Drugs for Men’s Health and Reproductive Disorders
Objectives
• Differentiate common conditions for which androgen therapy and antiandrogen therapy are indicated.
• Describe patients for whom androgen therapy is particularly risky.
• Assess patients for therapeutic and adverse effects of androgen therapy.
• Categorize commonly prescribed medications that can impair male sexual function.
Key Terms
Addison’s disease, p. 880
anabolism, p. 877
androgens, p. 874
antiandrogens, p. 878
cryptorchidism, p. 876
delayed puberty, p. 879
ejaculatory dysfunction, p. 880
erectile dysfunction, p. 880
gynecomastia, p. 876
hirsutism, p. 877
hypogonadism, p. 875
hypothyroidism, p. 880
inhibited sexual desire, p. 880
oligospermia, p. 876
priapism, p. 876
spermatogenesis, p. 876
testosterone, p. 874
virilization, p. 877
The authors gratefully acknowledge the work of Karen Carmody, who updated this chapter for the eighth edition.
Reproductive health requires the production of adequate quantities of various hypothalamic, pituitary, and gonadal hormones as well as the appropriate hormone receptors. It requires normal development and patency of the reproductive tract. In addition, reproductive health implies that men and women at developmentally appropriate life stages are fertile (i.e., able to produce gametes [sperm or eggs]). Finally, reproductive health entails the ability to engage in sexual intercourse with ejaculation by the male.
Alterations in male reproductive health reflect a wide range of developmental, endocrine, infectious, inflammatory, hypertrophic, malignant, and psycho-emotional processes. Review the introduction to this unit to gain a better understanding of ways in which reproductive health is affected, including anatomy and physiology, sperm production, regulation of male sexual functioning, and sexual intercourse.
The drug family most clearly associated with male reproductive processes is the androgens. Because synthetic anabolic steroids and antiandrogens affect male reproduction, they are also discussed.
Substances Related to Male Reproductive Disorders
Androgens
Androgens, or male sex hormones, control the development and maintenance of sexual processes, accessory sexual organs, cellular metabolism, and bone and muscle growth. Testosterone is the principal male sex hormone and is an anabolic steroid. It is the prototype of the androgen hormones, synthesized primarily in the testes and, to a lesser extent, in the adrenal cortex. In women, the ovaries synthesize small amounts of testosterone. In men, normal plasma concentrations of testosterone are 250 to 1000 ng/dL, with circadian fluctuations. Prototype Drug Chart 57-1 lists the natural and synthetic androgens and their dosages, uses, and considerations.
Pharmacokinetics
Testosterone secretion is greater in men than in women in most stages of life. About 98% of circulating testosterone is bound to both sex hormone–binding globulin (SHBG) and albumin protein, leaving about 2% unbound, or circulating free in the plasma. It is this unbound portion that is biologically active. Estrogen elevates the production of sex hormone–binding globulin, resulting in more protein-bound testosterone in women than in men. The half-life of endogenous (naturally occurring) free testosterone in the blood is 10 to 20 minutes.
Exogenous (supplemental) testosterone is absorbed when taken orally, but because as much as 50% is metabolized on its first pass through hepatic circulation, high doses are needed to achieve effective plasma levels. Synthetic androgens have longer half-lives, so only synthetic androgens are available in oral formulations in the United States. Testosterone can be combined with esters to form esterified testosterone, in an oil base for intramuscular (IM) injection.
Testosterone is excreted mainly in the urine as the metabolites androsterone and etiocholanolone. About 6% of the hormone is excreted unaltered in the feces. Synthetic androgens may be excreted as unaltered hormone or as metabolites. In some tissues the action of testosterone depends on its reduction to 5-alpha-dihydrotestosterone (DHT), whereas in other tissues testosterone itself is the active hormone. In the central nervous system, the metabolite estradiol affects hormonal action.
Pharmacodynamics
Testosterone is responsible for the development of male sex characteristics. The biologic effects of testosterone may be mediated directly by testosterone or by its metabolites. Testosterone and dihydrotestosterone act as androgens by way of a single androgen receptor officially designated NR3A. The hormones bind to sites on certain responsive genes, causing a change to take place in the target cell. The effects of the testosterone depend on which receptor it activates and the tissues in which these effects occur. The manufacture of protein within the target cells results in the buildup of cellular tissue (anabolism), especially in muscles. This leads to development of secondary sex characteristics such as pubic hair growth, beard and body hair growth, baldness, deepening of the male voice, thickening of the skin, sebaceous gland activity, increased musculature, bone development, and red blood cell formation.
Fetal testes begin to produce testosterone during the first 3 months in utero. After birth until just before puberty, production is negligible. During puberty, production increases rapidly and continues until later adulthood. As men age, the number of Leydig cells decreases, sperm production declines, and luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels rise. Levels of unbound testosterone are reduced in older men to one-third to one-fifth the peak value. If men experience osteoporosis and anemia, and if their testosterone levels are ≤300 ng/dL, testosterone replacement therapy should be considered.
Indications for Androgen Therapy
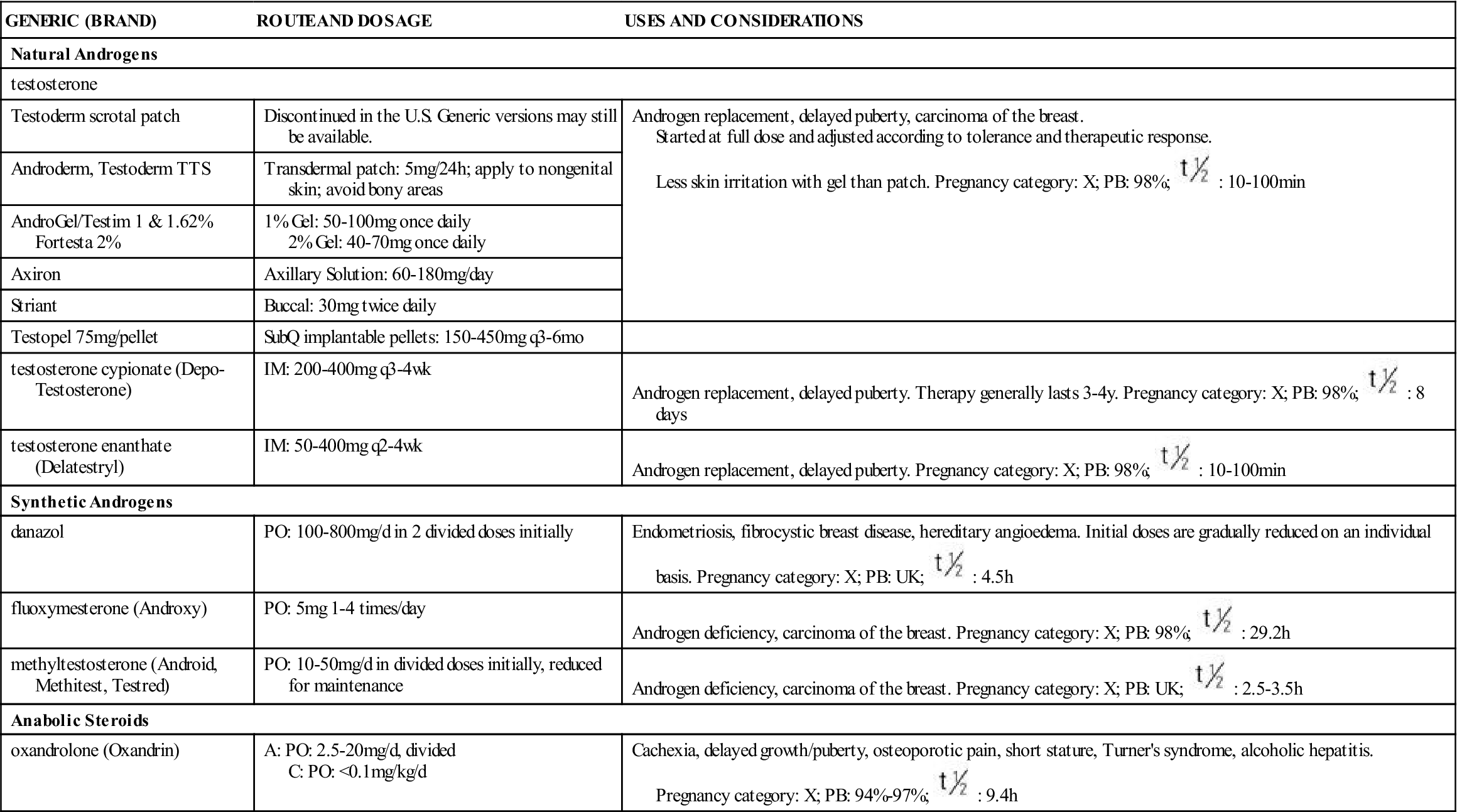
Table 57-1 lists the natural and synthetic androgens with their dosages, uses, and considerations. The most common clinical use is for androgen deficiency in males, specifically hypogonadism, replacement therapy for testicular failure in adult males, and delayed puberty in adolescents. Occasionally it is used to slow the decline of patients with certain muscle wasting diseases such as COPD and AIDS.
TABLE 57-1
| GENERIC (BRAND) | ROUTE AND DOSAGE | USES AND CONSIDERATIONS |
| Natural Androgens | ||
| testosterone | ||
| Testoderm scrotal patch | Discontinued in the U.S. Generic versions may still be available. | Androgen replacement, delayed puberty, carcinoma of the breast. Started at full dose and adjusted according to tolerance and therapeutic response. Less skin irritation with gel than patch. Pregnancy category: X; PB: 98%; 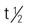 : 10-100 min : 10-100 min |
| Androderm, Testoderm TTS | Transdermal patch: 5 mg/24 h; apply to nongenital skin; avoid bony areas | |
| AndroGel/Testim 1 & 1.62% Fortesta 2% | 1% Gel: 50-100 mg once daily 2% Gel: 40-70 mg once daily | |
| Axiron | Axillary Solution: 60-180 mg/day | |
| Striant | Buccal: 30 mg twice daily | |
| Testopel 75 mg/pellet | SubQ implantable pellets: 150-450 mg q3-6mo | |
| testosterone cypionate (Depo-Testosterone) | IM: 200-400 mg q3-4wk | Androgen replacement, delayed puberty. Therapy generally lasts 3-4 y. Pregnancy category: X; PB: 98%; 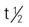 : 8 days : 8 days |
| testosterone enanthate (Delatestryl) | IM: 50-400 mg q2-4wk | Androgen replacement, delayed puberty. Pregnancy category: X; PB: 98%; 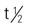 : 10-100 min : 10-100 min |
| Synthetic Androgens | ||
| danazol | PO: 100-800 mg/d in 2 divided doses initially | Endometriosis, fibrocystic breast disease, hereditary angioedema. Initial doses are gradually reduced on an individual basis. Pregnancy category: X; PB: UK; 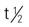 : 4.5 h : 4.5 h |
| fluoxymesterone (Androxy) | PO: 5 mg 1-4 times/day | Androgen deficiency, carcinoma of the breast. Pregnancy category: X; PB: 98%; 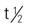 : 29.2 h : 29.2 h |
| methyltestosterone (Android, Methitest, Testred) | PO: 10-50 mg/d in divided doses initially, reduced for maintenance | Androgen deficiency, carcinoma of the breast. Pregnancy category: X; PB: UK; 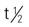 : 2.5-3.5 h : 2.5-3.5 h |
| Anabolic Steroids | ||
| oxandrolone (Oxandrin) | A: PO: 2.5-20 mg/d, divided C: PO: <0.1 mg/kg/d | Cachexia, delayed growth/puberty, osteoporotic pain, short stature, Turner’s syndrome, alcoholic hepatitis. Pregnancy category: X; PB: 94%-97%; 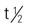 : 9.4 h : 9.4 h |
Hypogonadism.
The clearest indication for exogenous androgen therapy is hypogonadism. Male hypogonadism is a defect of the reproductive system that results in failure of the testes to produce testosterone, sperm, or both. Deficiency of sex hormones can result in defective primary or secondary sexual development. Defective sperm development can result in infertility. Hypogonadism is either primary, reflecting testicular abnormality, or secondary, reflecting hypothalamic or pituitary failure. A combination of disorders can also occur. Inadequate pituitary function will severely affect young boys and results in lack of secondary sex characteristics and infertility. Adult men may experience lack of libido, testicular atrophy, impotence, decreased bone density, loss of muscle tone, increased hair growth, or the onset of vasomotor flushing may occur.
The timing and extent of treatment depend on the clinical manifestations. Because accelerated bone maturation can lead to premature closure of bone epiphyses and short stature, androgen therapy should be used cautiously in children and only by specialists aware of the adverse effects on bone maturation. Skeletal maturation must be monitored every 6 months by x-ray of the hand and wrist. Artificial induction of puberty is undertaken only after boys reach age 15 to 17 years and hypothalamic and pituitary function has been assessed. A 4- to 6-month trial of androgen therapy is implemented, followed by a like period of rest for reevaluation. If prolonged therapy is required, testosterone cypionate or testosterone enanthate is used, 50 to 400 mg IM every 2 to 4 weeks. It should be given deep in the gluteal muscle. Inspect vials visually for particulate matter and discoloration before administration. Warm and shake the vial to dissolve any crystals that may have formed during storage. It takes 3 or 4 years for sexual development to occur. Plasma testosterone levels should be monitored and dosages adjusted as needed to maintain normal levels. If the serum testosterone level is below the normal range, the provider will adjust the dose upward. Therapy may be lifelong.
Some young, hypogonadal men are able to resolve erectile dysfunction with testosterone replacement therapy by way of oral, parenteral, or direct injection of drugs into the penis. These therapies may be associated with hypotension, dizziness, pain, and priapism; some of these drugs can cause hepatotoxicity. Given the success and high efficacy of oral erectile dysfunction therapy, discussed later, the use of these medications for adult hypogonadism has decreased markedly.
Testosterone may be administered buccally, orally, transdermally, or parenterally. The selection of an androgen or anabolic steroid depends on the balance of growth and sexual maturation desired and on the preferred route of administration. A buccal muco-adhesive system (Striant) is available at a 30-mg dose every 12 hours. Advise the patient to place the rounded side surface of the system against the gum above an incisor tooth and hold firmly in place with finger over lip and against product for 30 seconds. To remove, slide gently downwards toward tooth to avoid scratching gums. Sites must be rotated with each application. If the product falls off within the 12-hour dosing interval or falls out of position within 4 hours before next dose, remove and apply a new system. The patient should not chew or swallow the tablet. Advise the patient to regularly inspect gums where the system has been applied. With the exception of the buccal system, androgens are considered controlled substances Schedule III.
Many brands of testosterone transdermal patches are available. Testoderm patches are applied to the scrotum; scrotal skin is about five times more permeable than normal skin, and Testoderm will not achieve desired serum concentrations if applied to other skin sites. Serum concentrations of testosterone maximize after 2 to 4 hours, returning to baseline 2 hours after patch removal. Serum concentrations of testosterone approach those of normal males and reach a plateau after 3 to 4 weeks. Testoderm TTS patches achieve adequate serum concentrations when applied to the arm, back, or upper buttocks. Androderm patches can be applied to any healthy skin site other than the scrotum or bony areas. Daily application of two Androderm 2.5-mg skin patches at 10 PM results in serum testosterone concentrations approaching those of healthy young men. The first day of dosing results in morning serum testosterone concentrations within the normal range. There is no testosterone accumulation with continued use. After removal of Androderm, hypogonadal status returns within 24 hours. Keep testosterone gel out of reach of children.
An underarm solution (Axiron) is applied to one or both axillary areas at the same time each day. The patient should not apply Axiron to the genitals. These skin application products can be transferred to others through personal contact with skin or clothing. The treatment eliminates the need for injections and provides circadian fluctuations in dosage.
Other Uses.
Other uses of androgens include treatment of refractory anemia in men and women, hereditary angioedema, tissue wasting associated with severe or chronic illness, advanced carcinoma of the breast in women, and endometriosis. The effectiveness of androgens for treatment of cryptorchidism (undescended testis) and impotence has not been established. Androgens may be used in combination with estrogens for management of severe menopausal symptoms in women.
Side Effects.
Hypogonadal men on androgen therapy may experience frequent erections or priapism (painful, continuous erection), gynecomastia (mammary gland enlargement in men), or urinary urgency. Continued use of androgens by normal men can halt spermatogenesis (formation of spermatozoa). The sperm count may be low (oligospermia) for 3 or more months after therapy is stopped.
Other side effects of androgen therapy include abdominal pain, nausea, insomnia, diarrhea or constipation, hives or redness at the injection site, increased salivation, mouth soreness, and increased or decreased sexual desire. Advise the patient to notify the health care provider if side effects persist, worsen, or are bothersome.
Adverse Reactions.
Androgen therapy may cause hypercalcemia by stimulating bone resorption in patients with breast cancer or immobilized patients. The drug should be discontinued and appropriate measures instituted if signs of hypercalcemia occur including: nausea and vomiting, lethargy, decreased muscle tone, polyuria, and increased urine and serum calcium.
Virilization refers to the development of male secondary sex characteristics in women or hypogonadal males. Such characteristics include growth of facial hair, acne and skin oiliness, and vocal huskiness. Menstrual irregularities or amenorrhea, suppressed ovulation or lactation, baldness or increased hair growth (hirsutism), and hypertrophy of the clitoris may develop in women undergoing androgen therapy. Although most adverse effects slowly reverse themselves after short-term therapy is completed, vocal changes may be permanent. With long-term therapy, as in the treatment of breast cancer, adverse effects may be irreversible.
Children may experience profound virilization as well as impaired bone growth. During pregnancy, androgens can cross the placenta and cause masculinization of the fetus. Virilization can occur in those secondarily exposed to testosterone gel and may cause teratogenic effects in fetuses. Women and children should not handle the gel and should avoid contact with application sites in men using testosterone gel.
Less frequent adverse effects include dizziness, weakness, changes in skin color, frequent headaches, confusion, respiratory distress, depression, pruritus, allergic skin rash, edema of the lower extremities, jaundice, bleeding, paresthesias, chills, polycythemia, muscle cramps, and sodium and water retention. Hepatic carcinoma can occur in patients who have received selected androgens for long-term therapy.
Serum cholesterol may become elevated during androgen therapy. Other alterations in laboratory tests include increased hematocrit, altered thyroid and liver function tests, and elevated urine 17-ketosteroids (a by-product of the breakdown of androgens). Rare complications of long-term therapy include hepatic necrosis, hepatic peliosis (blood-filled cysts), hepatic tumors, and leukopenia.
Contraindications.
Androgen therapy is contraindicated during pregnancy and in individuals with nephrosis or the nephrotic phase of nephritis, hypercalcemia, pituitary insufficiency, hepatic dysfunction, benign prostatic hypertrophy, prostate cancer, or history of myocardial infarction. Men with breast cancer are not treated with androgens, nor are women whose breast cancer is not estrogen-dependent.
Caution must be exercised when using androgen therapy in individuals with hypertension, hypercholesterolemia, coronary artery disease, renal disease, or seizure disorder. It is used with caution in infants and prepubertal children because of the potential for growth disturbances and in older men because of their increased risk for benign prostatic hypertrophy and prostate cancer.
Drug Interactions.
Androgens potentiate the effects of oral anticoagulants, necessitating a decrease in anticoagulant dosage. Androgens antagonize calcitonin and parathyroid hormones. Because androgens can decrease blood glucose in patients with diabetes, dosages of insulin or other antidiabetic agents may need to be reduced. Concurrent use of corticosteroids exacerbates the edema that can occur with androgen therapy. Barbiturates, phenytoin, and phenylbutazone decrease the effects of androgens.
Anabolic Steroids
Anabolic steroids, or anabolic-androgenic steroids (AAS), are a class of steroid hormones related to the hormone testosterone. They increase protein synthesis within cells, which results in the buildup of cellular tissue (anabolism), especially in muscles. Anabolic steroids also have androgenic and virilizing properties, including the development and maintenance of masculine characteristics such as the growth of the vocal cords and body hair. The American College of Sports Medicine notes that AAS, combined with sufficient diet, can contribute to increased body weight, often as lean mass, and that the gain in muscular strength achieved through high-intensity exercise and proper diet can be additionally enhanced by the use of AAS in some individuals.
Testosterone precursors available as nutritional supplements include androstenediol, androstenedione, and dehydroepiandrosterone (DHEA). Older teens are the heaviest users, but more than one-half million junior high school students use them. Marketed as “sport supplements” or “teen formulas,” they can be purchased without a prescription in stores and on the Internet. A sudden dramatic increase in weight and body size, increased acne, and changes in mood and behavior can be signs of exogenous anabolic steroid use. Individuals using anabolic steroids may become more aggressive and physical. Health risks can result from long-term use or excessive intake of anabolic steroids; these effects include increased low-density lipoprotein (bad) cholesterol and decreased high-density lipoprotein (good) cholesterol, acne, high blood pressure, liver damage, and dangerous changes in the structure of the left ventricle of the heart. Adverse effects may not be recognized until years later.
Two other steroids that have gained popularity, especially with athletes, are human chorionic gonadotropin (hCG, Pregnyl, Novarel, Ovidrel) and tetrahydrogestrinone (THG). HCG is a hormone used to treat infertility and stimulate testosterone production. THG is a potent androgen developed to escape urine detection. It is not approved by the U.S. Food and Drug Administration (FDA) and is not legally marketed. All major athletic organizations prohibit the use of anabolic steroids, but their continued use despite bans has led to “anti-doping” investigations and punitive action.
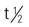 : 10-100 min
: 10-100 min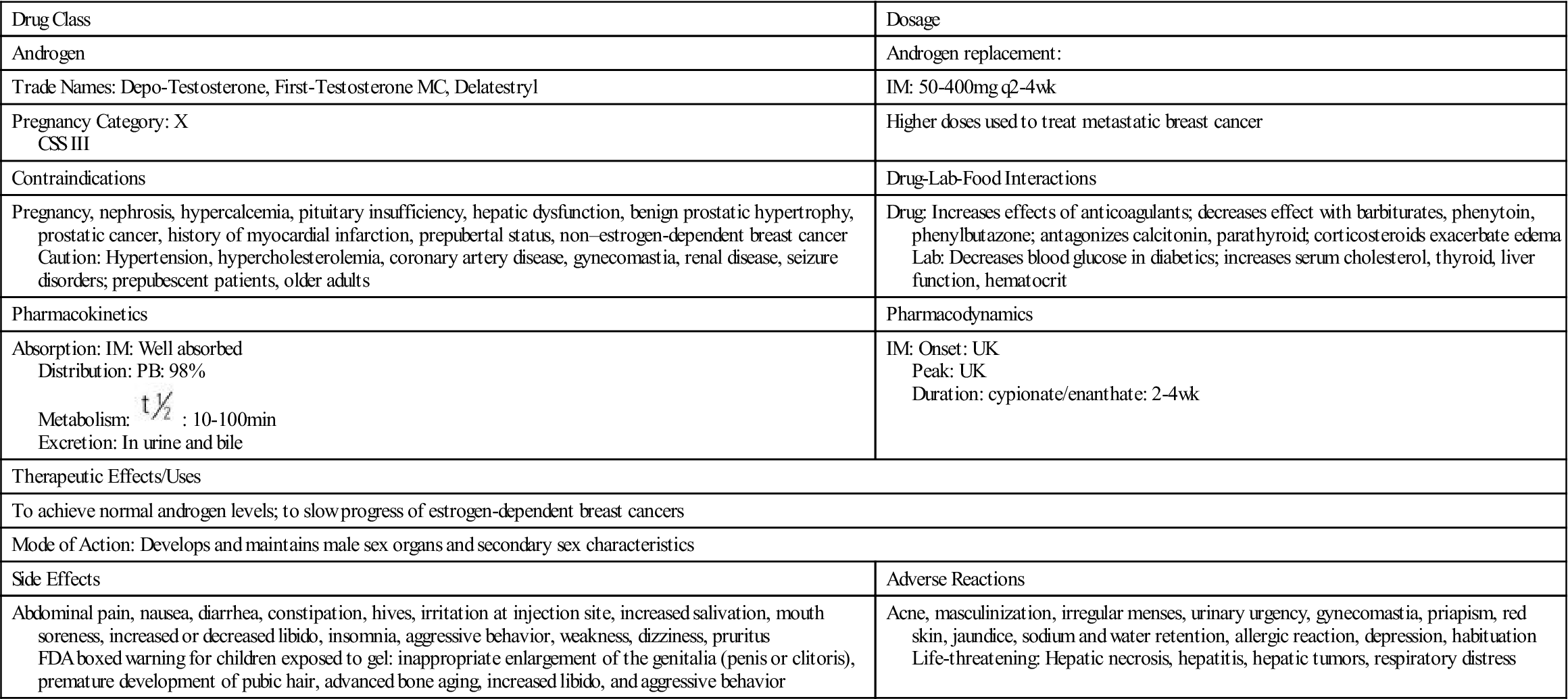
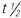 , half-life; UK, unknown; wk, week.
, half-life; UK, unknown; wk, week.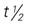 , half-life; UK, unknown; wk, week; y, year.
, half-life; UK, unknown; wk, week; y, year.







