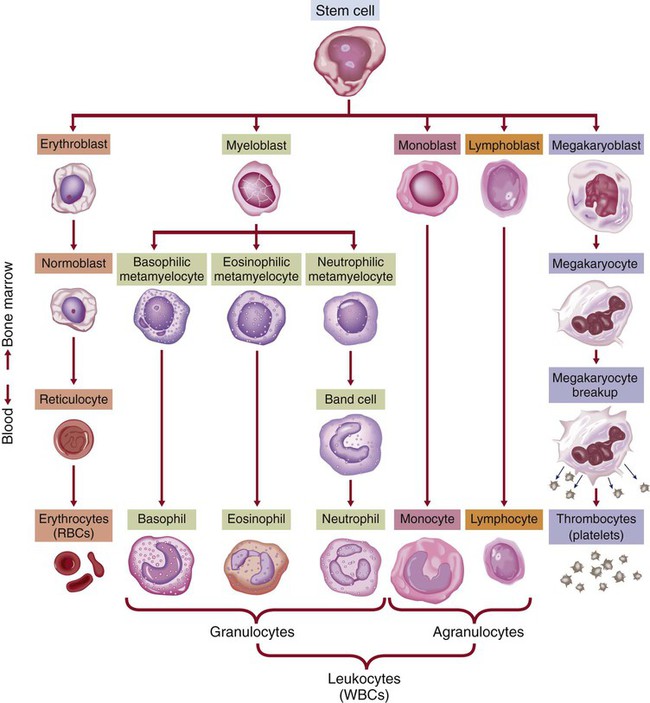
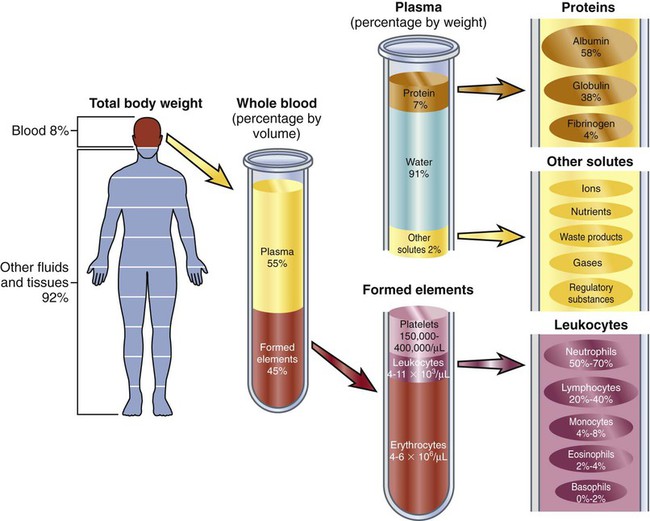
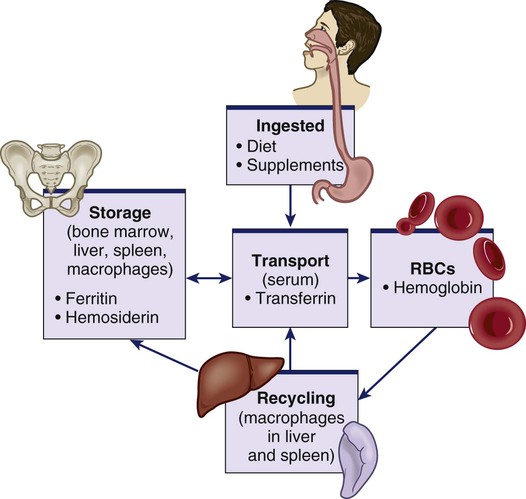
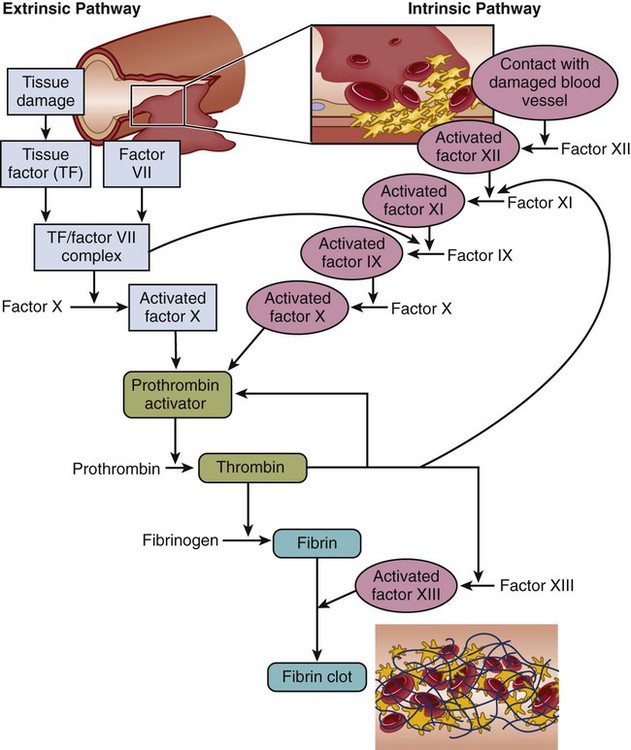
Chapter 30 1. Describe the structures and functions of the hematologic system. 2. Differentiate among the different types of blood cells and their functions. 3. Explain the process of hemostasis. 4. Link the age-related changes in the hematologic system to differences in findings of hematologic studies. 5. Select the significant subjective and objective assessment data related to the hematologic system that should be obtained from a patient. 6. Describe the components of a physical assessment of the hematologic system. 7. Differentiate normal from common abnormal findings of a physical assessment of the hematologic system. 8. Describe the purpose, significance of results, and nursing responsibilities related to diagnostic studies of the hematologic system. eTABLE 30-1 DRUGS AFFECTING HEMATOLOGIC FUNCTION* *This represents only a partial listing of drugs affecting the hematologic system. All three types of blood cells (red blood cells [RBCs], white blood cells [WBCs], and platelets) develop from a common hematopoietic stem cell within the bone marrow. The hematopoietic stem cell is best described as an immature blood cell that is able to self-renew and to differentiate into hematopoietic progenitor cells. As the cells mature and differentiate, several different types of blood cells are formed (Fig. 30-1). The marrow responds to increased demands for various types of blood cells by increasing production via a negative feedback system. The bone marrow is stimulated by various factors or cytokines (e.g., erythropoietin, granulocyte colony-stimulating factor [G-CSF], stem cell factor, thrombopoietin) that cause differentiation of the stem cells into one of the committed hematopoietic cells (e.g., RBC). For example, when tissue hypoxia occurs, erythropoietin is secreted by the kidney and liver. It circulates to the bone marrow and causes differentiation of proerythroblasts in the bone marrow.1 Blood is a type of connective tissue that performs three major functions: transportation, regulation, and protection (Table 30-1). Blood has two major components: plasma and blood cells. In an adult weighing between 150 and 180 lb, the volume of blood is usually between 4.7 and 5.5 L (5 to 6 quarts). TABLE 30-1 Approximately 55% of blood is plasma (Fig. 30-2). Plasma is composed primarily of water, but it also contains proteins, electrolytes, gases, nutrients (e.g., glucose, amino acids, lipids), and waste. The term serum refers to plasma minus its clotting factors. Plasma proteins include albumin, globulin, and clotting factors (mostly fibrinogen). Most plasma proteins are produced by the liver, except for antibodies (immunoglobulins), which are produced by plasma cells. Albumin is a protein that helps maintain oncotic pressure in the blood.1 About 45% of the blood (see Fig. 30-2) is composed of formed elements, or blood cells. The three types of blood cells are erythrocytes (RBCs), leukocytes (WBCs), and thrombocytes (platelets). The primary function of erythrocytes is oxygen transportation, whereas the leukocytes are involved in protecting the body from infection. Platelets promote blood coagulation. Erythrocytes are primarily composed of a large molecule called hemoglobin. Hemoglobin, a complex protein-iron compound composed of heme (an iron compound) and globin (a simple protein), binds with oxygen and carbon dioxide. As RBCs circulate through the capillaries surrounding alveoli within the lung, oxygen attaches to the iron on the hemoglobin. The oxygen-bound hemoglobin is referred to as oxyhemoglobin and is responsible for giving arterial blood its bright red appearance. As RBCs flow to body tissues, oxygen detaches from the hemoglobin and diffuses from the capillary into tissue cells. Carbon dioxide diffuses from tissue cells into the capillary, attaches to the globin portion of hemoglobin, and is transported to the lungs for removal. Hemoglobin also acts as a buffer and plays a role in maintaining acid-base balance. This buffering function is described further in Chapter 17. Erythropoiesis (the process of RBC production) is regulated by cellular oxygen requirements and general metabolic activity. Erythropoiesis is stimulated by hypoxia and controlled by erythropoietin, a glycoprotein growth factor synthesized and released primarily by the kidney. Erythropoietin stimulates the bone marrow to increase erythrocyte production. Normally the bone marrow releases 3 × 109 RBC/kg of body weight/day. The normal life span of an erythrocyte is about 120 days. Erythropoiesis is also influenced by the availability of nutrients. Many essential nutrients are necessary for erythropoiesis, including protein, iron, folate (folic acid), cobalamin (vitamin B12), riboflavin (vitamin B2), pyridoxine (vitamin B6), pantothenic acid, niacin, ascorbic acid, and vitamin E.1 Erythrocyte production is also affected by endocrine hormones, such as thyroxine, corticosteroids, and testosterone. For example, hypothyroidism is often associated with anemia.2 Several distinct cell types evolve during erythrocyte maturation (see Fig. 30-1). The reticulocyte is an immature erythrocyte. The reticulocyte count measures the rate at which new RBCs appear in the circulation. Reticulocytes can develop into mature RBCs within 48 hours of release into the circulation. Therefore assessing the number of reticulocytes is a useful means of evaluating the rate and adequacy of erythrocyte production. Hemolysis (destruction of RBCs) by monocytes and macrophages removes abnormal, defective, damaged, and old RBCs from circulation. Hemolysis normally occurs in the bone marrow, liver, and spleen. Because one of the components of RBCs is bilirubin, hemolysis of these cells results in increased bilirubin to be processed by the body. When hemolysis occurs via normal mechanisms, the liver is able to conjugate and excrete all bilirubin that is released (see Fig. 31-2). Leukocytes (WBCs) appear white when separated from blood. Like the RBCs, leukocytes originate from stem cells within the bone marrow (see Fig. 30-1). There are different types of leukocytes, each with a different function. Leukocytes containing granules within the cytoplasm are called granulocytes (also known as polymorphonuclear leukocytes). Granulocytes include three types: neutrophils, basophils, and eosinophils. Leukocytes that do not have granules within the cytoplasm are called agranulocytes and include lymphocytes and monocytes. Lymphocytes and monocytes are also referred to as mononuclear cells because they have only one discrete nucleus. Leukocytes have a widely variable life span. Granulocytes may live only for hours, yet some T lymphocytes may live for years. The primary function of the granulocytes is phagocytosis, a process by which WBCs ingest or engulf any unwanted organism and then digest and kill it. They are able to migrate through vessel walls and to the sites where they are needed. The neutrophil is the most common type of granulocyte, accounting for 50% to 70% of all WBCs. Neutrophils are the primary phagocytic cells involved in acute inflammatory responses. Once they engulf the pathogen, they die in 1 to 2 days.1 Neutrophil production and maturation are stimulated by hematopoietic growth factors (e.g., G-CSF and granulocyte-macrophage colony-stimulating factor [GM-CSF])1 (see Table 14-3). Eosinophils account for only 2% to 4% of all WBCs. They have a similar but reduced ability for phagocytosis. One of their primary functions is to engulf antigen-antibody complexes formed during an allergic response. An elevated level of eosinophils is also seen in some neoplastic disorders, such as Hodgkin’s lymphoma, and in various skin diseases and connective tissue disorders.3 Eosinophils are also able to defend against parasitic infections. Lymphocytes, one of the agranular leukocytes, constitute 20% to 40% of the WBCs. Lymphocytes form the basis of the cellular and humoral immune responses (see Chapter 14). Two lymphocyte subtypes are B cells and T cells. Although T cell precursors originate in the bone marrow, these cells migrate to the thymus gland for further differentiation into T cells. Natural killer (NK) cells are lymphocytes that do not require prior exposure to antigens to kill virus-infected cells and activate T cells and phagocytes. Most lymphocytes transiently circulate in the blood and also reside in lymphoid tissues. (Details of lymphocyte function are presented in Chapter 14.) Monocytes, the other type of agranular leukocytes, account for approximately 4% to 8% of the total WBCs. Monocytes are potent phagocytic cells that ingest small or large masses of matter, such as bacteria, dead cells, tissue debris, and old or defective RBCs. These cells are only present in the blood for a short time before they migrate into the tissues and become macrophages (see Chapter 13). In addition to macrophages that have differentiated from monocytes, tissues also contain resident macrophages. These resident macrophages are given special names (e.g., Kupffer cells in the liver, osteoclasts in the bone, alveolar macrophages in the lung). These macrophages protect the body from pathogens at these entry points and are more phagocytic than monocytes. Macrophages also interact with lymphocytes to facilitate the humoral and cellular immune responses (see Chapter 14). The other one third of iron is stored as ferritin and hemosiderin (degraded form of ferritin) in the bone marrow, spleen, liver, and macrophages (Fig. 30-3).When the stored iron is not replaced, hemoglobin production is reduced. As part of normal iron metabolism, iron is recycled after macrophages in the liver and spleen phagocytize, or ingest and destroy, old and damaged RBCs. Iron binds to transferrin in the plasma or is stored as ferritin or hemosiderin (see Fig. 30-3). Only about 3% is lost daily in urine, sweat, bile, and epithelial cells in the gastrointestinal (GI) tract. Therefore there is normally very little iron loss except from blood loss. When a blood vessel is injured, an immediate local vasoconstrictive response occurs. Vasoconstriction reduces the leakage of blood from the vessel not only by restricting the vessel size but also by pressing the endothelial surfaces together. The latter reaction enhances vessel wall stickiness and maintains closure of the vessel even after vasoconstriction subsides. Vascular spasm may last for 20 to 30 minutes, allowing time for the platelet response and plasma clotting factors to be activated. The platelet response and plasma clotting factors are triggered by endothelial injury and the release of substances such as tissue factor (TF).1 The formation of a visible fibrin clot on the platelet plug is the conclusion of a complex series of reactions involving different clotting (coagulation) factors. The plasma clotting factors are labeled with both names and Roman numerals (Table 30-2). Plasma proteins circulate in inactive forms until stimulated to initiate clotting through one of two pathways, intrinsic or extrinsic (see Fig. 30-4). The intrinsic pathway is activated by collagen exposure from endothelial injury when the blood vessel is damaged. The extrinsic pathway is initiated when tissue factor or tissue thromboplastin is released extravascularly from injured tissues. TABLE 30-2 Regardless of whether clotting is initiated by substances internal or external to the blood vessel, coagulation ultimately follows the same final common pathway of the clotting cascade. Thrombin, in the common pathway, is the most powerful enzyme in the coagulation process (see Fig. 30-4). It converts fibrinogen to fibrin, which is an essential component of a blood clot. The second means of maintaining blood in its fluid form is fibrinolysis, a process resulting in the dissolution of the fibrin clot. The fibrinolytic system is initiated when plasminogen is activated to plasmin (Fig. 30-5). Thrombin is one of the substances that can activate the conversion of plasminogen to plasmin, thereby promoting fibrinolysis. The plasmin attacks either fibrin or fibrinogen by splitting the molecules into smaller elements known as fibrin split products (FSPs) or fibrin degradation products (FDPs). (More information about FSPs can be found in Table 30-7 later in this chapter and in the discussion of disseminated intravascular coagulation in Chapter 31.) The lymph nodes, which are also a part of the lymphatic system, are round, oval, or bean shaped and vary in size according to their location. Structurally, the nodes are small clumps of lymphatic tissue and are found in groups along lymph vessels at various sites. More than 200 lymph nodes are found throughout the body, with the greatest number being in the abdomen surrounding the GI tract. Lymph nodes are situated both superficially and deep. The superficial nodes can be palpated, but evaluation of the deep nodes requires radiologic examination.4 A primary function of lymph nodes is filtration of pathogens and foreign particles that are carried by lymph to the nodes. The liver functions as a filter. It also produces all the procoagulants that are essential to hemostasis and blood coagulation. Additionally, it stores iron that is in excess of tissue needs, which can occur with frequent blood transfusions or diseases that cause iron overload. Hepcidin, produced by the liver, is a key regulator of iron balance. The synthesis of hepcidin is stimulated by iron overload or inflammation. Hepcidin reduces the release of stored iron from enterocytes (in the intestines) and macrophages.5 Thus when iron is deficient, hepatocytes produce less hepcidin. Other functions of the liver are described in Chapters 39 and 44. Physiologic aging is a gradual process that involves cell loss and organ atrophy. Aging leads to a decrease in bone marrow mass and cellularity and an increase in bone marrow fat.6 However, peripheral blood cell concentrations in healthy older adults are similar to those of younger adults.7 Although the older adult is still capable of maintaining adequate blood cell levels, the reserve capacity leaves the older adult more vulnerable to possible problems with clotting, transporting oxygen, and fighting infection, especially during periods of increased demand. This results in a diminished ability of an older adult to compensate for an acute or chronic illness.6,7 Hemoglobin levels begin to decrease in both men and women after middle age, with the low-normal levels seen in most older people. Total serum iron, total iron-binding capacity, and intestinal iron absorption are all decreased in older adults. Iron deficiency is usually responsible for the low hemoglobin levels. Healthy older patients are not able to produce reticulocytes in response to hemorrhage or hypoxemia as well as younger adults.1 The total WBC count and differential are generally not affected by aging. However, decreases in humoral antibody response and T cell function may occur.6,8 During an infection, the older adult may have only a minimal elevation in the total WBC count. These laboratory findings suggest a diminished bone marrow reserve of granulocytes in older adults and reflect the possible impaired stimulation of hematopoiesis. The number of platelets is unaffected by the aging process, but functionally they may have increased adhesiveness.9 Changes in vascular integrity related to aging can manifest as easy bruising. The effects of aging on hematologic studies9–11 are presented in Table 30-3. Immune changes related to aging are presented in Chapter 14. TABLE 30-3 GERONTOLOGIC ASSESSMENT DIFFERENCES MCHC, Mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume. A complete medication history of prescription and over-the-counter drugs is an important component of a hematologic assessment. Specifically address the use of vitamins, herbal products, or dietary supplements because many patients may not consider them to be drugs. Many drugs may interfere with normal hematologic function (see eTable 30-1 available on the website for this chapter).12–14 Herbal therapy can interfere with clotting (see Complementary & Alternative Therapies box in Chapter 38 on p. 851). Antineoplastic agents used to treat malignant disorders (see Chapter 16) and antiretroviral agents used to treat human immunodeficiency virus (HIV) infection (see Chapter 15) may cause bone marrow depression. A patient previously treated with chemotherapy agents, particularly alkylating agents, has a higher risk of developing a secondary malignancy of leukemia or lymphoma. A patient on long-term anticoagulant therapy such as warfarin (Coumadin) could be at risk for bleeding problems.
Nursing Assessment
Hematologic System
Structures and Functions of Hematologic System
Bone Marrow
Drug Class
Hematologic Effects
Antidysrhythmics (e.g., procainamide [Pronestyl], digoxin)
Anemia, hemolytic anemia, thrombocytopenia, neutropenia
Antiplatelet agents (e.g., abciximab [ReoPro], clopidogrel [Plavix], ticlopidine [Ticlid]))
Interfere with platelet function, thrombocytopenia, hemolysis
Antiseizure/antiparkinsonian (e.g., phenytoin [Dilantin], carbamazepine [Tegretol], levodopa)
Anemia, hemolytic anemia, neutropenia, thrombocytopenia
Antihypertensives (e.g., captopril)
Neutropenia
Antimicrobials
• Aminoglycosides (e.g., vancomycin)
Thrombocytopenia, neutropenia
• amphotericin B (Fungizone)
Anemia, thrombocytopenia
• Cephalosporins (e.g., cefotaxime)
Anemia, hemolytic anemia, neutropenia
• penicillin, piperacillin
Anemia, hemolytic anemia, neutropenia, thrombocytopenia, platelet dysfunction
dapsone
Anemia, hemolytic anemia, neutropenia
isoniazid (INH), rifampin
Neutropenia, anemia, hemolytic anemia, thrombocytopenia
ganciclovir
Anemia, neutropenia, thrombocytopenia
trimethoprim/sulfamethoxazole (Bactrim, Septra)
Anemia, neutropenia, thrombocytopenia
Antineoplastics (e.g., alkylating agents, antitumor antibiotics, platinol agents, antimitotics)
Anemia, neutropenia, leukopenia, thrombocytopenia
Antiretrovirals (e.g., raltegravir [Isentress])
Anemia, neutropenia
Corticosteroids (e.g., dexamethasone [Decadron], hydrocortisone, prednisolone)
Lymphopenia, neutrophilia, thrombosis
Diuretics (e.g., loop diuretics, thiazide diuretics)
Anemia, neutropenia, thrombocytopenia, thrombosis, interfere with platelet function
Histamine (H2)-blocking agents (e.g., ranitidine [Zantac], cimetidine [Tagamet])
Aplastic anemia, neutropenia, thrombocytopenia, interfere with platelet production
Hormonal agents (e.g., tamoxifen, oral contraceptives)
Increase in coagulation and thromboemboli formation
Immunosuppressants (e.g., azathioprine [Imuran], cyclosporine, tacrolimus [Prograf])
Lymphopenia, thrombocytopenia (TTP), hemolytic anemia
Nonsteroidal antiinflammatory agents (e.g., ibuprofen [Motrin, Advil], naproxen [Naprosyn])
Anemia, leukopenia, neutropenia, thrombocytopenia, inhibit platelet aggregation
Phenothiazines (e.g., chlorpromazine [Thorazine], prochlorperazine [Compazine])
Aplastic anemia, thrombocytopenia, neutropenia
Salicylates (e.g., aspirin and aspirin-containing compounds [e.g., Percodan])
Interfere with platelet function, thrombocytopenia, neutropenia, aplastic anemia
Tricyclic antidepressants
Interfere with platelet function, neutropenia, thrombocytopenia
Miscellaneous
allopurinol (Zyloprim)
Neutropenia, anemia, thrombocytopenia
dextran
Interferes with platelet function
Anticoagulants (e.g., heparin, argatroban)
Interfere with clotting factors, thrombocytopenia, thrombosis, anemia
Blood
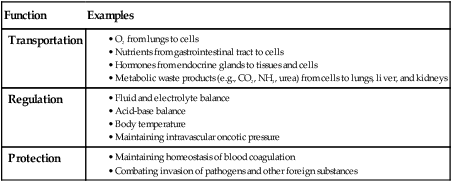
Function
Examples
Transportation
Regulation
Protection
Plasma.
Blood Cells.
Erythrocytes.
Leukocytes.
Granulocytes.
Lymphocytes.
Monocytes.
Normal Iron Metabolism
Normal Clotting Mechanisms
Vascular Response.
Plasma Clotting Factors.
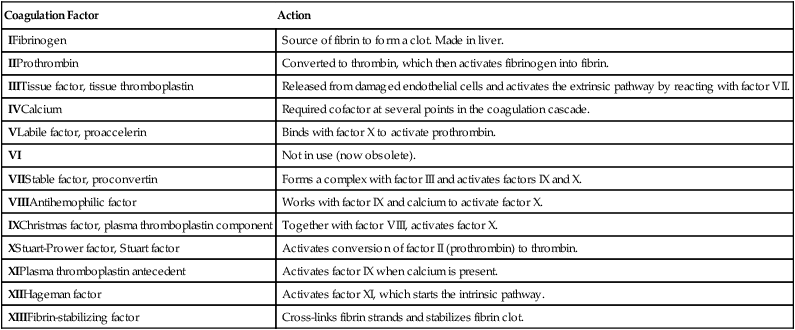
Coagulation Factor
Action
IFibrinogen
Source of fibrin to form a clot. Made in liver.
IIProthrombin
Converted to thrombin, which then activates fibrinogen into fibrin.
IIITissue factor, tissue thromboplastin
Released from damaged endothelial cells and activates the extrinsic pathway by reacting with factor VII.
IVCalcium
Required cofactor at several points in the coagulation cascade.
VLabile factor, proaccelerin
Binds with factor X to activate prothrombin.
VI
Not in use (now obsolete).
VIIStable factor, proconvertin
Forms a complex with factor III and activates factors IX and X.
VIIIAntihemophilic factor
Works with factor IX and calcium to activate factor X.
IXChristmas factor, plasma thromboplastin component
Together with factor VIII, activates factor X.
XStuart-Prower factor, Stuart factor
Activates conversion of factor II (prothrombin) to thrombin.
XIPlasma thromboplastin antecedent
Activates factor IX when calcium is present.
XIIHageman factor
Activates factor XI, which starts the intrinsic pathway.
XIIIFibrin-stabilizing factor
Cross-links fibrin strands and stabilizes fibrin clot.
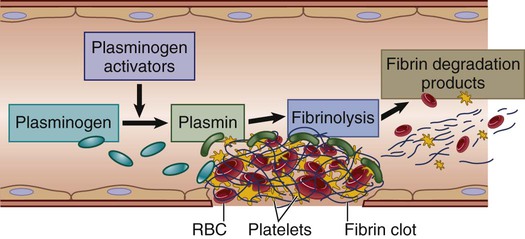
Lysis of Clot.
Lymph System
Liver
Gerontologic Considerations
Hematologic System
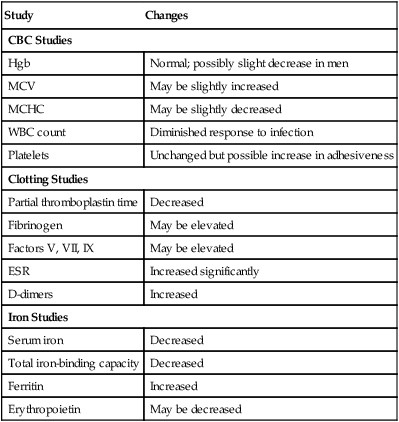
Effects of Aging on Hematologic Studies
Study
Changes
CBC Studies
Hgb
Normal; possibly slight decrease in men
MCV
May be slightly increased
MCHC
May be slightly decreased
WBC count
Diminished response to infection
Platelets
Unchanged but possible increase in adhesiveness
Clotting Studies
Partial thromboplastin time
Decreased
Fibrinogen
May be elevated
Factors V, VII, IX
May be elevated
ESR
Increased significantly
D-dimers
Increased
Iron Studies
Serum iron
Decreased
Total iron-binding capacity
Decreased
Ferritin
Increased
Erythropoietin
May be decreased
Assessment of Hematologic System
Subjective Data
Important Health Information
Medications.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nursing Assessment: Hematologic System
Get Clinical Tree app for offline access