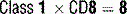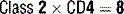Chapter 15 Immunology
Basic concepts
4 What is adaptive/acquired immunity?
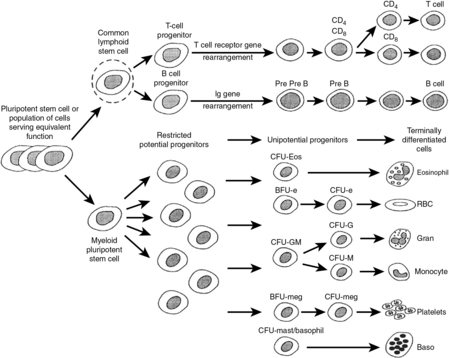
Adaptive immunity involves the response of B and T cells following exposure to an antigen to which they are specific. It is characterized by memory, resulting in rapid, stronger, and more efficient elimination of the source of the offending antigen upon repeat exposure to said antigen (Table 15-1). It is very important to recognize that stimulation of the adaptive immune response depends on prior activation of the innate immune system. Dendritic cells are considered to form the bridge between the innate and adaptive immune systems, because they present antigen to naive T cells. Stimulation of dendritic cell PRRs results in antigen processing and presentation to naive T cells via major histocompatibility complex (MHC)-T-cell receptor (TCR) interactions.
Table 15-1 Characteristics of Innate and Acquired Immunity
| Innate (Natural) | Acquired (Adaptive) | |
|---|---|---|
| Effector cells | Neutrophils (polymorphonuclear neutrophils), macrophages, eosinophils, basophils, mast cells, natural killer (NK) cells | B cells and plasma cells, T helper cells (e.g., TH1, TH2 cells), cytotoxic T lymphocytes (CTLs) |
| Chemical mediators | Complement, lysosomal enzymes, cytokines, interferons, acute-phase proteins | Antibodies (immunoglobulins), cytokines, granzyme, perforin |
| Response characteristics | Rapid, nonspecific; same intensity against all antigens, no memory | Slow, antigen-specific; long-term memory and enhanced response generated after first exposure (e.g., more rapid and intense) |
9 What are the five classes (isotypes) of immunoglobulins? Describe their respective distributions in the body
Table 15-2 Immunoglobulin Isotypes
| Immunoglobulin Isotype | Location | Functions |
|---|---|---|
| IgA | Dimeric IgA, joined by a J chain, is found in secretions Monomeric IgA is found in the blood | Found in mucosal secretions (e.g., tears, saliva, colostrum, gastrointestinal secretions); mediates mucosal immunity Poor activator of complement |
| IgD | Surface of mature B cells; some found in the blood | Unclear |
| IgE | Bound to FcεR1 receptors on the surface of tissue mast cells and blood basophils | Mediates type 1 hypersensitivity Mediates parasitic killing via ADCC (eosinophils possessing Fcε receptors are the effector cells and induce damage with major basic protein) |
| IgG | Found in the blood; crosses placenta Has the longest half-life of all isotopes and is thus used for passive immunization | Ligand for Fc receptors Activates classical complement pathway Most abundant immunoglobulin in secondary immune response Highest affinity for antigen (greatest strength of interaction between antigen and antibody) |
| IgM | Found in the blood, often in pentamers (joined by J chains), on the surface of immature and mature B cells | Activates classical complement pathway Earliest antibody produced in any humoral response Most abundant immunoglobulin in primary immune response Highest avidity for antigen (greatest number of antigen-binding sites as a result of pentameric form) |
ADCC, antibody-dependent cellular cytotoxicity.
10 Most humans can produce 106 to 109 unique immunoglobulin (Ig) molecules. However, the number of immunoglobulin genes is orders of magnitude less than this. How is this possible?
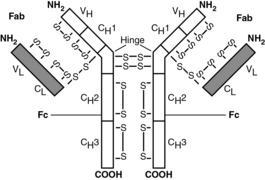
For the purpose of the USMLE, it is not important to memorize the detailed mechanism by which V(D)J recombination occurs. Rather, focus on understanding the significance of recombination—that is, the diversity of antigen recognition sites results from the recombination process. It is, however, particularly high-yield to understand details regarding general antibody structure (Fig. 15-2).
11 What are complement proteins and how do they function in an immune response?
Complement proteins comprise a network of soluble plasma proteins that become activated as a cascade by IgM and IgG (classical pathway) or by surface molecules of microorganisms (alternative and lectin pathways). The complement proteins have many important biologic activities. The membrane attack complex mediates cell lysis, whereas other components participate in opsonization, chemotaxis, neutralization of pathogens, and clearance of immune complexes (Fig. 15-3 and Table 15-3).
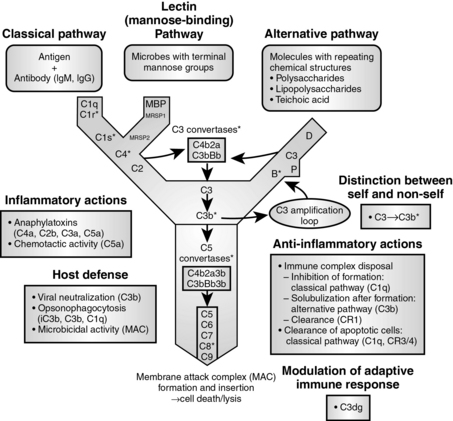
Figure 15-3 The complement cascade.
(From Mandell GL, Bennett JE, Dolin R: Principles and Practice of Infectious Disease, 6th ed. Philadelphia, Churchill Livingstone, 2005.)
Table 15-3 Complement Pathway Components and Activity
| Biologic Activity | Complement Component(s) |
|---|---|
| Cell lysis | C5b-C9 (membrane attack complex [MAC]) |
| Degranulation of mast cells and basophils | C3a, C4a, C5a (anaphylatoxins) |
| Opsonization of particulate antigens | C3b, C4b, iC3b (opsonins) |
| Chemotaxis of leukocytes (mainly polymorphonuclear neutrophils) | C5a, C3a, C5b67 (chemotactic factors) |
| Viral neutralization | C3b, C5b-9 |
| Solubilization and clearance of immune complexes | C3b |
12 Describe the ramifications of the most common complement protein deficiencies
Table 15-4 Complement Protein Deficiencies
| Complement Protein(s) | Deficiencies |
|---|---|
| C1 esterase inhibitor | Deficiency of C1 esterase inhibitor results in C1 esterase overactivity and overproduction of anaphylatoxins, leading to recurrent episodes of angioedema; this is also known as hereditary angioneurotic edema and is inherited in an autosomal dominant fashion. C1 esterase inhibitor also is inhibited directly by bradykinin, which explains the rare but life-threatening angioedema that may result as a side effect of ACE inhibitors (ACE is responsible for the degradation of bradykinin). |
| C2 or C4 | These deficiencies often resemble autoimmune diseases (e.g., systemic lupus erythematosus, vasculitis) but frequently are asymptomatic. C2 deficiency is the most common complement deficiency and also may be associated with septicemia (typically due to S. pneumoniae). |
| C3 | Reduced levels of C3b predispose patient to recurrent pyogenic infections as a consequence of decreased opsonization. Red blood cells also recognize antigen-antibody-C3b complexes in circulation and transport them to the liver or spleen for phagocytic degradation. Decreased C3b levels in serum thus predispose patients to type III hypersensitivity reactions due to decreased immune complex clearance from circulation. |
| C5-C8 or mannan-binding lectin (MBL), or mannose-binding protein | These greatly increase risk of Neisseria infections; of note, C5b-C9 forms the MAC, which can kill most unencapsulated gram-negative organisms. |
| Decay-accelerating factor (DAF) or CD55 and CD59) | This protein is located on the surface of all human cells and destabilizes C3 convertase and C5 convertase, preventing MAC formation, thereby protecting human cells from lysis; deficiency is manifested as an increase in complement-mediated hemolysis, clinically apparent as paroxysmal nocturnal hemoglobinuria. DAF deficiency is diagnosed with the Ham test, which checks to see whether the fragility of red blood cells increases when placed in a mildly acidic solution. |
ACE, angiotensin-converting enzyme; MAC, membrane attack complex.
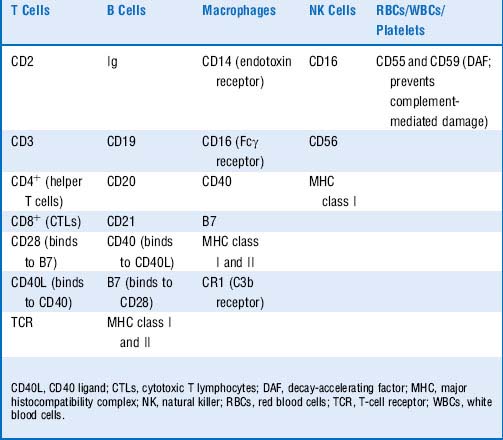
14 As a review, list the effector functions of the major leukocyte classes
Table 15-5 Leukocytes and their Effector Functions
| Cell | General Description | Effector Mechanism |
|---|---|---|
| Monocyte | A phagocytic cell that constitutes 4-10% of the WBCs in peripheral blood. Several hours after release from the bone marrow, monocytes will die or migrate into the tissue and differentiate in macrophages or dendritic cells. | Phagocytosis |
| Macrophage | This is a highly phagocytic tissue-dwelling cell. Major functions include phagocytosis of particulate material, antigen presentation to T cells, and secretion of IL-1, TNF, IL-8, and IL-12. | Antimicrobicidal activity includes generation of both oxygen-dependent mediators (e.g., superoxide anion, hydrogen peroxide, hypoclorous acid) and oxygen-independent mediators (e.g., TNF-α, lysozyme, defensins, hydrolytic enzymes). |
| Dendritic cell | This potent antigen-presenting cell forms an extensive web in tissues for trapping antigen (e.g., Langerhan cells in the epidermis). | Phagocytosis |
| Neutrophil | This type of granulocyte makes up ~ 70% of WBCs in peripheral blood. Neutrophils are active phagocytes that are the first cell to arrive at sites of inflammation. | Like macrophages, neutrophils employ both oxygen-dependent and oxygen-independent pathways to generate antimicrobial substances. |
| Eosinophil | This type of granulocyte makes up 2-5% of the WBCs in peripheral blood. It is a phagocytic cell that migrates into the tissue spaces, where it plays a role in defense against parasitic organisms. | Exocytosis of granules contains extremely basic proteins. |
| Basophil | This nonphagocytic granulocyte makes up 0.5-1% of peripheral WBCs. Basophils play a major role in allergic responses. | Basophils release pharmacologically active substances from cytoplasmic granules (histamine and other vasoactive amines) on cross-linking of surface-bound IgE by allergen. |
| Mast cell | This tissue-dwelling cell plays a role in allergic responses similar to that of basophils. Mast cells have Fcε receptors (for IgE) and histamine-containing granules. | This cell releases pharmacologically active substances from cytoplasmic granules (histamine and other vasoactive amines) on cross-linking of surface-bound IgE by allergen. |
| Helper T cell | This CD4+ lymphocyte matures in the thymus and functions in cytokine production. Helper T cells play a central role in the immune response by regulating the function of cells such as CTLs, B cells, NK cells, and macrophages. Helper T cells are activated by foreign antigen in the context of MHC class II molecules. | TH1 cells: IL-12 induces their differentiation (from TH0 cells); secretes IFN-γ (activates macrophages), and IL-2 activates CTLs and propagates the response) TH2 cells: IL-4 induces their differentiation; activate B cells to plasma cells with IL-4 and IL-5. |
| Cytotoxic T cell | This CD8+ lymphocyte matures in the thymus and functions in direct cell killing upon activation by foreign antigen presented by MHC class I molecules. | Perforins, granzymes, IFN-γ, TNF-β, FasL |
| B cell | This CD19+CD20+ lymphocyte has membrane-bound immunoglobulin. Upon activation by helper T cells, B cells may differentiate into plasma cells, which produce large volumes of antibodies. By presenting endocytosed antigen in the cleft of MHC class II, B cells also function as antigen-presenting cells in the activation of helper T cells. | Antibody |
| NK cell | This is a large granular lymphocyte that has no markers in common with B or T cells and is not MHC-restricted. NK cells act to lyse virally infected cells (via ADCC) and tumor cells with decreased levels of MHC class I. | Perforins, granzymes, IFN-γ, TNF-α |
ADCC, antibody-dependent cellular cytotoxicity; CTLs, cytotoxic T lymphocytes; FasL, Fas ligand; IFN-γ, interferon-γ; IgE, immunoglobulin E; IL, interleukin; MHC, major histocompatibility complex; NK, natural killer; TNF, tumor necrosis factor; WBCs, white blood cells.
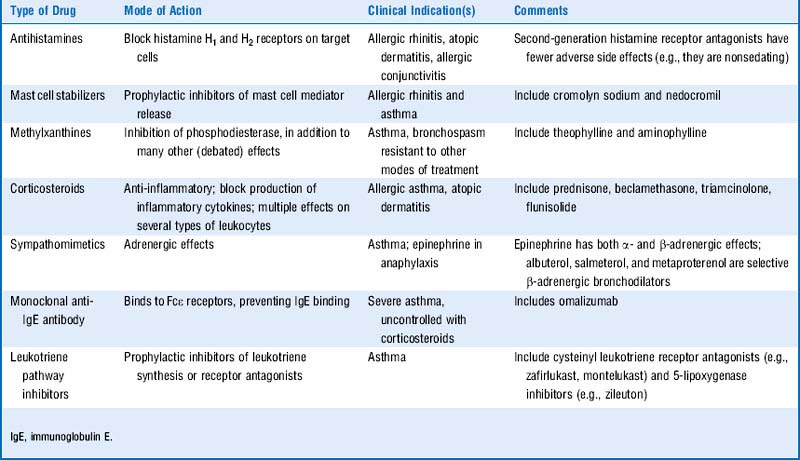
15 List the functions of the major cytokines secreted by various classes of immune cells
7 Why did the child NOT have a reaction to amoxicillin when it was first administered for his previous ear infection?
8 What clinical testing can be performed to confirm that this immediate hypersensitivity reaction was caused by amoxicillin?
10 Why was the child given diphenhydramine and methylprednisolone?
Diphenhydramine (Benadryl) is an H1 receptor antagonist and will ameliorate the histamine-mediated components of anaphylaxis.
Methylprednisolone is a corticosteroid that acts synergistically with epinephrine by upregulating adrenergic receptors. It also blocks a portion of the late phase of the allergic response by demarginalizing neutrophils (preventing subsequent chemotaxis), reducing eosinophil counts, and blocking phospholipase A2 (the enzyme that produces arachidonic acid, which is eventually converted into prostaglandins and leukotrienes). Note that although corticosteroid administration decreases plasma counts of many leukocyte subtypes, it increases neutrophil count secondary to decreased adhesion molecule synthesis. This reduces adhesion of polymorphonuclear cells (PMNs) to the endothelial wall and thus allows more to enter the circulation.
Related questions
11 What was the motivation for developing the second-generation H1 receptor antagonists such as fexofenadine (Allegra) and loratadine (Claritin)?
12 List the common classes of drugs that are used to treat type I hypersensitivity disorders and their general mode of action
Summary Box: Anaphylactic Shock
 Anaphylactic shock has many potential manifestations, including bronchospasm, urticaria, hypotension, and gastrointestinal (GI) problems.
Anaphylactic shock has many potential manifestations, including bronchospasm, urticaria, hypotension, and gastrointestinal (GI) problems.
 Anaphylactic shock is a type I, IgE-mediated hypersensitivity reaction, with histamine and leukotrienes playing the major mediator roles.
Anaphylactic shock is a type I, IgE-mediated hypersensitivity reaction, with histamine and leukotrienes playing the major mediator roles.
 Epinephrine, diphenhydramine (an antihistamine), and corticosteroids are the most effective treatments for anaphylactic shock.
Epinephrine, diphenhydramine (an antihistamine), and corticosteroids are the most effective treatments for anaphylactic shock.
 See Table 15-8 for more information.
See Table 15-8 for more information.
4 What is the significance of a positive direct Coombs’ test and how does it support the diagnosis considered in this patient?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree