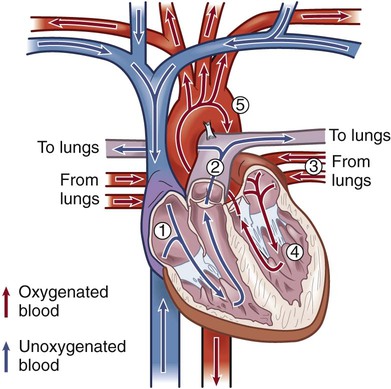
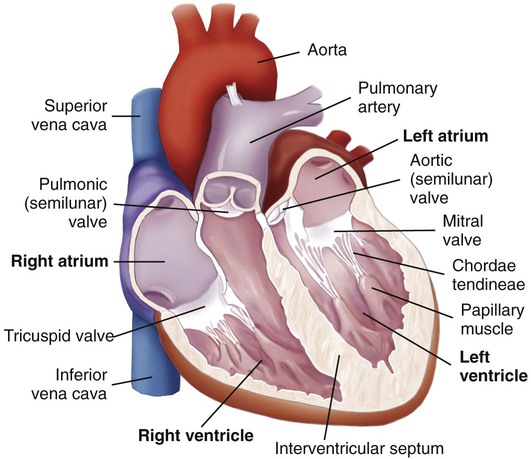
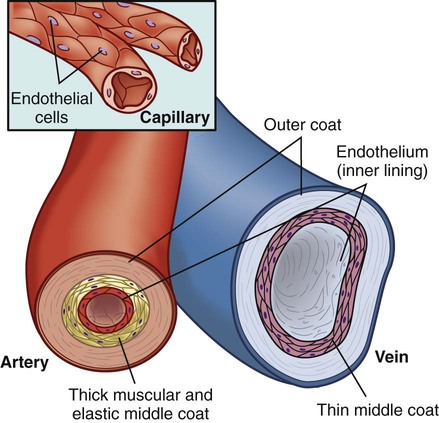
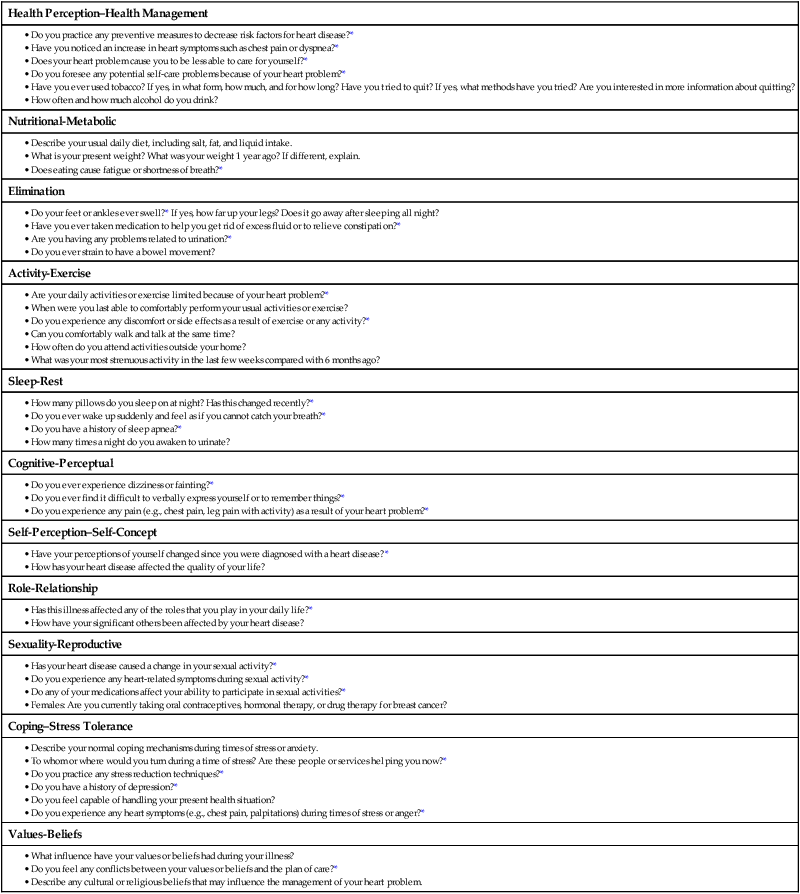
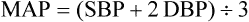Chapter 32 Angela DiSabatino and Linda Bucher 1. Differentiate the anatomic location and function of the following cardiac structures: pericardial layers, atria, ventricles, semilunar valves, and atrioventricular valves. 2. Relate the coronary circulation to the areas of heart muscle supplied by the major coronary arteries. 3. Differentiate the structure and function of arteries, veins, capillaries, and endothelium. 4. Describe the mechanisms involved in the regulation of blood pressure. 5. Relate the various waveforms on a normal electrocardiogram to the associated cardiac events. 6. Select essential assessment data related to the cardiovascular system that should be obtained from a patient and/or caregiver. 7. Select appropriate techniques to use in the physical assessment of the cardiovascular system. 8. Differentiate normal from abnormal findings of a physical assessment of the cardiovascular system. 9. Link the age-related changes of the cardiovascular system to the differences in assessment findings. 10. Describe the purpose, significance of results, and nursing responsibilities related to diagnostic studies of the cardiovascular system. The heart is a four-chambered hollow muscular organ normally about the size of a fist. It lies within the thorax in the mediastinal space that separates the right and left pleural cavities. The heart is composed of three layers: a thin inner lining, the endocardium; a layer of muscle, the myocardium; and an outer layer, the epicardium. The heart is covered by a fibroserous sac called the pericardium. This sac consists of two layers: the inside (visceral) layer of the pericardium (part of the epicardium) and the outer (parietal) layer. A small amount of pericardial fluid (approximately 10 to 15 mL) lubricates the space between the pericardial layers (pericardial space) and prevents friction between the surfaces as the heart contracts.1 The heart is divided vertically by the septum. The interatrial septum creates a right and left atrium, and the interventricular septum creates a right and left ventricle. The thickness of the wall of each chamber is different. The atrial myocardium is thinner than that of the ventricles, and the left ventricular wall is two or three times thicker than the right ventricular wall.1 The thickness of the left ventricle is necessary to produce the force needed to pump the blood into the systemic circulation. The blood flow through the heart is illustrated in Fig. 32-1. The four valves of the heart serve to keep blood flowing in a forward direction. The cusps of the mitral and tricuspid valves are attached to thin strands of fibrous tissue termed chordae tendineae (Fig. 32-2). Chordae are anchored in the papillary muscles of the ventricles. This support system prevents the eversion of the leaflets into the atria during ventricular contraction. The pulmonic and aortic valves (also known as semilunar valves) prevent blood from regurgitating into the ventricles at the end of each ventricular contraction. The myocardium has its own blood supply, the coronary circulation (Fig. 32-3). Blood flow into the two major coronary arteries occurs primarily during diastole (relaxation of the myocardium). The left coronary artery arises from the aorta and divides into two main branches: the left anterior descending artery and the left circumflex artery. These arteries supply the left atrium, the left ventricle, the interventricular septum, and a portion of the right ventricle. The right coronary artery also arises from the aorta, and its branches supply the right atrium, the right ventricle, and a portion of the posterior wall of the left ventricle. In 90% of people the atrioventricular (AV) node and the bundle of His receive blood supply from the right coronary artery. For this reason, blockage of this artery often causes serious defects in cardiac conduction. The conduction system is specialized nerve tissue responsible for creating and transporting the electrical impulse, or action potential. This impulse starts depolarization and subsequently cardiac contraction (Fig. 32-4, A). The electrical impulse is normally started by the sinoatrial (SA) node (the pacemaker of the heart). Each impulse coming from the SA node travels through interatrial pathways to depolarize the atria, resulting in a contraction. The electrical activity of the heart can be detected on the body surface using electrodes and is recorded on an electrocardiogram (ECG). The letters P, QRS, T, and U are used to identify the separate waveforms (Fig. 32-4, B). The first wave, P, begins with the firing of the SA node and represents depolarization of the atria. The QRS complex represents depolarization from the AV node throughout the ventricles. There is a delay of impulse transmission through the AV node that accounts for the time between the beginning of the P wave and the beginning of the QRS wave. The T wave represents repolarization of the ventricles. The U wave, if seen, may represent repolarization of the Purkinje fibers, or it may be associated with hypokalemia.2 Intervals between these waves (PR, QRS, and QT intervals) reflect the time it takes for the impulse to travel from one area of the heart to another. These time intervals can be measured, and changes from these time references often indicate pathologic conditions. (See Chapter 36 for a complete discussion of ECG monitoring.) Frank-Starling law states that, to a point, the more the myocardial fibers are stretched, the greater their force of contraction. The volume of blood in the ventricles at the end of diastole, before the next contraction, is called preload. Preload determines the amount of stretch placed on myocardial fibers. Preload can be increased by a number of conditions such as myocardial infarction, aortic stenosis, and hypervolemia.3 Contractility can be increased by epinephrine and norepinephrine released by the sympathetic nervous system. Increasing contractility raises the SV by increasing ventricular emptying. Afterload is the peripheral resistance against which the left ventricle must pump. Afterload is affected by the size of the ventricle, wall tension, and arterial blood pressure (BP). If the arterial BP is elevated, the ventricles meet increased resistance to ejection of blood, increasing the work demand. Eventually this results in ventricular hypertrophy, an enlargement of the cardiac muscle tissue without an increase in CO or the size of chambers (see Fig. 35-1, B). The arterial system differs from the venous system by the amount and type of tissue that make up arterial walls (Fig. 32-5). The large arteries have thick walls composed mainly of elastic tissue. This elastic property cushions the impact of the pressure created by ventricular contraction and provides recoil that propels blood forward into the circulation. Large arteries also contain some smooth muscle. Examples of large arteries are the aorta and the pulmonary artery. Veins are large-diameter, thin-walled vessels that return blood to the right atrium (see Fig. 32-5). The venous system is a low-pressure, high-volume system. The larger veins contain semilunar valves at intervals to maintain the blood flow toward the heart and to prevent backward flow. The amount of blood in the venous system is affected by a number of factors, including arterial flow, compression of veins by skeletal muscles, alterations in thoracic and abdominal pressures, and right atrial pressure. The autonomic nervous system consists of the sympathetic nervous system and the parasympathetic nervous system (see Chapter 56). The source of neural control of blood vessels is the sympathetic nervous system. The alpha1 (α1)-adrenergic receptors are located in vascular smooth muscles. Stimulation of α1-adrenergic receptors results in vasoconstriction. Decreased stimulation to α1-adrenergic receptors causes vasodilation. (Sympathetic nervous system receptors that influence BP are presented in Table 33-1.) Chemoreceptors are located in the aortic and carotid bodies and the medulla. They are capable of causing changes in respiratory rate and BP in response to increased arterial CO2 pressure (hypercapnia) and, to a lesser degree, decreased plasma pH (acidosis) and arterial O2 pressure (hypoxia). When the chemoreceptors in the medulla are triggered, they stimulate the vasomotor center to increase BP.4 The two main factors influencing BP are cardiac output (CO) and systemic vascular resistance (SVR): SVR is the force opposing the movement of blood. This force is created primarily in small arteries and arterioles. Normal blood pressure is SBP less than 120 mm Hg and DBP less than 80 mm Hg5 (see Chapter 33). BP can be measured by invasive and noninvasive techniques. The invasive technique consists of catheter insertion into an artery. The catheter is attached to a transducer, and the pressure is measured directly (see Chapter 66). As the pressure in the cuff is lowered, auscultate the artery for Korotkoff sounds. There are five phases of Korotkoff sounds. The first phase is a tapping sound caused by the spurt of blood into the constricted artery as the pressure in the cuff is gradually deflated. This sound is noted as the SBP. The fifth phase occurs when the sound disappears, which is noted as the DBP.6 BP is recorded as SBP/DBP (e.g., 120/80 mm Hg). Sometimes, an auscultatory gap occurs, which is a loss of sound between the SBP and the DBP. Proper BP technique (e.g., using the correct cuff size, positioning arm at heart level) is essential for accurate readings6 (see Table 33-11). Another noninvasive way to measure BP indirectly is an automated device that uses oscillometric measurements to assess BP. Though this method does not involve auscultation, the same attention to proper technique is essential for accuracy. Finally, SBP (and pulse) can be assessed using a Doppler ultrasonic flowmeter. The hand-held transducer is positioned over the artery (identified by audible, pulsatile sounds). The cuff is applied above the artery, inflated until the sounds disappear, and then another 20 to 30 mm Hg beyond that point. The cuff is then slowly deflated until sounds return. This point is the SBP.6 One of the greatest risk factors for cardiovascular disease (CVD) is age. CVD remains the leading cause of death in adults older than age 85. It is the most common cause of hospitalization and the second leading cause of death in adults younger than age 85. The most common cardiovascular problem is coronary artery disease (CAD) secondary to atherosclerosis. It is difficult to separate normal aging changes from the pathophysiologic changes of atherosclerosis. Many of the physiologic changes in the cardiovascular system of older adults are a result of the combined effects of the aging process, disease, environmental factors, and lifetime health behaviors rather than just age alone.7 Age-related changes in the cardiovascular system and differences in assessment findings are presented in Table 32-1. With increased age, the amount of collagen in the heart increases and elastin decreases. These changes affect the myocardium’s ability to stretch and contract. One of the major changes in the cardiovascular system is the response to physical or emotional stress. In times of increased stress, CO and SV decrease due to reduced contractility and HR response. The resting supine HR is not markedly affected by aging. When the patient changes positions (e.g., sits upright), the sympathetic nerve pathway may be affected by fibrous tissue and fatty deposits, resulting in a blunted HR response.7 TABLE 32-1 GERONTOLOGIC ASSESSMENT DIFFERENCES The number of pacemaker cells in the SA node decreases with age. By age 75, a person may have only 10% of the normal number of pacemaker cells. Although this is compatible with adequate SA node function, it may account for the frequency of some sinus dysrhythmias in older adults. Similar decreases also occur in the number of conduction cells in the internodal tracts, bundle of His, and bundle branches. These changes contribute to the development of atrial dysrhythmias and heart blocks. About 50% of older adults have an abnormal resting ECG that shows increases in the PR, QRS, and/or QT intervals.8 Arterial and venous blood vessels thicken and become less elastic with age.9 Arteries increase their sensitivity to vasopressin (antidiuretic hormone). With aging both of these changes contribute to a progressive increase in SBP and a decrease or no change in DBP. Thus an increase in the pulse pressure is found. Hypertension is not a normal consequence of aging, and should be treated. Valves in the large veins in the lower extremities have a reduced ability to return the blood to the heart, often resulting in dependent edema. Orthostatic hypotension, which is estimated to be present in more than 30% of patients over age 70 with systolic hypertension, may be related to medications and/or decreased baroreceptor function.8 Postprandial hypotension (decrease in BP of at least 20 mm Hg that occurs within 75 minutes after eating) may also occur in about a third of otherwise healthy older adults. Both orthostatic and postprandial hypotension may be related to falls in older adults. Despite the changes associated with aging, the heart is able to function adequately under most circumstances. Assess the patient’s current and past use of medications. This includes over-the-counter (OTC) drugs, herbal supplements, and prescription drugs. For example, aspirin prolongs the blood clotting time, and is found in many drugs used to treat cold symptoms. List all of the patient’s drugs. Include dosage, time of last dose, and the patient’s understanding of the drug’s purpose and side effects. Many noncardiac drugs can adversely affect the cardiovascular system and should be assessed (Table 32-2). TABLE 32-2 CARDIOVASCULAR EFFECTS OF NONCARDIAC DRUGS* †Second-generation NSAIDs, known as COX-2 inhibitors, have been linked to an increased risk of serious adverse cardiovascular events. The strong correlation between components of a patient’s lifestyle and cardiovascular health supports the need to review each functional health pattern. Key questions to ask a person with a cardiovascular problem are listed in Table 32-3. TABLE 32-3 HEALTH HISTORY
Nursing Assessment
Cardiovascular System
Structures and Functions of Cardiovascular System
Heart
Structure.
Blood Flow Through Heart.
Cardiac Valves.
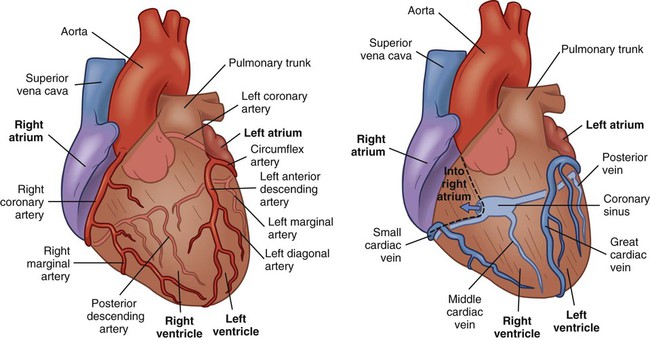
Blood Supply to Myocardium.
Conduction System.
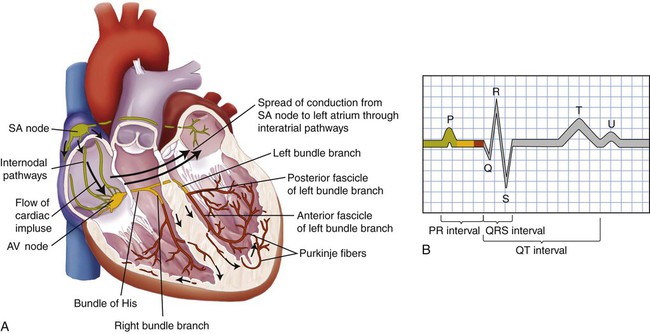
B, The normal electrocardiogram (ECG) pattern. The P wave represents depolarization of the atria. The QRS complex indicates depolarization of the ventricles. The T wave represents repolarization of the ventricles. The U wave, if present, may represent repolarization of the Purkinje fibers, or it may be associated with hypokalemia. The PR, QRS, and QT intervals reflect the time it takes for the impulse to travel from one area of the heart to another.
Electrocardiogram.
Mechanical System.
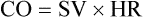
Factors Affecting Cardiac Output.
Vascular System
Blood Vessels.
Arteries and Arterioles.
Veins and Venules.
Regulation of Cardiovascular System
Autonomic Nervous System.
Effect on Blood Vessels.
Chemoreceptors.
Blood Pressure
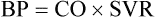
Measurement of Arterial Blood Pressure.
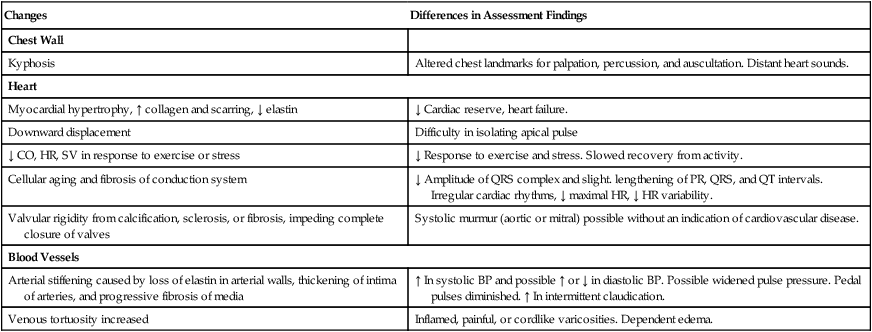
Gerontologic Considerations
Effects of Aging on the Cardiovascular System
Cardiovascular System
Changes
Differences in Assessment Findings
Chest Wall
Kyphosis
Altered chest landmarks for palpation, percussion, and auscultation. Distant heart sounds.
Heart
Myocardial hypertrophy, ↑ collagen and scarring, ↓ elastin
↓ Cardiac reserve, heart failure.
Downward displacement
Difficulty in isolating apical pulse
↓ CO, HR, SV in response to exercise or stress
↓ Response to exercise and stress. Slowed recovery from activity.
Cellular aging and fibrosis of conduction system
↓ Amplitude of QRS complex and slight. lengthening of PR, QRS, and QT intervals. Irregular cardiac rhythms, ↓ maximal HR, ↓ HR variability.
Valvular rigidity from calcification, sclerosis, or fibrosis, impeding complete closure of valves
Systolic murmur (aortic or mitral) possible without an indication of cardiovascular disease.
Blood Vessels
Arterial stiffening caused by loss of elastin in arterial walls, thickening of intima of arteries, and progressive fibrosis of media
↑ In systolic BP and possible ↑ or ↓ in diastolic BP. Possible widened pulse pressure. Pedal pulses diminished. ↑ In intermittent claudication.
Venous tortuosity increased
Inflamed, painful, or cordlike varicosities. Dependent edema.
Assessment of Cardiovascular System
Subjective Data
Important Health Information
Medications.
Drug Classification
Examples
Cardiovascular Effects
Anticancer agents
daunorubicin (Cerubidine)
doxorubicin (Adriamycin)
Dysrhythmias, cardiomyopathy
Antipsychotics
chlorpromazine (Thorazine)
haloperidol (Haldol)
Dysrhythmias, orthostatic hypotension
Corticosteroids
cortisone (Cortone)
prednisone (Orasone)
Hypotension, edema, potassium depletion
Hormone therapy, oral contraceptives
estrogen + progestin (Ortho-Novum, Prempro, Tri-Norinyl)
Myocardial infarction, thromboembolism, stroke, hypertension
Nonsteroidal antiinflammatory drugs (NSAIDs) †
ibuprofen (Motrin)
celecoxib (Celebrex)
Hypertension, myocardial infarction, stroke
Psychostimulants
cocaine
amphetamines
Tachycardia, angina, myocardial infarction, hypertension, dysrhythmias
Tricyclic antidepressants
amitriptyline (Elavil)
doxepin (Sinequan)
Dysrhythmias, orthostatic hypotension
Functional Health Patterns.
Cardiovascular System

Nursing Assessment: Cardiovascular System
Get Clinical Tree app for offline access