Robin Webb Corbett and Laura K. Williford Owens • Describe the drugs that alter uterine muscle contractility. • Compare systemic and regional medications for pain control during labor. • Describe the drugs used in gestational hypertension. eclampsia, p. 793 gestational hypertension, p. 793 HELLP syndrome, p. 793 L/S (lecithin/sphingomyelin) ratio, p. 792 preeclampsia, p. 793 preterm labor, p. 788 progesterone, p. 782 teratogens, p. 778 tocolytic therapy, p. 789 Because pregnancy is a change in the normal physiology of the body, the normal and expected pharmacokinetics and pharmacodynamics of medications also change. Changes in drug action during pregnancy include (1) effect of circulating steroid hormones on the liver’s metabolism of drugs; (2) reduced gastrointestinal motility and increased gastric pH; (3) increased glomerular filtration rate and increased renal perfusion, resulting in more rapid renal excretion of drugs; (4) expanded maternal circulating blood volume, resulting in dilution of drugs; and (5) alteration in the clearance of drugs in later pregnancy, resulting in a decrease in serum and tissue concentrations of drugs. Because of alterations in the normal physiology of the body, medications should not be ordered in lower doses with longer intervals between doses because of the possibility of subtherapeutic serum and tissue concentrations. Other factors, such as late pregnancy and labor, can alter the half-lives of some medications. Antibiotics and barbiturates are examples of medications that have shorter half-lives during pregnancy. In contrast to later pregnancy, labor can actually increase the half-life of some medications (e.g., analgesics, hypnotics, antibiotics); it is believed that drug clearance decreases as a result of transient reduced blood flow associated with uterine contractions when the patient is in a supine position. There is also concern about the effects of certain disease states on medication during pregnancy. Disorders like diabetes mellitus and gestational hypertension may result in decreased renal perfusion and subsequent drug accumulation. The placenta plays an important role in drug use and metabolism. It was once thought that the placenta played a barrier role, but it is now known that the placenta has an important function as the organ of exchange for numerous substances, including medications. It allows some substances to transfer quickly or slowly between mother and fetus, depending on variables such as (1) maternal and fetal blood flow; (2) molecular weight of the substance (low–molecular-weight substances cross more readily than do high–molecular-weight substances; most medications have a low molecular weight, so they readily cross the placenta); (3) degree of ionization of the drug molecule (the more ionized the molecule, the less readily it crosses the placenta); (4) degree of protein-binding (highly bound drugs do not cross readily); (5) metabolic activity of the placenta (the metabolic activity can biotransform molecules into active metabolites that can affect the fetus); and (6) maternal dose. Guidelines for medication administration during pregnancy must include determination that the benefits of prescribing a drug outweigh potential short- or long-term risks to the maternal-fetal system. Careful selection and monitoring for the minimum effective dose for the shortest interval in the therapeutic range are required. Consideration must be given to alterations related to the physiologic changes of pregnancy. Liver metabolism of medications is much slower in the fetus as a result of immaturity of the liver. Therefore, drug metabolism is slower in the fetus, which can cause more evident or longer drug effects than on the mother. The degree of fetal exposure to a drug and its breakdown products are more important to fetal outcome than the rate at which the drug is transported to the fetus. The mechanisms by which drugs cross the placenta are analogous to the way in which drugs infiltrate breast tissue. Lactation results in increased blood flow to the breasts, and drugs accumulate in adipose breast tissue through simple diffusion. Long-term effects on infants from drugs in breast milk are unknown, but medications that accumulate in breast milk are known, and the breastfeeding patient should be alerted to the potential accumulation. Despite prenatal education, public service announcements, and information conveyed through the media, use of legal and illicit drugs by pregnant patients continues. Additionally, health care providers may prescribe drugs for maternal disorders that indirectly affect the fetus. However, it is important to note that most drugs required by pregnant patients can be used safely. It is estimated that half the medications taken by pregnant patients are over-the-counter (OTC) drugs. The drugs most commonly ingested during pregnancy are iron supplements and vitamins, antiemetics, antacids, stool softeners, nasal decongestants, mild analgesics, and antibiotics. Pregnant patients should be discouraged from using OTC medications until they consult with their health care provider or pharmacist. Drugs conclusively determined to be safe for the embryo are limited in number. Clinical trials can be resources for reliable drug information; however, it is unethical to test for the safety and efficacy of medications in pregnant patients. Animal studies are required during drug testing, but the information obtained from such studies is difficult to extrapolate to humans. Case reports used for such information can be of limited value because they usually present isolated occurrences. A commonly used source of information about drug safety in pregnancy is the U.S. Food and Drug Administration’s (FDA) category system. The categories were created to assist with safe prescribing and informed counseling of the pregnant patient requiring medication. TABLE 53-1 GENERAL ADVERSE EFFECTS OF SUBSTANCES COMMONLY ABUSED DURING PREGNANCY *Children with prenatal drug exposure scored significantly lower on measures of language, school readiness skills, impulse control, and visual attention span/sequencing at age 5 years when compared with nondrug-exposed children from a comparable environment. The most common indications for use of medications during pregnancy are nutritional supplementation with iron, vitamins, and minerals and treatment of nausea and vomiting, gastric acidity, and mild discomforts, but caution must be exercised (Herbal Alert 53-1). During pregnancy, approximately twice the normal amount of iron is needed to meet fetal and maternal daily requirements: 27 mg/day during pregnancy compared with 18 mg/day for nonpregnant patients 19 to 30 years of age. Supplementation with iron is not generally necessary until the second trimester, when the fetus begins to store iron; the goal is to prevent maternal iron-deficiency anemia, not to supply the fetus. The fetus is adequately supplied through the placenta, although the mother is deficient. Greatest iron demand occurs in the third trimester: 22.4 mg/day compared with 6.4 mg/day and 18.8 mg/day for the first and second trimesters, respectively. Although a normal diet generally provides the 18-mg recommended daily allowance (RDA) of iron for nonpregnant patients, nonanemic pregnant patients are usually instructed to supplement using a dosage that provides 60 mg of elemental iron; anemic patients should receive 120 mg of elemental iron. The elemental iron content of the most common iron salts includes ferrous sulfate 20% (300 mg of ferrous sulfate is equivalent to 60 mg elemental iron), exsiccated ferrous sulfate 30%, ferrous gluconate 12%, and ferrous fumarate 33%. The estimated net iron cost of pregnancy is approximately 800 to 1000 mg. This iron cost is calculated as 250 mg basal losses + 320 mg deposition in fetal and placental tissue + 500 mg increased hemoglobin mass + 350 mg iron loss in blood associated with delivery. Patients are advised to continue supplements for 6 weeks postpartum. Pregnant patients generally have decreased hematocrit early in the third trimester. Those with levels less than 30% will have their supplemental iron dosages increased and complete blood counts with platelet and ferritin measured. In those with true iron-deficiency anemia, response to iron supplementation is usually noted in 5 to 7 days, with a modest reticulocytosis and an increase in hemoglobin in 3 weeks. No teratogenic effects have been reported with physiologic doses. In contrast, increasing evidence has associated prenatal iron supplementation with glucose impairment and hypertension in midpregnancy. Many OTC and prescription iron products (Table 53-2) are available in varying dosages, which differ in the amount of elemental iron contained in the form of iron salts. TABLE 53-2 Common side effects of iron supplements include nausea, constipation, black tarry stools, gastrointestinal irritation, epigastric pain, vomiting, discoloration of urine, and diarrhea. Liquid forms can cause temporary tooth discoloration and therefore should be diluted and administered through a plastic straw. Iron supplements are best absorbed on an empty stomach and when administered with water or juice (concurrent administration of 200 mg ascorbic acid/vitamin C per 30 mg of elemental iron increases the absorption of iron). However, if gastric irritation does occur, administer with food. Iron supplementation may inhibit the absorption of several medications, and appropriate separation of doses should be followed (e.g., iron supplementation should be administered 2 hours before or 4 hours after antacids). Additional examples of medications that may require separation in dose include levodopa, levothyroxine (Levothroid, Synthroid), methyldopa, penicillamine (Cuprimine, Depen), quinolones, and tetracyclines. For the same reasons, do not administer iron with milk, cereal, tea, coffee, or eggs. Folic acid supplementation as part of preconception planning improves the outcome of pregnancy. During pregnancy, folic acid (vitamin B9, folate) is needed in increased amounts. Folic acid deficiency early in pregnancy can result in spontaneous abortion or birth defects, especially neural tube defects (failure of the embryonic neural tube to close properly, leading to spina bifida or skull and brain malformations). Deficiency of folic acid may also contribute to premature birth, low birth weight, and premature separation of the placenta (abruptio placentae). In the United States, approximately 4,000 pregnancies a year are affected by neural tube defects. Controlled clinical trials have demonstrated that folic acid supplementation can reduce this incidence by as much as 50%. The neural tube closes within the first 4 weeks of pregnancy (18 to 26 days after conception); therefore, it is important that women consume the recommended amounts of folic acid per day. For patients who have had a pregnancy that was affected by a neural tube defect, higher doses of folic acid are recommended: 4 mg starting 1 to 3 months before conception. The recommended amount should be ingested from folate-enriched foods and supplementation, because the amount of naturally occurring folic acid ingested in foods varies from day to day, and the folic acid from these sources is not well absorbed. Examples of folate-enriched foods are bread, cornmeal, rice, pasta, and cereal. Side effects of folic acid supplementation are not common but include allergic bronchospasm, rash, pruritus, erythema, and general malaise. Patients should be aware that folic acid supplementation may cause urine to turn more intensely yellow. Prenatal vitamin preparations are routinely recommended for pregnant women. These preparations generally supply vitamins A, D, E, C, B complex, B12, iron, calcium, and other minerals. The role of prenatal vitamins in preventing congenital defects (e.g., cleft lip or palate, limb defects) has not been determined. Inadequate nutrition cannot be rectified through supplements alone; vitamins are used most effectively by the body when taken with meals, and calories and protein are not supplied by supplements. Megadoses of vitamins and minerals during pregnancy will not improve health and may cause harm to the pregnant patient, the fetus, or both. Large doses of vitamin A can be teratogenic, and excessive ingestion of vitamins D, E, and K may also be toxic. The average prenatal patient uses three drugs during pregnancy, two of which are vitamin and mineral supplements. Drug ingestion is most likely during the first and third trimesters, when the discomforts of pregnancy tend to be most bothersome. Many complaints associated with pregnancy are related to the gastrointestinal (GI) tract (nausea and vomiting, heartburn, constipation). The etiology of nausea and vomiting is unclear. Physiologically, nausea is purported to be related to increased human chorionic gonadotropin (hCG) levels during pregnancy. Increased progesterone during pregnancy, which relaxes smooth muscle, contributes to heartburn and constipation. The physiologic reason is that elevated female sex hormones during pregnancy change the motility of the GI tract. Additionally, the enlarging uterus displaces the bowel. Nausea and vomiting (morning sickness) during early pregnancy are major complaints for most (about 88%) pregnant patients, but hyperemesis gravidarum (severe nausea and vomiting that may require hospitalization for hydration and nutrition) occurs with much lower incidence (1% to 3%). Nonpharmacologic measures to decrease nausea and vomiting include (1) eating crackers, dry toast, or other carbohydrates before rising; (2) avoiding high-fat or highly seasoned foods; (3) eating small, frequent meals; (4) drinking fluids between rather than with meals; (5) drinking apple juice or flat soda between meals; (6) eating a high-protein bedtime snack; (7) stopping smoking; and (8) taking an iron supplement at bedtime. These measures work well for most patients, but if vomiting is severe, fluid replacement and pharmacologic measures may be necessary. The FDA has approved one drug for morning sickness, doxylamine succinate and pyridoxine hydrocholoride (Diclegis). Antiemetic drug studies often find that affected patients rate even placebo agents as helpful. Table 53-3 lists drugs used for management of nausea and vomiting during pregnancy with their dosages, uses, and considerations. TABLE 53-3 DRUGS FOR MANAGEMENT OF NAUSEA AND VOMITING DURING PREGNANCY* Many patients may use ginger to help treat nausea and vomiting associated with pregnancy, but there is insufficient evidence of its safety and efficacy during pregnancy. Ingestion of large amounts may act as an abortifacient. Studies suggest that ginger can be safely used in moderation, but as with all medications and herbal supplements, encourage the pregnant patient to discuss use of ginger with her health care provider. Ginger can increase the risk for bleeding, particularly if the patient has a history of bleeding disorder. Research has demonstrated safe dosage levels up to 1,000 mg/day in pregnancy; therefore, pregnant patients are advised not to take more than 1,000 mg/day. Women who experience nausea and vomiting may experience gastric distress if they are also taking supplemental iron; taking the iron supplement with food or at bedtime, or temporarily suspending therapy, may help. Prenatal vitamins should be taken at the time of day the patient is least likely to experience emesis, because a high incidence of nausea and vomiting is associated with prenatal vitamins. For patients with continued iron-induced gastric distress, many health care providers recommend taking two children’s chewable multivitamins with iron. Salting food to taste may help replace vomited chloride; foods rich in potassium and magnesium may also help replace lost nutrients. Patients whose symptoms persist and who experience weight loss and dehydration may require intravenous (IV) rehydration, including replacement of electrolytes and vitamins. Antiemetic therapy (probably with phenothiazines) may be used, and ondansetron (Zofran) may be administered in severe cases of hyperemesis gravidarum. Heartburn (pyrosis) is a burning sensation in the epigastric and sternal regions that occurs with reflux of acidic stomach contents. The incidence of heartburn during pregnancy is common, up to 80%. Pregnant patients experience decreased motility in the GI tract as a result of the normal increase in the hormone progesterone. Progesterone also relaxes the cardiac sphincter (the sphincter leading into the stomach from the esophagus, also called the lower esophageal sphincter), making reflux activity (reverse peristalsis) more likely. Digestion and gastric emptying are slower than in the nonpregnant state. Heartburn is common when a pregnant patient sits or lies down soon after eating a normal meal, only to have her gravid uterus exert upward pressure on her stomach, causing increased reflux activity and the perception of hyperacidity. Heartburn is a disorder of the second and third trimesters of pregnancy. Nonpharmacologic measures are preferred in the management of heartburn. These include (1) limiting the size of meals; (2) avoiding highly seasoned or greasy foods; (3) avoiding gas-forming foods (e.g., cabbage, onions); (4) eating slowly and chewing thoroughly; (5) avoiding citrus juices; (6) drinking adequate fluids, but not with meals; and (7) avoiding reclining immediately after eating. Antacids should be considered first-line therapy if the patient does not respond to nonpharmacologic therapy. The antacids of choice for the pregnant patient include nonsystemic low-sodium products (those considered dietetically sodium-free) containing aluminum and magnesium (in the form of hydroxide) in combination. Discourage long-term use or large doses of magnesium antacids, because fetal renal, respiratory, cardiovascular, and muscle problems may result. Sucralfate (Carafate) is likely safe during pregnancy, because the drug is not systemically absorbed. Calcium carbonate antacid preparations may be avoided in pregnancy because of the rebound effect following acid neutralization. TUMS are frequently taken by pregnant patients for heartburn, but because TUMS are calcium based, excessive use may contribute to constipation. Most patients do not realize that remedies commonly used by nonpregnant patients (e.g., baking soda, antacids such as Alka-Seltzer) can be harmful during pregnancy. Selection of the wrong antacid can result in diarrhea, constipation, or electrolyte imbalance. A combination of nonpharmacologic measures and minimal use of safe antacids should effectively meet the pregnant patient’s needs. Liquid antacids are the preparations most commonly used in pregnancy because of their uniform dissolution, rapid action, and greater activity. Tablets are also acceptable, particularly for convenience, provided they are thoroughly chewed and the patient maintains adequate fluid intake. Histamine2 receptor antagonists can be used during pregnancy, but only if their use is recommended by a health care provider and initial treatment with antacids has failed. The teratogenicity of these medications is unknown; however, cimetidine (Tagamet), ranitidine (Zantac), famotidine (Pepcid), and nizatidine (Axid) have received the FDA’s pregnancy category B rating. H2 receptor antagonists work by competitively and reversibly binding to the histamine receptors of the parietal cells, causing a reduction in gastric acid secretion. The onset of action is generally in 1 hour and can persist for 6 to 12 hours. There is even less experience with the use of proton pump inhibitors. These medications work to suppress gastric acid secretion by inhibiting the proton pump on the surface of the parietal cells. With the release of omeprazole (Prilosec OTC), pregnant patients may wonder about its use for heartburn during pregnancy. Encourage patients to discuss the options with their health care provider. Currently, the use of omeprazole is limited to cases in which the benefits of therapy far outweigh the risks. Table 53-4 presents medications for heartburn commonly used during pregnancy. TABLE 53-4 OVER-THE-COUNTER ANTACIDS COMMONLY USED IN PREGNANCY *Dosage recommendations for antacid preparations should be clarified by the health care provider; however, as a general rule, no more than 12 tablets or 12 tsp should be taken in a 24-hour period, depending on the strength of the product. Major side effects are change in bowel habits (diarrhea or constipation), nausea, vomiting, alkalosis, and hypermagnesemia. Antacids figure in numerous drug interactions because of their increased action on gastric pH and their propensity to bind with other drugs to form poorly absorbed complexes. Antacids should not be taken within 2 hours of taking iron, digitalis products, tetracycline, or phenothiazine. Castor oil should be avoided during pregnancy, because it can stimulate uterine contractions. Mineral oil should also be avoided, because it can reduce the absorption of fat-soluble vitamins like vitamin K. (Low levels of vitamin K in the neonate can result in hemorrhage.) Through week 26 of pregnancy, headaches resulting from hormonally induced body changes, sinus congestion, or eye strain are quite common. It is not unusual for the pregnant patient to experience backaches, joint pains, round ligament pain (resulting in mild abdominal aches and twinges), and pain from minor injuries. Nonpharmacologic pain relief measures should be tried initially, including rest; calming environment; relaxation exercises; alteration in routine; mental imagery; ice packs; warm, moist heat; postural changes; correct body mechanics; and changes in footwear. Acetaminophen (Tylenol), a para-aminophenol analgesic, is a pregnancy category B drug. It is the most commonly ingested nonprescription drug during pregnancy. Acetaminophen may be used during all trimesters of pregnancy in therapeutic doses on a short-term basis for its analgesic and antipyretic effects. The drug is a weak prostaglandin inhibitor and does not have significant antiinflammatory effects. Prototype Drug Chart 26-1 lists the pharmacologic data for acetaminophen. The rate of absorption of acetaminophen is dependent on the rate of gastric emptying. Acetaminophen is 20% to 50% protein-bound and crosses the placenta during pregnancy; it is also found in low concentrations in breast milk. Acetaminophen is partially hepatically metabolized into inactive metabolites; however, a highly active metabolite (N-acetyl-p-benzoquinone) produced when the drug is taken in large doses can have potential liver and kidney toxicity. The half-life is 2 to 3 hours. There is no concrete evidence of fetal anomalies associated with the use of acetaminophen, and no adverse effects have been noted in breastfed infants of patients who used the drug while pregnant or breastfeeding. The maximum daily dose of acetaminophen (Tylenol) is 4000 mg per day, and the use of acetaminophen during pregnancy should not exceed 12 tablets per 24 hours of a 325-mg formulation (regular strength) or 8 tablets per 24 hours of a 500-mg (extra strength) formulation (because of the potential for kidney and liver toxicity). The drug should be taken at 4- to 6-hour intervals. Onset of effects after oral ingestion is within 10 to 30 minutes; peak action occurs at 1 to 2 hours; duration is from 3 to 5 hours. Manufacturers of OTC products containing acetaminophen have lowered the maximum daily dose recommendation to 3,000 mg due to the frequency of overdose with acetaminophen. One reason for the high frequency of overdose is that patients may take multiple OTC products that all contain acetaminophen. Most patients without preexisting renal or hepatic disease tolerate acetaminophen well. Patients with hypersensitivity to the compound should not use it. Acetaminophen should be used cautiously in patients who are at risk for infection because of the possibility of masking signs and symptoms. The most frequent adverse reactions are skin eruptions, urticaria, unusual bruising, erythema, hypoglycemia, jaundice, hemolytic anemia, neutropenia, leukopenia, pancytopenia, and thrombocytopenia. Aspirin, a salicylate, is classified as a mild analgesic. Aspirin is a pregnancy category C drug (which changes to category D if full-dose aspirin is used in the third trimester). It is a prostaglandin synthetase inhibitor with antipyretic, analgesic, and antiinflammatory properties. Teratogenic effects have not been shown conclusively, but the risk for anomalies is perceived to be small. Aspirin can inhibit the initiation of labor and actually prolong labor through its effects on uterine contractility; therefore, its use is not recommended during pregnancy. Aspirin use late in pregnancy is also associated with greater maternal blood loss at delivery, and there may be increased risk for anemia in pregnancy and of antepartum hemorrhage. Hemostasis is affected in the newborn whose mother ingested aspirin during the last 2 months of pregnancy (even without use during the week of delivery). Platelets are unable to aggregate to form clots, and it appears that this is not a reversible effect after delivery; the infant must wait for its own bone marrow to produce new platelets. (Refer to Chapter 26 for more information on aspirin.) Ibuprofen is classified as a pregnancy category C drug (which changes to category D if used in the third trimester). If taken late in pregnancy, it may cause premature closure of the ductus arteriosus. Ibuprofen is a prostaglandin synthetase inhibitor with antipyretic, analgesic, and antiinflammatory properties. Bleeding risks are similar to those reported with aspirin, though ibuprofen causes less inhibition of platelet aggregation than aspirin formulations.
Female Reproductive Cycle I
Pregnancy and Preterm Labor Drugs
Objectives
Key Terms
![]() http://evolve.elsevier.com/KeeHayes/pharmacology/
http://evolve.elsevier.com/KeeHayes/pharmacology/
Physiology of Pregnancy
![]() There are many known teratogens (substances that cause developmental abnormalities). Timing, dose, and duration of exposure are of crucial importance in determining the teratogenicity of a given drug. In humans, the teratogenic period begins 2 weeks after conception. During the first 2 weeks, the embryo is not susceptible to teratogenesis. At this time of development, exposure to teratogens may result in either death of the embryo or minor cellular damage without congenital birth defects. From 2 gestational weeks through the next 10 weeks is the period of organogenesis (development of major structures and organs). Table 53-1 shows adverse effects of selected illicit substances commonly used during pregnancy.
There are many known teratogens (substances that cause developmental abnormalities). Timing, dose, and duration of exposure are of crucial importance in determining the teratogenicity of a given drug. In humans, the teratogenic period begins 2 weeks after conception. During the first 2 weeks, the embryo is not susceptible to teratogenesis. At this time of development, exposure to teratogens may result in either death of the embryo or minor cellular damage without congenital birth defects. From 2 gestational weeks through the next 10 weeks is the period of organogenesis (development of major structures and organs). Table 53-1 shows adverse effects of selected illicit substances commonly used during pregnancy.
SUBSTANCE
MATERNAL EFFECTS
FETAL EFFECTS*
Alcohol (high risk: 6 oz or more/d)
1 oz (2 drinks) absolute alcohol 2 times/wk: increased risk for spontaneous abortion (2-4 times)
Fetal alcohol syndrome (FAS): mild to moderate mental retardation, altered facial features, growth retardation, low birth weight, small head circumference, hypotonia, and poor motor coordination. Full FAS seen only in some children; others display only fetal alcohol effect (FAE).
Caffeine
2 cups increase epinephrine concentrations after 30 min and decrease intervillous blood flow with potential for spontaneous abortion (dosage and gestational period related)
Excess consumption (>6-8 cups/d) likely toxic to embryo. No evidence of teratogenicity
Cocaine
48-h clearance via urine. Increased incidence of spontaneous abortion in first trimester. Continued use or sporadic use related to premature delivery and abruptio placentae secondary to placental vasoconstriction and hyperextension
4-5 d clearance time via urine of newborn because of liver immaturity and lack of cholinesterase. Intrauterine growth retardation, decreased head circumference, intrauterine cerebral infarction. No true withdrawal syndrome, but increased irritability, hyperreflexia, and tremulousness. Deficient organization and interactive abilities. By month 4, still exhibits hypertonicity, tremulousness, and impaired motor development. By month 6, effects may appear self-limiting, but long-term research is needed.
Heroin
First-trimester spontaneous abortion, premature delivery, inadequate maternal calorie and protein intake
Neonatal meconium aspiration syndrome; decreased weight and length through postnatal month 9 (weight and length catch up by month 12); smaller head circumference (with no catch-up); impaired interactive abilities (hard to console and engage); inconsistent behavioral responses; increased tremulousness and irritability
Marijuana
Heavy use (5 or more marijuana cigarettes per week): shortened gestation (<37 wk); may hasten delivery through uterine stimulation
No higher incidence of serious birth defects caused solely by marijuana. Higher incidence of meconium passage during labor
Tobacco/nicotine
Degenerative placental lesions with areas of poor oxygen exchange; higher incidence of abruptio placentae; placenta previa, vaginal bleeding during pregnancy; possible PROM; possible amnionitis; less likely to choose to breastfeed
Short stature, smaller head and arm circumferences; no increase in mortality rate or congenital anomalies (some evidence of increased oral clefts); increased respiratory infections beyond perinatal period; possible shorter attention span beyond perinatal period
Methadone
If taken before pregnancy, will need to slow detoxification during pregnancy and decrease dose 5 mg every 2 wk. Do not detoxify before week 14 of gestation because of increased risk for spontaneous abortion
Smaller weight and length through postnatal month 9 (catch up on weight and length by month 12); smaller head circumference (no catch-up); withdrawal-induced fetal distress if mother detoxifies after 32 weeks’ gestation
Barbiturates
CNS depression; lethargy; sleepiness; subtle mood alterations and impaired judgment/fine-motor skills for 24 h
No known inhibitory effect on uterine tone or contractility. Selective anticonvulsant activity without anesthesia effects may warrant use in pregnancy for seizure disorders
Active labor with imminent delivery is a contraindication, because no antagonist drug is available
Rapidly cross placenta; with excessive use/high doses cause CNS depression, leading to respiratory depression, hyperactivity, and decreased sucking reflex
Tranquilizers
Dose-dependent; toxic reactions include ataxia, syncope, vertigo, and drowsiness; control of acute eclamptic seizures during labor
Benzodiazepine (diazepam [Valium]) use in first trimester not associated with oral clefts or other anomalies. Chronic third trimester or labor exposure in high doses associated with hypotonia, hypothermia, hyperbilirubinemia, and poor sucking reflex. Effects may be enhanced if systemic analgesics also given to mother. Fetal effects are prolonged.
Therapeutic Drug and Herbal Use in Pregnancy
Iron
GENERIC (BRAND)
ROUTE AND DOSAGE
USES AND CONSIDERATIONS
ferrous sulfate (Fer-In-Sol, Feosol, Fero-Gradumet, Mol-Iron, Fer-Iron)
A: PO: 300-600 mg/d in divided doses
325 mg/d sufficient to meet needs of non–iron-deficient pregnant patient; with iron deficiency, administer 325 mg 2-3 times/d
Hematinic; for iron-deficiency anemia; prophylaxis for iron deficiency in pregnancy. Replaces iron stores needed for RBC development
Absorption PO is 5%-30% in intestines; therefore GI side effects. Toxic reactions include pallor, hematemesis, shock, cardiovascular collapse, and metabolic acidosis. Contraindicated in patients with hypersensitivity and peptic ulcer. Decreased absorption of tetracycline, penicillamine, and antacids; increased absorption with ascorbic acid; decreased absorption with eggs, milk, coffee, tea. Can reduce availability of zinc from diet
Take at bedtime to avoid GI upset. Absorption promoted when taken with orange juice or other vitamin C source. Use straw (elixir); swallow tab/cap whole; take with water on empty stomach. Sit upright 30 min after dose to decrease reflux. Increase fluids, activity, and dietary bulk. Keep out of reach of children
Peak reticulocytosis: 5-10 d; hemoglobin values increase: 2-4 wk
Pregnancy category: A; PB: UK; 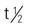 : UK; onset: 3-10 d; duration: 3-4 mo
: UK; onset: 3-10 d; duration: 3-4 mo
ferrous gluconate (Fergon)
A: PO: 200-600 mg t.i.d.
Same as above
ferrous fumarate (Fumasorb, Femiron, Feostat, Fumerin)
A: PO: 200 mg t.i.d./q.i.d.
Same as above
Adverse Reactions
Nursing Implications
Folic Acid
![]() Normally, the RDA for folic acid is 180 mcg, but the United States Preventive Services Task Force recommends that women who are planning pregnancy take a supplement containing 0.4 mg to 0.8 mg of folic acid 1 month before and for the first 2 to 3 months after conception. The American College of Obstetricians and Gynecologists (ACOG) recommends that all women of childbearing age ingest 400 mcg of folic acid daily for birth defect prevention (during pregnancy, the RDA rises to 600 mcg). The reasoning behind the ACOG’s recommendation is the high incidence of unplanned and unrecognized pregnancies.
Normally, the RDA for folic acid is 180 mcg, but the United States Preventive Services Task Force recommends that women who are planning pregnancy take a supplement containing 0.4 mg to 0.8 mg of folic acid 1 month before and for the first 2 to 3 months after conception. The American College of Obstetricians and Gynecologists (ACOG) recommends that all women of childbearing age ingest 400 mcg of folic acid daily for birth defect prevention (during pregnancy, the RDA rises to 600 mcg). The reasoning behind the ACOG’s recommendation is the high incidence of unplanned and unrecognized pregnancies.
Adverse Reactions
Multiple Vitamins
Drugs for Minor Discomforts of Pregnancy
Nausea and Vomiting
GENERIC (BRAND)
ROUTE AND DOSAGE
USES AND CONSIDERATIONS
Vitamin
pyridoxine (B6)
PO: Up to 25 mg t.i.d.
Coenzyme for various metabolic functions, including metabolism of proteins, carbohydrates, and fats.
Side effects are rare and include headache, nausea, somnolence, and sensory neuropathy
Pregnancy category: A; absorbed in jejunum and metabolized primarily in the liver.excreted in urine;  : 0.5 d; Peak plasma time: 5-6 h (pyridoxine)
: 0.5 d; Peak plasma time: 5-6 h (pyridoxine)
Antihistamines
meclizine (Antivert)
PO: 20-50 mg/d
Blocks CTZ, which acts on vomiting center; available OTC. Sites of action are labyrinth and CNS.
Side effects include dizziness, drowsiness, dry mouth and nose, blurred vision, diplopia, urinary retention, urticaria, rash, and headache. Cardiovascular effects include hypotension, palpitations, and tachycardia
Contraindicated in patients with hypersensitivity to drug or any component
Contraindicated in patients with closed-angle glaucoma Increased effect of alcohol, tranquilizers, and narcotics
Pregnancy category: B; metabolized in liver and excreted unchanged in feces and as metabolites in urine.  : 6 h; Onset: 1-2 h; Duration: 8-24 h
: 6 h; Onset: 1-2 h; Duration: 8-24 h
doxylamine (Unisom) (unlabeled use)
PO: 25 mg ( 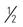 tab b.i.d.)
tab b.i.d.)
Considered mild, available OTC. Competes with free histamine for binding at H1 receptor sites. If pyrixodine (B6) 25 mg is not effective, health care provider may suggest docylamine 25 mg and pyridoxine 25 mg t.i.d. (1 tab h.s. and 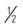 tab in morning and afternoon).
tab in morning and afternoon).
May cause sedative effects, thick bronchial secretions, drowsiness, dizziness, headache, constipation, abdominal pain with cramps, blurred vision, decreased coordination, and dry mouth
Contraindicated in patients with acute asthma and possibly hypertension, cardiovascular disorders, and hyperthyroidism
Caution: Take only under direction of health care provider.
Pregnancy category: A; 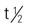 :1.25 h; Onset: 1-2 h
:1.25 h; Onset: 1-2 h
Antihistamine Plue Vitamin B6 Analog
doxylamine succineate (10 mg)/pyridoxine 10 mg (Diclegis)
PO: 2 tablets h.s. If dose controls symptoms the next day, continue taking 2 tablets at h.s. If symptoms persist into the afternoon of day 2, take 2 tablets at h.s., then 3 tablets starting on day 3 (1 tablet in the morning and 2 tablets at h.s.). If 3 tablets adequately control symptoms on day 4, continue taking 3 tablets daily. If symptoms persist, take 4 tablets starting on day 4 (1 tablet in morning, 1 tablet in mid-afternoon, and 2 tablets at h.s.
Mechanism of action: Unknown
Side effects: Somnolence, falls or other accidents in combined use with CNS depressants, including alcohol
Warnings/precautions: Contraindicated in women with known hypersensitivity to doxylamine succinate, other ethanolamine derivative antihistamines, and pyridoxine hydrochloride. Monoamine oxidase (MAO) inhibitors intensify and prolong the adverse central nervous system effects of Diclegis. Has anticholinergic properties and should be used with caution in women with asthma, increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, and urinary bladder-neck obstruction. Nursing mothers should not use.
Pregnancy category: A; absorbed in the jejunum and primarily metabolized in the liver; doxylamine is primarily excreted by the kidney; 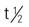 : 0.5 h; Peak plasma time: 7-8 h (doxylamine); 5-6 h (pyridoxine)
: 0.5 h; Peak plasma time: 7-8 h (doxylamine); 5-6 h (pyridoxine)
Phenothiazines
promethazine (Phenergan)
PO/IV/IM/PR: 12.5-25 mg q4-6h PRN
Blocks postsynaptic mesolimbic dopaminergic receptors in brain; exhibits strong alpha-adrenergic blocking effect and depresses release of hypothalamic and hypophyseal hormones; competes with histamine for H1 receptor
Side effects include dizziness, drowsiness, excitation, fatigue, insomnia, photosensitivity reactions, nausea, vomiting, and constipation
Contraindicated in patients with hypersensitivity to drug or any component, CNS depression, or coma
Use caution in patients with cardiovascular disease; not recommended for subQ or intraarterial administration; injection may contain sulfites, which may cause allergic reaction
Pregnancy category: C; 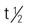 : 9-16 h; Onset: IM: 20 min; IV: 3-5 min; Duration: 2-6 h
: 9-16 h; Onset: IM: 20 min; IV: 3-5 min; Duration: 2-6 h
Anticholinergics
scopolamine
PO/IM/IV/subQ: 0.3-0.65 mg q4-6h
Antagonizes histamine and serotonin
Side effects include confusion, drowsiness, headache, fatigue, dry skin, constipation, vomiting, and bloated feeling. Cardiovascular side effects include orthostatic hypotension, ventricular fibrillation, tachycardia, and palpitations
Contraindicated in patients with hypersensitivity to drug or any component and those with narrow-angle glaucoma, acute hemorrhage, GI or GU obstruction, tachycardia secondary to cardiac insufficiency, and myasthenia gravis
Use with caution in patients with hepatic or renal impairment
Pregnancy category: C; Onset: IM: 0.5-1 h; Duration: 4-6 h
Prokinetic Agents
metoclopramide (Reglan)
PO: 10-15 mg q.i.d. 30 min a.c.
Blocks dopamine receptors in CTZ of CNS; causes enhanced motility and accelerated gastric emptying without stimulating secretions
Side effects include restlessness, drowsiness, diarrhea, weakness, and insomnia
Contraindicated in patients with hypersensitivity to drug or any component; GI obstruction, perforation, or hemorrhage; pheochromocytoma; or history of seizure disorder
Pregnancy category: B; 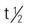 : 4-7 h; Onset: 0.5-1 h; Duration: 1-2 h
: 4-7 h; Onset: 0.5-1 h; Duration: 1-2 h
Other
trimethobenzamide (Tigan, T-Gen)
200 mg PR q6-8h
Obscure action; may be mediated through CTZ. Does not inhibit direct impulse to vomiting center. Chemically classified as ethanolamine derivative. Contraindicated in patients with cardiac dysrhythmias, narrow-angle glaucoma, asthma, and pyloroduodenal obstruction. Rectal doses of drug are more unpredictable
Side effects include drowsiness, headache, blurred vision, diarrhea, depression, hypotension, muscle cramps, allergic reactions, extrapyramidal symptoms, and blood dyscrasias
Contraindicated with benzocaine and in patients with hypersensitivity to drug. Use suppository form in neonates or preterm infants
Caution: Do not use in patients with acute emesis to avoid masking symptoms
Drug interactions with phenothiazines/barbiturates, belladonna
Pregnancy category: C; 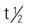 : UK; Onset: PO/PR: 10-40 min; IM: 15-35 min; Duration: 3-4 h
: UK; Onset: PO/PR: 10-40 min; IM: 15-35 min; Duration: 3-4 h
ondansetron (Zofran)
8 mg PO/IV every 8 h
Antiemetic; selective 5-HT3 receptor antagonist. Blocks serotonin, both peripherally on vagal nerve terminals and centrally in CMZ of CNS
Side effects include headache, constipation, diarrhea, malaise/fatigue
Contraindicated in patients with hypersensitivity to drug or other selective 5-HT3 antagonists
Caution: Use as scheduled, not PRN. Use with caution in patients who have congenital/medical conditions that cause prolonged QT interval or take medications that prolong QT interval
Drug interactions: drugs altering activity of liver enzymes
Pregnancy category: B; 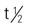 : 3-6 h; Onset: 30 min; Duration: 4-8 h
: 3-6 h; Onset: 30 min; Duration: 4-8 h
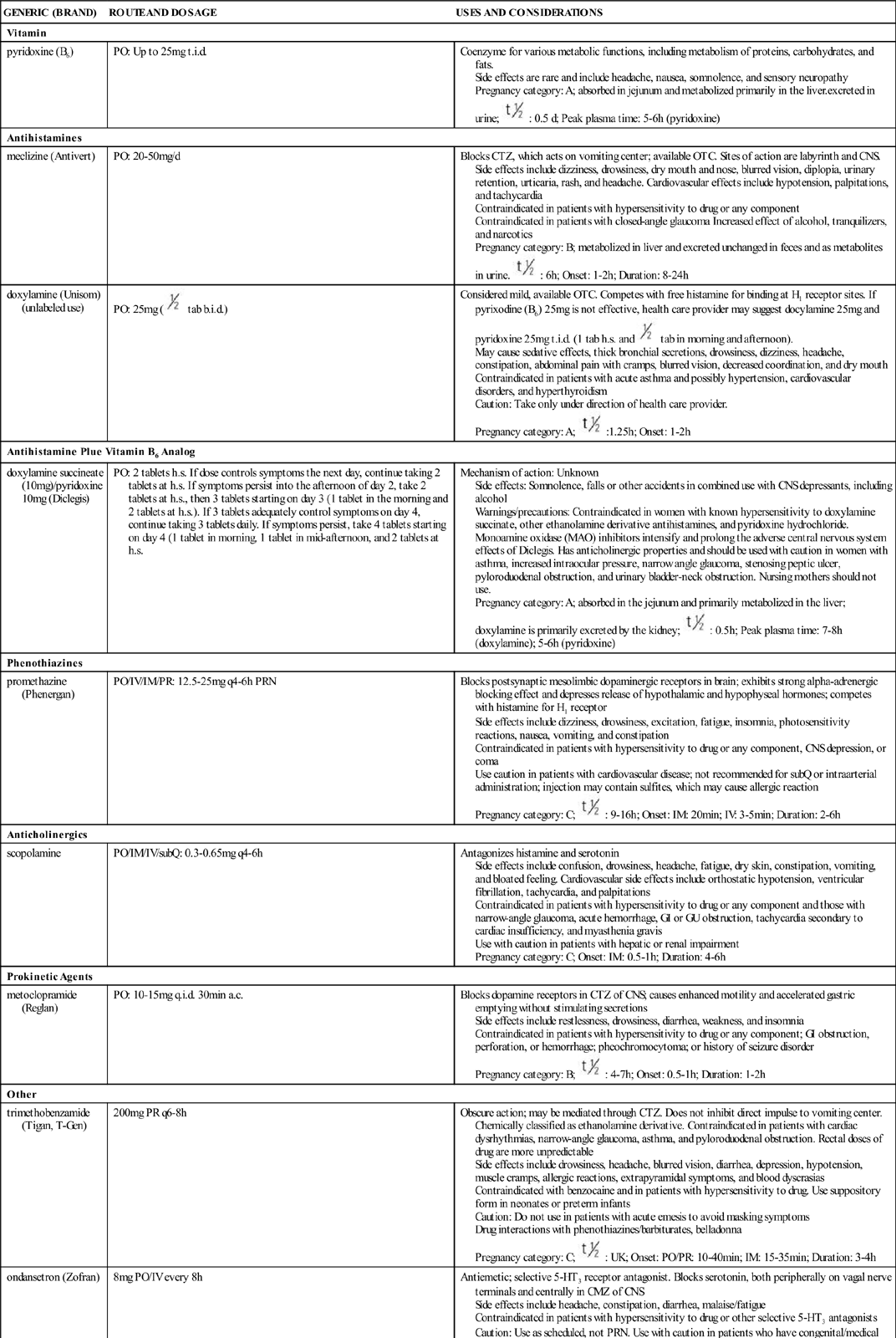

Heartburn
GENERIC (BRAND)
ROUTE AND DOSAGE
USES AND CONSIDERATIONS
aluminum hydroxide (Amphojel)
A: PO: As directed*
Contains 320 mg of aluminum hydroxide gel per 300-mg tab or per 5 mL; ANC 8; contains saccharin and sorbitol. OTC preparation.
For heartburn secondary to reflux
Neutralizes gastric acidity
Side effects include constipation; adverse reactions include dehydration, hypophosphatemia (long-term use), GI obstruction
Decreased effects with tetracycline, phenothiazine, benzodiazepines, isoniazid, digoxin; follow dose with water
Pregnancy category: C; PB: UK; 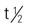 : UK; Onset: 15-30 min; Peak: 0.5 h; Duration: 1-3 h
: UK; Onset: 15-30 min; Peak: 0.5 h; Duration: 1-3 h
magnesium hydroxide and aluminum hydroxide with simethicone (Mylanta Extra Strength Liquid, Almacone)
40-125 mg PO q.i.d. after meals and h.s.; up to 500 mg/d
Mylanta Extra Strength Liquid: Each 5 mL contains 400 mg aluminum hydroxide, 400 mg magnesium hydroxide, 40 mg simethicone, parabens, saccharin, sorbitol, and 2 mg sodium
OTC preparation
Almacone tabs: Each tab contains 200 mg aluminum hydroxide, 200 mg magnesium hydroxide, and 20 mg simethicone
OTC preparation. Tabs must be chewed thoroughly.
Same as above with addition of antiflatulence action.
Neutralizes gastric acidity and antiflatulent.
Adverse effects include acid rebound. Aluminum-based antacids may cause constipation, while magnesium-based antacids have a laxative effect. Aluminum and magnesium–based combination antacids are given to balance the constipation and laxative effects. Do not administer magnesium-based antacids in patients with renal disease.
Drug interactions: Concurrent administration with digoxin, indomethacin (Indocin), or iron salts may decrease absorption of these drugs. Concurrent administration with chlordiazepoxide hydrochloride decreases rate of chlordiazepoxide absorption. Decreased pharmacologic effect with antacids and benzodiazepines, captopril, corticosteroids, fluoroquinolones, histamine H2 antagonists, hydantoins, ketoconazole, penicillamine (Cuprimine, Depen), phenothiazines, salicylates, and ticlopidine (Ticlid). Increased pharmacologic effect with levodopa, sulfonylureas, and valproic acid.
Pregnancy category: C; PB: UK; 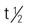 : UK
: UK
Constipation
![]() Constipation is a frequent occurrence during pregnancy. Its cause may be related to hormonal changes—specifically progesterone, which decreases GI motility. As with heartburn, nonpharmacologic treatments for constipation should be tried first. These include (1) increased fluid intake, (2) increased dietary fiber intake, and (3) moderate physical exercise. If these methods do not work, treatment is indicated, and the safest agents are bulk-forming preparations containing fiber (e.g., Metamucil), because they are not systemically absorbed. Also, docusate sodium (Colace), a stool softener, would be appropriate as first-line treatment during pregnancy. Agents that should be reserved for occasional use include milk of magnesia, magnesium citrate, lactulose (Constulose, Enulose), sorbitol, bisacodyl (Correctol, Dulcolax), and senna (Senokot, Ex-Lax).
Constipation is a frequent occurrence during pregnancy. Its cause may be related to hormonal changes—specifically progesterone, which decreases GI motility. As with heartburn, nonpharmacologic treatments for constipation should be tried first. These include (1) increased fluid intake, (2) increased dietary fiber intake, and (3) moderate physical exercise. If these methods do not work, treatment is indicated, and the safest agents are bulk-forming preparations containing fiber (e.g., Metamucil), because they are not systemically absorbed. Also, docusate sodium (Colace), a stool softener, would be appropriate as first-line treatment during pregnancy. Agents that should be reserved for occasional use include milk of magnesia, magnesium citrate, lactulose (Constulose, Enulose), sorbitol, bisacodyl (Correctol, Dulcolax), and senna (Senokot, Ex-Lax).
Pain
Acetaminophen
Pharmacokinetics
Pharmacodynamics
Aspirin and Ibuprofen
Antidepressant Drugs
![]() Exposure to antidepressant drugs and depressive disorders have both been associated with adverse birth outcomes. Adverse outcomes have included low birth weight, small for gestational age, preterm delivery, and increased neonatal irritability and decreased attentiveness. Use of selective serotonin reuptake inhibitors (SSRIs) in pregnancy is associated with low birth weight and small for gestational age infants. Preterm delivery (before 37 gestational weeks) is significantly higher in patients taking SSRIs and tricyclics. Although tricyclic antidepressant use in pregnancy has not been associated with structural malformations, in utero exposure has been linked to neonatal jitteriness and irritability. Poor neonatal adaptation, a term for transient symptoms like tachypnea, irritability, hypoglycemia, and weak cry, has been reported in neonates exposed to SSRIs in late pregnancy. Options for treatment for pregnant patient include psychotherapy alone or in conjunction with pharmacologic therapy as determined by the health care provider.
Exposure to antidepressant drugs and depressive disorders have both been associated with adverse birth outcomes. Adverse outcomes have included low birth weight, small for gestational age, preterm delivery, and increased neonatal irritability and decreased attentiveness. Use of selective serotonin reuptake inhibitors (SSRIs) in pregnancy is associated with low birth weight and small for gestational age infants. Preterm delivery (before 37 gestational weeks) is significantly higher in patients taking SSRIs and tricyclics. Although tricyclic antidepressant use in pregnancy has not been associated with structural malformations, in utero exposure has been linked to neonatal jitteriness and irritability. Poor neonatal adaptation, a term for transient symptoms like tachypnea, irritability, hypoglycemia, and weak cry, has been reported in neonates exposed to SSRIs in late pregnancy. Options for treatment for pregnant patient include psychotherapy alone or in conjunction with pharmacologic therapy as determined by the health care provider.
Female Reproductive Cycle I: Pregnancy and Preterm Labor Drugs
Get Clinical Tree app for offline access

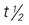 , half-life; t.i.d., three times a day; tab, tablet; UK, unknown; wk, week.
, half-life; t.i.d., three times a day; tab, tablet; UK, unknown; wk, week.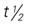 , half-life; t.i.d., three times a day; tab, tablet; q.i.d., four times a day; UK, unknown.
, half-life; t.i.d., three times a day; tab, tablet; q.i.d., four times a day; UK, unknown.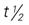 , half-life; tab, tablet; tsp, teaspoon; UK, unknown.
, half-life; tab, tablet; tsp, teaspoon; UK, unknown.








