephron is composed of the glomerulus, Bowman’s capsule, and a tubular system. The tubular system consists of the proximal convoluted tubule, loop of Henle, distal convoluted tubule, and collecting tubules (Fig. 45-3). The glomerulus, Bowman’s capsule, proximal tubule, and distal tubule are located in the cortex of the kidney. The loop of Henle and collecting tubules are located in the medulla. Several collecting tubules join to form a single collecting duct. The collecting ducts eventually merge into a pyramid that empties via the papilla into a minor calyx. Blood flow to the kidneys, approximately 1200 mL/min, accounts for 20% to 25% of the cardiac output. Blood reaches the kidneys via the renal artery, which arises from the aorta and enters the kidney through the hilus. The renal artery divides into secondary branches and then into still smaller branches, each of which forms an afferent arteriole. The afferent arteriole divides into a capillary network, the glomerulus, which is a tuft of up to 50 capillaries (see Fig. 45-3). The capillaries of the glomerulus unite in the efferent arteriole. This efferent arteriole splits to form a capillary network, the peritubular capillaries, which surround the tubular system. All peritubular capillaries drain into the venous system; the renal vein empties into the inferior vena cava. Urine formation begins at the glomerulus, where blood is filtered. The glomerulus is a semipermeable membrane that allows filtration (see Fig. 45-3). The hydrostatic pressure of the blood within the glomerular capillaries causes a portion of blood to be filtered across the semipermeable membrane into Bowman’s capsule, where the filtered portion of the blood (the glomerular filtrate) begins to pass down to the tubule. Filtration is more rapid in the glomerulus than in ordinary tissue capillaries because the glomerular membrane is porous. The ultrafiltrate is similar in composition to blood except that it lacks blood cells, platelets, and large plasma proteins. Under normal conditions, the capillary pores are too small to allow the loss of these large blood components. In many kidney diseases, capillary permeability is increased, which permits plasma proteins and blood cells to pass into the urine. Since the glomerular membrane is a selective filtration membrane that filters primarily by size, provision is made for the reabsorption of essential materials and the excretion of nonessential ones (Table 45-1). The tubules and collecting ducts carry out these functions by means of reabsorption and secretion. Reabsorption is the passage of a substance from the lumen of the tubules through the tubule cells and into the capillaries. This process involves both active and passive transport mechanisms. Tubular secretion is the passage of a substance from the capillaries through the tubular cells into the lumen of the tubule. Reabsorption and secretion cause numerous changes in the composition of the glomerular filtrate as it moves through the entire length of the tubule. TABLE 45-1 Acid-base regulation involves reabsorbing and conserving most of the bicarbonate (HCO3−) and secreting excess H+. The distal tubule has different ways to maintain the pH of ECF within a range of 7.35 to 7.45 (see Chapter 17). Vitamin D is a hormone that can be obtained in the diet or synthesized by the action of ultraviolet radiation on cholesterol in the skin. These forms of vitamin D are inactive and require two more steps to become metabolically active. The first step in activation occurs in the liver; the second step occurs in the kidneys. Active vitamin D is essential for the absorption of calcium from the gastrointestinal (GI) tract. The patient with kidney failure (also called renal failure) has a deficiency of the active metabolite of vitamin D and manifests problems of altered calcium and phosphate balance (see Chapter 47). Renin is important in the regulation of blood pressure. Renin is produced and secreted by the kidney’s juxtaglomerular cells (Fig. 45-4). Renin is released into the bloodstream in response to decreased renal perfusion, decreased arterial blood pressure, decreased ECF, decreased serum Na+ concentration, and increased urinary Na+ concentration. The plasma protein angiotensinogen (from the liver) is activated to angiotensin I by renin. Angiotensin I is subsequently converted to angiotensin II by angiotensin-converting enzyme (ACE). ACE is located on the inner surface of all blood vessels, with particularly high levels in the vessels of the lungs. Angiotensin II stimulates the release of aldosterone from the adrenal cortex, which causes Na+ and water retention, leading to increased ECF volume. Angiotensin II also causes increased peripheral vasoconstriction. Release of renin is inhibited by an elevation in blood pressure. Excessive renin production caused by impaired renal perfusion may be a contributing factor in the etiology of hypertension (see Chapters 33 and 47). Most body tissues synthesize prostaglandins (PGs) from the precursor, arachidonic acid, in response to appropriate stimuli. (See Chapter 12 and Fig. 12-2 for a more detailed discussion of PGs.) In the kidney, PG synthesis (primarily PGE2 and PGI2) occurs primarily in the medulla. These PGs have a vasodilating action, thus increasing renal blood flow, and promote Na+ excretion. They counteract the vasoconstrictor effect of substances such as angiotensin and norepinephrine. Renal PGs may have a systemic effect in lowering blood pressure by decreasing systemic vascular resistance. The significance of renal PGs is related to the kidneys’ role in causing hypertension. In renal failure with a loss of functioning tissue, these renal vasodilator factors are also lost, which may contribute to hypertension (see Chapter 47). The ureters are tubes that carry urine from the renal pelvis to the bladder (see Fig. 45-1). Arranged in a meshlike outer layer, circular and longitudinal smooth muscle fibers contract to promote the peristaltic, one-way flow of urine through the ureters. Distention, neurologic and endocrine influences, and drugs can affect these muscle contractions. Each ureter is approximately 10 to 12 in (25 to 30.5 cm) long and 0.08 to 0.3 in (0.2 to 0.8 cm) in diameter. The urinary bladder is a stretchable (able to fill at relatively low pressures) organ positioned behind the symphysis pubis and anterior to the vagina and rectum (Fig. 45-5). Its primary functions are to serve as a reservoir for urine and to eliminate waste products from the body. Normal adult urine output is approximately 1500 mL/day, which varies with food and fluid intake. The volume of urine produced at night is less than half of that formed during the day because of hormonal influences (e.g., ADH). This diurnal pattern of urination is normal. Typically, an individual will urinate five or six times during the day and occasionally at night. Anatomic changes in the aging kidney include a 20% to 30% decrease in size and weight between ages 30 and 90 years. By the seventh decade of life, 30% to 50% of glomeruli have lost their function. Atherosclerosis accelerates the decrease of renal size with age. Despite these changes, older individuals maintain body fluid homeostasis unless they encounter diseases or other physiologic stressors.1 The prostate surrounds the proximal urethra. As men age, the prostate enlarges and may affect urinary patterns, causing hesitancy, retention, slow stream, and bladder infections.2 Age-related changes in the urinary system and differences in assessment findings are presented in Table 45-2. TABLE 45-2 GERONTOLOGIC ASSESSMENT DIFFERENCES An assessment of the patient’s current and past use of medications is important. This should include over-the-counter drugs, prescription medications, and herbs. Drugs affect the urinary tract in several ways. Many drugs are known to be nephrotoxic (Table 45-3). Certain drugs may alter the quantity and character of urine output (e.g., diuretics). A number of drugs such as phenazopyridine (Pyridium) and nitrofurantoin (Macrodantin) change the color of urine. Anticoagulants may cause hematuria. Many antidepressants, calcium channel blockers, antihistamines, and drugs used for neurologic and musculoskeletal disorders affect the ability of the bladder or sphincter to contract or relax normally.
Nursing Assessment
Urinary System
Structures and Functions of Urinary System
Kidneys
Macrostructure.
Microstructure.
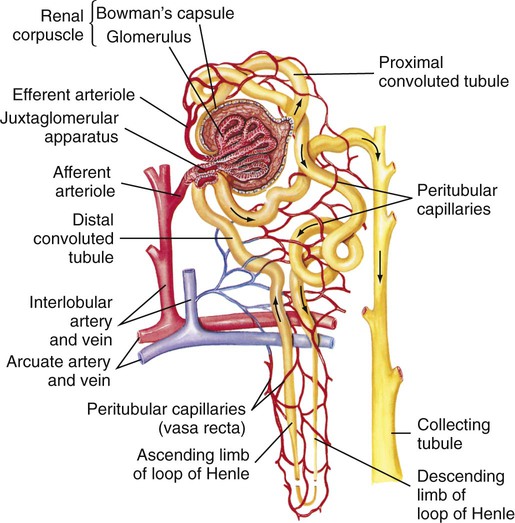
Blood Supply.
Physiology of Urine Formation.
Glomerular Function.
Tubular Function.
Component
Function
Glomerulus
Selective filtration.
Proximal tubule
Reabsorption of 80% of electrolytes and water, glucose, amino acids, HCO3−. Secretion of H+ and creatinine.
Loop of Henle
Reabsorption of Na+ and Cl− in ascending limb and water in descending loop. Concentration of filtrate.
Distal tubule
Secretion of K+, H+, ammonia. Reabsorption of water (regulated by ADH) and HCO3−. Regulation of Ca2+ and PO42− by parathyroid hormone. Regulation of Na+ and K+ by aldosterone.
Collecting duct
Reabsorption of water (ADH required).
Other Functions of Kidneys.
Ureters
Bladder
Gerontologic Considerations
Effects of Aging on Urinary System
Urinary System
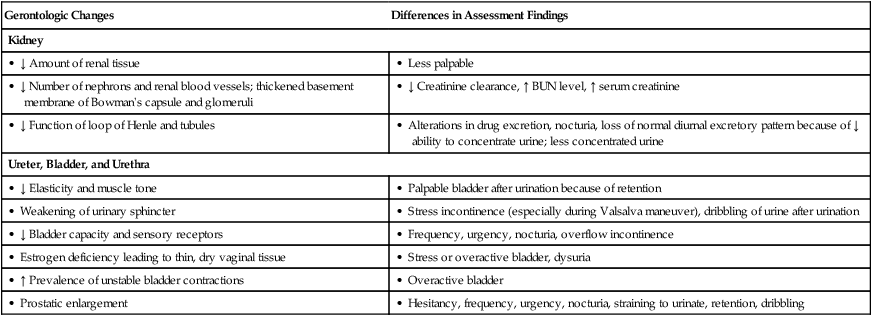
Gerontologic Changes
Differences in Assessment Findings
Kidney
• ↓ Amount of renal tissue
• Less palpable
• ↓ Number of nephrons and renal blood vessels; thickened basement membrane of Bowman’s capsule and glomeruli
• ↓ Creatinine clearance, ↑ BUN level, ↑ serum creatinine
• ↓ Function of loop of Henle and tubules
• Alterations in drug excretion, nocturia, loss of normal diurnal excretory pattern because of ↓ ability to concentrate urine; less concentrated urine
Ureter, Bladder, and Urethra
• ↓ Elasticity and muscle tone
• Palpable bladder after urination because of retention
• Weakening of urinary sphincter
• Stress incontinence (especially during Valsalva maneuver), dribbling of urine after urination
• ↓ Bladder capacity and sensory receptors
• Frequency, urgency, nocturia, overflow incontinence
• Estrogen deficiency leading to thin, dry vaginal tissue
• Stress or overactive bladder, dysuria
• ↑ Prevalence of unstable bladder contractions
• Overactive bladder
• Prostatic enlargement
• Hesitancy, frequency, urgency, nocturia, straining to urinate, retention, dribbling
Assessment of Urinary System
Subjective Data
Important Health Information
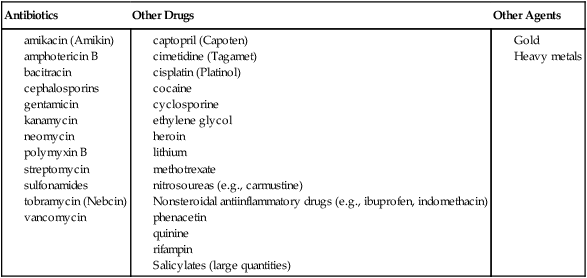
Medications.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nursing Assessment: Urinary System
Get Clinical Tree app for offline access