Drugs for Dermatologic Disorders
Objectives
• Differentiate acne vulgaris, psoriasis, drug-induced dermatitis, and contact dermatitis.
• Describe nonpharmacologic measures used to treat mild acne vulgaris.
• Compare the topical antibacterial agents used to prevent and treat burn tissue infection.
Key Terms
acne vulgaris, p. 728
contact dermatitis, p. 733
keratolysis, p. 729
macules, p. 727
papules, p. 727
plaques, p. 728
psoriasis, p. 731
tinea capitis, p. 727
tinea pedis, p. 727
vesicles, p. 728
Numerous skin lesions and eruptions require mild to aggressive drug therapy. Some skin disorders include acne vulgaris, psoriasis, eczema dermatitis, contact dermatitis, drug-induced dermatitis, and burn infection. Skin eruptions may result from viral infections (e.g., herpes simplex, herpes zoster), fungal infections (e.g., tinea pedis [athlete’s foot], tinea capitis [ringworm]), and bacterial infections.
Most treatments for skin eruptions include topical creams, ointments, pastes, gels, lotions, and solutions. Skin lesions may appear as macules (flat with varying colors), papules (raised, palpable, and less than 1 cm in diameter), vesicles (raised, filled with fluid, and less than 1 cm in diameter), or plaques (hard, rough, raised, and flat on top). Selected skin disorders and their drug therapy regimens are discussed separately.
Acne Vulgaris
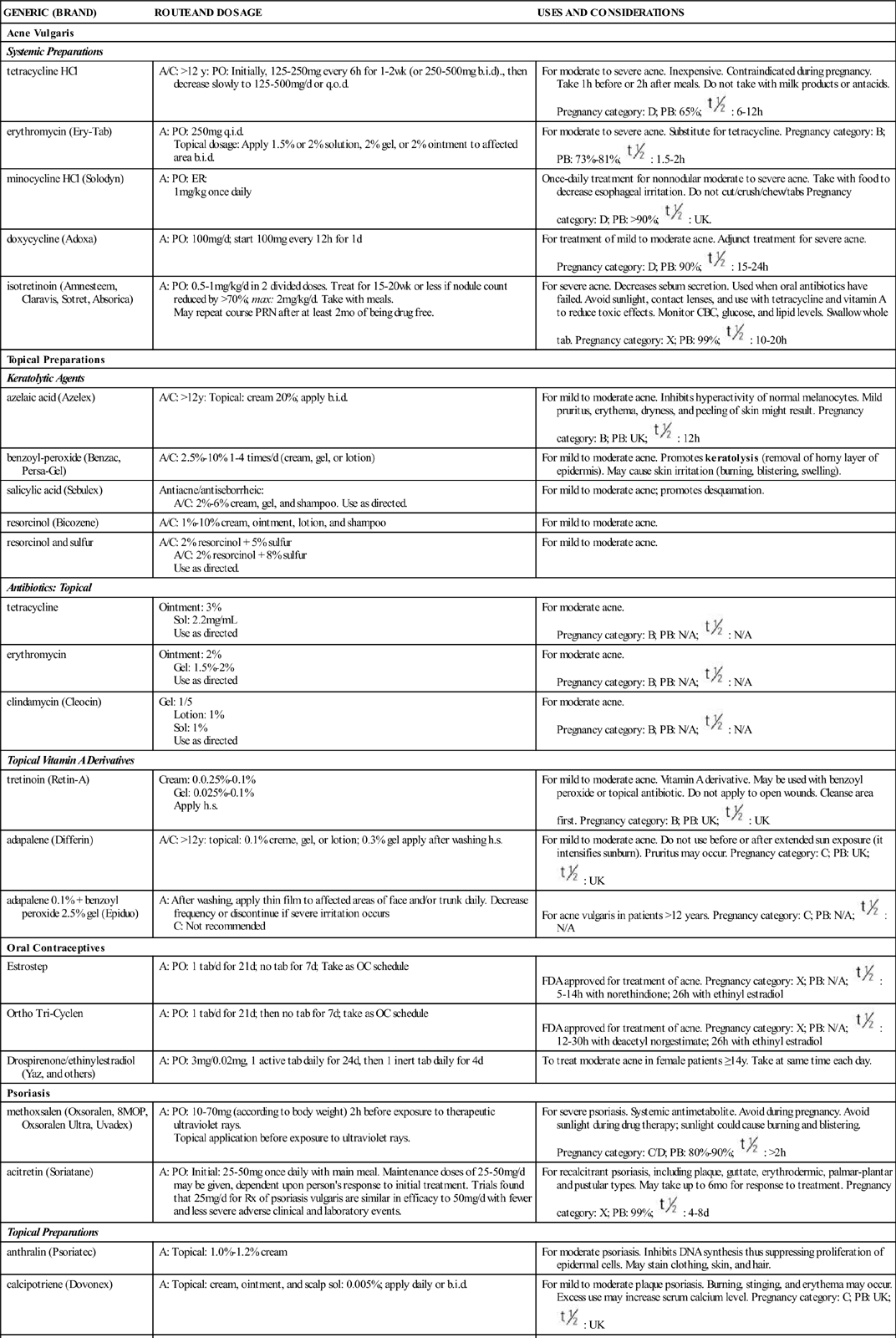
Acne is the most common skin disorder in the United States. Acne vulgaris is the formation of papules, nodules, and cysts on the face, neck, shoulders, and back resulting from keratin plugs at the base of the pilosebaceous oil glands near the hair follicles. Of persons with acne, 90% are adolescents. The increase in androgen production that occurs during adolescence increases the production of sebum, an oily skin lubricant. The sebum combines with keratin to form a plug, which results in acne. Table 50-1 lists the drugs commonly used to control acne vulgaris and their dosages, uses, and considerations.
TABLE 50-1
DRUGS FOR ACNE VULGARIS AND PSORIASIS
| GENERIC (BRAND) | ROUTE AND DOSAGE | USES AND CONSIDERATIONS |
| Acne Vulgaris | ||
| Systemic Preparations | ||
| tetracycline HCl | A/C: >12 y: PO: Initially, 125–250 mg every 6 h for 1–2 wk (or 250-500 mg b.i.d)., then decrease slowly to 125–500 mg/d or q.o.d. | For moderate to severe acne. Inexpensive. Contraindicated during pregnancy. Take 1 h before or 2 h after meals. Do not take with milk products or antacids. Pregnancy category: D; PB: 65%; 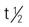 : 6–12 h : 6–12 h |
| erythromycin (Ery-Tab) | A: PO: 250 mg q.i.d. Topical dosage: Apply 1.5% or 2% solution, 2% gel, or 2% ointment to affected area b.i.d. | For moderate to severe acne. Substitute for tetracycline. Pregnancy category: B; PB: 73%–81%; 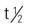 : 1.5–2 h : 1.5–2 h |
| minocycline HCl (Solodyn) | A: PO: ER: 1 mg/kg once daily | Once-daily treatment for nonnodular moderate to severe acne. Take with food to decrease esophageal irritation. Do not cut/crush/chew/tabs Pregnancy category: D; PB: >90%; 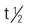 : UK. : UK. |
| doxycycline (Adoxa) | A: PO: 100 mg/d; start 100 mg every 12 h for 1 d | For treatment of mild to moderate acne. Adjunct treatment for severe acne. Pregnancy category: D; PB: 90%; 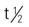 : 15-24 h : 15-24 h |
| isotretinoin (Amnesteem, Claravis, Sotret, Absorica) | A: PO: 0.5–1 mg/kg/d in 2 divided doses. Treat for 15–20 wk or less if nodule count reduced by >70%; max: 2 mg/kg/d. Take with meals. May repeat course PRN after at least 2 mo of being drug free. | For severe acne. Decreases sebum secretion. Used when oral antibiotics have failed. Avoid sunlight, contact lenses, and use with tetracycline and vitamin A to reduce toxic effects. Monitor CBC, glucose, and lipid levels. Swallow whole tab. Pregnancy category: X; PB: 99%; 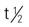 : 10–20 h : 10–20 h |
| Topical Preparations | ||
| Keratolytic Agents | ||
| azelaic acid (Azelex) | A/C: >12 y: Topical: cream 20%; apply b.i.d. | For mild to moderate acne. Inhibits hyperactivity of normal melanocytes. Mild pruritus, erythema, dryness, and peeling of skin might result. Pregnancy category: B; PB: UK; 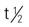 : 12 h : 12 h |
| benzoyl-peroxide (Benzac, Persa-Gel) | A/C: 2.5%–10% 1–4 times/d (cream, gel, or lotion) | For mild to moderate acne. Promotes keratolysis (removal of horny layer of epidermis). May cause skin irritation (burning, blistering, swelling). |
| salicylic acid (Sebulex) | Antiacne/antiseborrheic: A/C: 2%–6% cream, gel, and shampoo. Use as directed. | For mild to moderate acne; promotes desquamation. |
| resorcinol (Bicozene) | A/C: 1%–10% cream, ointment, lotion, and shampoo | For mild to moderate acne. |
| resorcinol and sulfur | A/C: 2% resorcinol + 5% sulfur A/C: 2% resorcinol + 8% sulfur Use as directed. | For mild to moderate acne. |
| Antibiotics: Topical | ||
| tetracycline | Ointment: 3% Sol: 2.2 mg/mL Use as directed | For moderate acne. Pregnancy category: B; PB: N/A; 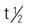 : N/A : N/A |
| erythromycin | Ointment: 2% Gel: 1.5%–2% Use as directed | For moderate acne. Pregnancy category: B; PB: N/A; 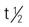 : N/A : N/A |
| clindamycin (Cleocin) | Gel: 1/5 Lotion: 1% Sol: 1% Use as directed | For moderate acne. Pregnancy category: B; PB: N/A;  : N/A : N/A |
| Topical Vitamin A Derivatives | ||
| tretinoin (Retin-A) | Cream: 0.0.25%–0.1% Gel: 0.025%–0.1% Apply h.s. | For mild to moderate acne. Vitamin A derivative. May be used with benzoyl peroxide or topical antibiotic. Do not apply to open wounds. Cleanse area first. Pregnancy category: B; PB: UK;  : UK : UK |
| adapalene (Differin) | A/C: >12 y: topical: 0.1% creme, gel, or lotion; 0.3% gel apply after washing h.s. | For mild to moderate acne. Do not use before or after extended sun exposure (it intensifies sunburn). Pruritus may occur. Pregnancy category: C; PB: UK;  : UK : UK |
| adapalene 0.1% + benzoyl peroxide 2.5% gel (Epiduo) | A: After washing, apply thin film to affected areas of face and/or trunk daily. Decrease frequency or discontinue if severe irritation occurs C: Not recommended | For acne vulgaris in patients >12 years. Pregnancy category: C; PB: N/A;  : N/A : N/A |
| Oral Contraceptives | ||
| Estrostep | A: PO: 1 tab/d for 21 d; no tab for 7 d; Take as OC schedule | FDA approved for treatment of acne. Pregnancy category: X; PB: N/A;  : 5–14 h with norethindione; 26 h with ethinyl estradiol : 5–14 h with norethindione; 26 h with ethinyl estradiol |
| Ortho Tri-Cyclen | A: PO: 1 tab/d for 21 d; then no tab for 7 d; take as OC schedule | FDA approved for treatment of acne. Pregnancy category: X; PB: N/A;  : 12–30 h with deacetyl norgestimate; 26 h with ethinyl estradiol : 12–30 h with deacetyl norgestimate; 26 h with ethinyl estradiol |
| Drospirenone/ethinylestradiol (Yaz, and others) | A: PO: 3 mg/0.02 mg, 1 active tab daily for 24 d, then 1 inert tab daily for 4 d | To treat moderate acne in female patients ≥14 y. Take at same time each day. |
| Psoriasis | ||
| methoxsalen (Oxsoralen, 8MOP, Oxsoralen Ultra, Uvadex) | A: PO: 10–70 mg (according to body weight) 2 h before exposure to therapeutic ultraviolet rays. Topical application before exposure to ultraviolet rays. | For severe psoriasis. Systemic antimetabolite. Avoid during pregnancy. Avoid sunlight during drug therapy; sunlight could cause burning and blistering. Pregnancy category: C/D; PB: 80%–90%;  : >2 h : >2 h |
| acitretin (Soriatane) | A: PO: Initial: 25-50 mg once daily with main meal. Maintenance doses of 25-50 mg/d may be given, dependent upon person’s response to initial treatment. Trials found that 25 mg/d for Rx of psoriasis vulgaris are similar in efficacy to 50 mg/d with fewer and less severe adverse clinical and laboratory events. | For recalcitrant psoriasis, including plaque, guttate, erythrodermic, palmar-plantar and pustular types. May take up to 6 mo for response to treatment. Pregnancy category: X; PB: 99%;  : 4–8 d : 4–8 d |
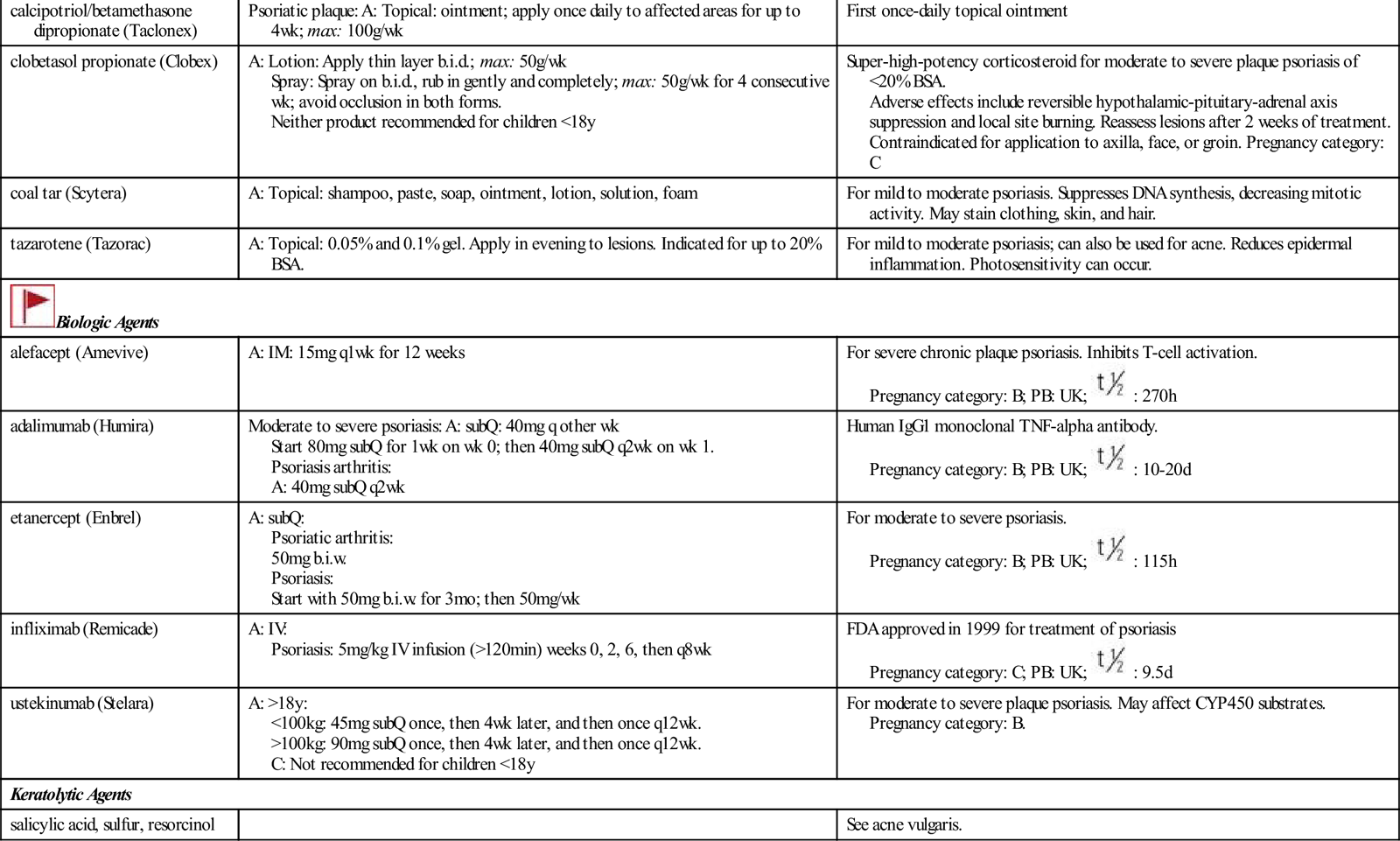
| Topical Preparations | ||
| anthralin (Psoriatec) | A: Topical: 1.0%-1.2% cream | For moderate psoriasis. Inhibits DNA synthesis thus suppressing proliferation of epidermal cells. May stain clothing, skin, and hair. |
| calcipotriene (Dovonex) | A: Topical: cream, ointment, and scalp sol: 0.005%; apply daily or b.i.d. | For mild to moderate plaque psoriasis. Burning, stinging, and erythema may occur. Excess use may increase serum calcium level. Pregnancy category: C; PB: UK; 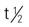 : UK : UK |
| calcipotriol/betamethasone dipropionate (Taclonex) | Psoriatic plaque: A: Topical: ointment; apply once daily to affected areas for up to 4 wk; max: 100g/wk | First once-daily topical ointment |
| clobetasol propionate (Clobex) | A: Lotion: Apply thin layer b.i.d.; max: 50 g/wk Spray: Spray on b.i.d., rub in gently and completely; max: 50g/wk for 4 consecutive wk; avoid occlusion in both forms. Neither product recommended for children <18 y | Super-high-potency corticosteroid for moderate to severe plaque psoriasis of <20% BSA. Adverse effects include reversible hypothalamic-pituitary-adrenal axis suppression and local site burning. Reassess lesions after 2 weeks of treatment. Contraindicated for application to axilla, face, or groin. Pregnancy category: C |
| coal tar (Scytera) | A: Topical: shampoo, paste, soap, ointment, lotion, solution, foam | For mild to moderate psoriasis. Suppresses DNA synthesis, decreasing mitotic activity. May stain clothing, skin, and hair. |
| tazarotene (Tazorac) | A: Topical: 0.05% and 0.1% gel. Apply in evening to lesions. Indicated for up to 20% BSA. | For mild to moderate psoriasis; can also be used for acne. Reduces epidermal inflammation. Photosensitivity can occur. |
| alefacept (Amevive) | A: IM: 15 mg q1wk for 12 weeks | For severe chronic plaque psoriasis. Inhibits T-cell activation. Pregnancy category: B; PB: UK; 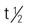 : 270 h : 270 h |
| adalimumab (Humira) | Moderate to severe psoriasis: A: subQ: 40 mg q other wk Start 80 mg subQ for 1 wk on wk 0; then 40 mg subQ q2wk on wk 1. Psoriasis arthritis: A: 40 mg subQ q2wk | Human IgG1 monoclonal TNF-alpha antibody. Pregnancy category: B; PB: UK; 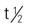 : 10–20 d : 10–20 d |
| etanercept (Enbrel) | A: subQ: Psoriatic arthritis: 50 mg b.i.w. Psoriasis: Start with 50 mg b.i.w. for 3 mo; then 50 mg/wk | For moderate to severe psoriasis. Pregnancy category: B; PB: UK; 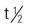 : 115 h : 115 h |
| infliximab (Remicade) | A: IV: Psoriasis: 5 mg/kg IV infusion (>120 min) weeks 0, 2, 6, then q8wk | FDA approved in 1999 for treatment of psoriasis Pregnancy category: C; PB: UK;  : 9.5 d : 9.5 d |
| ustekinumab (Stelara) | A: >18 y: <100 kg: 45 mg subQ once, then 4 wk later, and then once q12wk. >100 kg: 90 mg subQ once, then 4 wk later, and then once q12wk. C: Not recommended for children <18 y | For moderate to severe plaque psoriasis. May affect CYP450 substrates. Pregnancy category: B. |
| Keratolytic Agents | ||
| salicylic acid, sulfur, resorcinol | See acne vulgaris. | |
Nonpharmacologic Approach
Nonpharmacologic measures should be tried before drug therapy is initiated. A prescribed or suggested cleansing agent is necessary for all types of acne. The skin should be gently cleansed several times a day; vigorous scrubbing should be avoided. A well-balanced diet is indicated. Decreasing emotional stress and increasing emotional support are suggested. If drug therapy is necessary, nonpharmacologic measures should be maintained as well.
Topical Antiacne Drugs
Mild acne may require gentle cleansing and the use of keratolytics (keratin dissolvers such as benzoyl peroxide, resorcinol, and salicylic acid). Benzoyl peroxide is applied as a cream, lotion, or gel once or twice a day. This agent loosens the outer horny layer of the epidermis.
Tretinoin (Retin-A, Renova), a derivative of vitamin A, is a topical drug for mild to moderate acne that alters keratinization. Other antiacne agents, such as adapalene (Differin), azelaic acid (Azelex), and tazarotene (Tazorac), are used to treat mild to moderate acne. Adapalene is similar in action to tretinoin. It has antiinflammatory and comedolytic (eliminates blackheads) properties and tends to be more effective than tretinoin in reducing the number of acne lesions. Adapalene should not be used before or after extended sun exposure or sunburn. It can increase the risk of sunburn and intensify existing sunburn. Azelaic acid is as effective as benzoyl peroxide and tretinoin. Adapalene and azelaic acid can cause burning, pruritus, and erythema after several applications; however, this is less common with azelaic acid. Adapalene and tazarotene bind to select retinoid receptors; thus fewer adverse effects are anticipated.
Moderate acne requires a stronger concentration of benzoyl peroxide (10%), and topical antibiotics (e.g., tetracycline, erythromycin, clindamycin) may be added to the treatment regimen. Erythromycin and clindamycin are the most frequently prescribed topical antibiotics and have the fewest side effects.
Systemic Antiacne Drugs
For severe acne, oral antibiotics (doxycycline and minocycline [drugs of choice], tetracycline, erythromycin) and topical glucocorticoids may be prescribed. Pregnant patients should not take tetracycline because of possible teratogenic effects on the fetus. Once the condition is stabilized, the patient commonly progresses to the use of a topical preparation.
Isotretinoin (Amnesteem) is used for treatment of severe cystic acne. It can be administered orally or topically. It decreases sebum formation and secretion and has antiinflammatory and antikeratinizing (keratolytic) effects. The typical patient takes this drug for 4 to 6 months. It can cause adverse reactions such as chelitis (90%), dizziness, conjunctivitis, skin irritation, pruritus, epistaxis, hirsutism, and photosensitivity. Isotretinoin must not be used during pregnancy because of teratogenic effects. Using vitamin A or tetracycline with isotretinoin may increase its adverse effects. Baseline blood tests are required before initiating isotretinoin therapy and at intervals throughout therapy. Additional cautions associated with isotretinoin include the following: Do not breastfeed, give blood during or for 1 month after therapy, take other medications or herbal products without first consulting the health care provider, drive at night without knowing the effect of isotretinoin on night vision, or have cosmetic procedures to smooth skin. Instruct the patient to avoid excessively vigorous activity and to contact the health care provider and stop taking isotretinoin if experiencing muscle weakness, which may be an indication of serious muscle damage. Monitoring of complete blood count (CBC), glucose and lipid levels, and urinalysis on a regular basis is important. Isotretinoin must not be used during pregnancy; its pregnancy category is X. Because of this drug’s powerful teratogenicity, a risk-management system to prevent isotretinoin-related teratogenicity was designed by the FDA Dermatologic and Ophthalmic Advisory Committee in 2002. Named the System to Manage Accutane-Related Teratogenicity (SMART), its purpose was to ensure that no patient is pregnant when treatment is initiated and no patient becomes pregnant while taking the drug or for at least 1 month after completing a course of isotretinoin. The iPLEDGE program replaced SMART in December 2005. An Internet-based registry system, iPLEDGE uses a central database to track isotretinoin users and account for all information and access to the drug. This comprehensive program has rules for the health care provider, patient, pharmacist, and wholesaler, as follows:
iPLEDGE has recently become less stringent for female patients not of childbearing age and for male patients.
Psoriasis
Psoriasis is a chronic skin disorder that affects 2% to 4% of the U.S. population. Onset usually occurs before age 30 years, but it may occur as early as age 10 years. Psoriasis is characterized by erythematous papules and plaques covered with silvery scales. It appears on the scalp, elbows, palms of the hands, knees, and soles of the feet. With psoriasis, epidermal cell growth and epidermal turnover are accelerated to approximately five times the normal expected epidermal growth. Antipsoriatic drug therapy uses preparations such as coal tar products and anthralin to keep the psoriasis in check; however, there are usually periods of remission and exacerbation.
Topical and Systemic Preparations
Psoriatic scales may be loosened with keratolytics (salicylic acid, sulfur). Topical glucocorticoids are sometimes used for mild psoriasis. Other topical preparations for psoriasis include anthralin (Dithro-Crème Psoriatec, Zithranol-RR) and coal tar (Estar), Oxipor VHC, Balnetar, and Scytera. Applications of 1% anthralin may cause erythema to occur. This agent can stain clothing, skin, and hair. Coal tar products are available in shampoos, lotions, and creams. They have an unpleasant odor and can cause burning and stinging, so they are rarely used. A topical product for mild to moderate psoriasis is calcipotriene (Dovonex), a synthetic vitamin D3 derivative useful for suppressing cell proliferation. This drug may cause local irritation and the serious adverse effects of hypercalciuria and hypercalcemia (increased urine and serum calcium levels). A topical antipsoriatic, tazarotene (Tazorac), is used to treat mild to moderate psoriasis. Photosensitivity is a side effect of tazarotene; therefore, the patient should use sunscreen to avoid severe sunburn.
![]() The anticancer drug methotrexate is a systemic drug that slows high growth fraction. It is prescribed to decrease the acceleration of epidermal cell growth in severe psoriasis.
The anticancer drug methotrexate is a systemic drug that slows high growth fraction. It is prescribed to decrease the acceleration of epidermal cell growth in severe psoriasis.
Ultraviolet A (UVA) may be used to suppress mitotic (cell division) activity. Photochemotherapy, a combination of ultraviolet radiation and the psoralen derivative methoxsalen (photosensitive drug), is used to decrease proliferation of epidermal cells. This type of therapy is called psoralen and ultraviolet A (PUVA). PUVA permits lower doses of methoxsalen and ultraviolet A to be given.
High-cost biologic agents are helpful in the management of psoriasis in patients who are refractory to ultraviolet B (UVB) phototherapy and need improved control. FDA-approved agents include the T-cell antagonist alefacept (Amevive) and the tumor necrosis factor (TNF) inhibitors etanercept (Enbrel), infliximab (Remicade), adalimumab (Humira), and interleukin antagonist ustekinumab (Stelara). All TNF inhibitors have a risk of severe opportunistic infection. These biologic agents are expensive but tend to have fewer side effects with comparable efficacy to other systemic treatments.
Alefacept (Amevive) is a recombinant protein that modifies the inflammatory process and inhibits the activation of memory effector T lymphocytes. No increased rate of opportunistic infections or malignancy has been observed. The drug is administered IM weekly for 12 weeks. Chills are the most common side effect. A second 12-week cycle may be initiated, but with decreased benefits from the first cycle. Weekly monitoring of CBC and T-cell counts is required, and alefacept must be discontinued if the CD4+ T-cell count falls lower than 250 cells per microliter.
Etanercept (Enbrel) is a soluble recombinant human TNF-alpha inhibitor. This agent worsens infections, especially tuberculosis (TB). A tuberculin test (PPD) is required before initiation of therapy. Etanercept may also worsen heart failure (HF). The drug is administered twice weekly for 12 weeks, and then weekly.
Infliximab (Remicade) is a monoclonal antibody that neutralizes TNF-alpha purified protein derivative (PPD). The drug is administered IV at 0, 2, and 6 weeks, and then every 8 weeks as tolerated. Opportunistic infection rate is higher than with other agents. A tuberculin test before the start and during treatment is recommended. This drug is associated with demyelinating disease. The major side effect is increased risk for infection.
Adalimumab (Humira) is a TNF-alpha inhibitor. The drug is administered subcutaneously every other week.
Table 50-1 lists the drugs used to control psoriasis.
Side Effects and Adverse Reactions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 , half-life; tab, tablet; TNF, tumor necrosis factor; UK, unknown; wk, week; y, year; >, greater than; <, less than.
, half-life; tab, tablet; TNF, tumor necrosis factor; UK, unknown; wk, week; y, year; >, greater than; <, less than.