Categorize the types of pain.
 Recognize the pathophysiology associated with pain.
Recognize the pathophysiology associated with pain.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the opioid agonists.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the opioid agonists.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the opioid agonists/antagonists.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the opioid agonists/antagonists.
 Discuss administration of preemptive analgesia in the treatment of pain related to surgery.
Discuss administration of preemptive analgesia in the treatment of pain related to surgery.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the opioid antagonists.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the opioid antagonists.
 Understand how to implement the nursing process in the care of the patient receiving opioid medications for pain.
Understand how to implement the nursing process in the care of the patient receiving opioid medications for pain.
Clinical Application Case Study
Darlene Hoffman, a 50-year-old woman, is receiving treatment for ovarian cancer. After surgery, she arrives on the unit from the postanesthesia care unit (PACU) complaining of lower back pain of 2 out of 10 on a 10-point pain scale. She received a total of 10 mg of morphine sulfate in the PACU. Her vital signs are temperature 98.2°F, pulse 82 beats/min, respirations 22 breaths/min, and blood pressure 124/72 mm Hg, with an 02 saturation of 94% on 2 L/min nasal cannula.
KEY TERMS
Analgesics: drugs that relieve pain without loss of consciousness
Breakthrough pain: episodic bursts of intense pain that “breaks through” the pain control of the medication regime
Ceiling effect: a phenomenon of certain drugs that limits the ability to produce a further effect above a particular dosage level
Endogenous analgesia system: nerve signals that relieve pain by suppressing the transmission of pain signals from peripheral nerves; can be activated by nerve signals entering the brain or by morphine-like drugs
Endorphins: peptides (i.e., endorphins, enkephalins, and dynorphins) that interact with receptors to inhibit perception and transmission of pain signals
Nociceptors: nerve endings that selectively respond to painful stimuli and send pain signals to the brain and spinal cord
Pain: unpleasant, sensory, emotional sensation that is associated with actual or potential tissue damage
Patient-controlled analgesia: any method used by patients to administer their own pain medication, typically used to indicate administration through a controlled intravenous pump
Preemptive analgesia: a strategy to reduce postsurgical pain by administering medications prior to the occurrence of the noxious stimuli or pain
Introduction
Pain is an unpleasant, sensory, emotional sensation associated with actual or potential tissue injury. The perception of pain is part of the clinical presentation in many acute and chronic disorders and is one of the most difficult sensations for patients to cope with during the course of a disease. It impels a person to remove the cause of the damage or seek relief from the pain. This chapter aids in the understanding of drug actions by discussing pain and pain-relieving opioid drugs.
Overview of Pain
Pain is the most common symptom prompting people to seek health care. When not managed effectively, pain may greatly impair quality of life and ability to perform activities of daily living. The Joint Commission includes assessment and management of pain for all patients (the fifth vital sign) in the standards by which it evaluates health care organizations. Opioid analgesics are drugs that provide pain relief by affecting people’s perception and tolerance of moderate to severe pain.
Etiology
The causes of pain include nerve damage, actual tissue injury, cancer, or surgery. Pain may be classified according to its origin in body structures (e.g., somatic, visceral, neuropathic), duration (e.g., acute, chronic), or cause (e.g., cancer). Box 48.1 describes these types of pain.
BOX 48.1 Types of Pain
Acute pain may result from injury, trauma, spasm, disease processes, and treatment or diagnostic procedures that damage body tissues. Patients often describe it as sharp or cutting. The intensity of the pain is usually proportional to the amount of tissue damage and the pain serves as a warning system by demanding the sufferer’s attention and compelling behavior to withdraw from or avoid the pain- producing stimulus.
Acute pain is called fast pain because it is felt quickly after a pain stimulus is applied. It is aroused by mechanical and thermal stimuli and conducted to the spinal cord by A-delta fibers in the peripheral nerves. Glutamate is the neurotransmitter secreted in the spinal cord at the A-delta nerve fiber endings.
Acute pain is often accompanied by anxiety and objective signs of discomfort (e.g., facial expressions of distress; moaning or crying; positioning to protect the affected part; tenderness, edema, and skin color or temperature changes in the affected part; and either restlessness and excessive movement or limited movement, if movement increases pain). It usually responds to treatment with analgesic drugs and resolves as tissue repair mechanisms heal the damaged area.
Chronic (noncancer) pain (i.e., lasting 3 months or longer) demands attention less urgently, may not be characterized by visible signs, and is often accompanied by emotional stress, increased irritability, depression, social withdrawal, financial distress, loss of libido, disturbed sleep patterns, diminished appetite, weight loss, and decreased ability to perform usual activities of daily living. It may occur with or without evidence of tissue damage and may include acute pain that persists beyond the typical recovery time for the precipitating tissue injury, pain related to a chronic disease, pain without an identifiable cause, and pain associated with cancer. It may arise from essentially any part of the body. It may be continuous or episodic or a combination of both.
Chronic (noncancer) pain may also be called slow pain. It can be elicited by mechanical, thermal, and chemical stimuli and is described as burning, aching, or throbbing. Slow, chronic pain is transmitted by C nerve fibers to the spinal cord and brain. Substance P is the neurotransmitter at C nerve fiber endings; it is released slowly and accumulates over seconds or minutes.
Cancer pain has characteristics of both acute and chronic pain, and it may be constant or intermittent. Chronic cancer pain is often caused by tumor spread into pain-sensitive tissues and the resulting tissue destruction. It usually progresses as the disease advances and can be severe and debilitating. Acute pain is often associated with diagnostic procedures or treatment measures (e.g., surgery, chemotherapy). It may also occur with disease progression, with activity, or with the development of drug tolerance.
Somatic pain results from stimulation of nociceptors in skin, bone, muscle, and soft tissue. It is usually well localized and described as sharp, burning, gnawing, throbbing, or cramping. It may be intermittent or constant and acute or chronic. Sprains and other traumatic injuries are examples of acute somatic pain; the bone and joint pain of arthritis is an example of chronic somatic pain, although acute exacerbations may also occur. Somatic pain of low to moderate intensity may stimulate the sympathetic nervous system and produce increased blood pressure, pulse, and respiration; dilated pupils; and increased skeletal muscle tension, such as rigid posture or clenched fists.
Visceral pain, which is diffuse and not well localized, results when nociceptors are stimulated in abdominal or thoracic organs and their surrounding tissues. It includes pain associated with cholecystitis, pancreatitis, uterine disorders, and liver disease and is often described as deep, dull, aching, or cramping. It may be referred to a different part of the body (e.g., pain from the liver can be referred to the right shoulder area; ischemic pain from the heart can be referred to the left arm or neck area). Severe visceral pain stimulates the parasympathetic nervous system and produces decreased blood pressure and pulse, nausea and vomiting, weakness, syncope, and possibly loss of consciousness.
Neuropathic pain is caused by lesions or physiologic changes that injure peripheral pain receptors, nerves, or the central nervous system. It is characterized by excessive excitability in the damaged area or surrounding normal tissues, so that nerve cells discharge more easily. As a result, pain may arise spontaneously or from a normally nonpainful stimulus such as a light touch. It is a relatively common cause of chronic pain and is usually described as severe, shooting, burning, or stabbing. It occurs with peripheral neuropathies associated with diabetes mellitus (diabetic neuropathy), herpes zoster infections (postherpetic neuralgia), traumatic nerve injuries, and some types of cancer or cancer treatments. It is difficult to treat because standard analgesics (e.g., nonsteroidal anti-inflammatory drugs and opioids) are less effective in neuropathic pain than in other types of pain. Antidepressants and anticonvulsants are often used along with analgesics.
Pathophysiology
Pain occurs when tissue damage activates the free nerve endings (pain receptors) of peripheral nerves. Nociceptors, nerve endings that selectively respond to painful stimuli, are abundant in arterial walls, joint surfaces, muscle fascia, periosteum, skin, and soft tissues; they are scarce in most internal organs. Causes of tissue damage may be physical (e.g., heat, cold, pressure, stretch, spasm, and ischemia) or chemical (e.g., pain-producing substances are released into the extracellular fluid surrounding the nerve fibers that carry the pain signal). These pain-producing substances activate pain receptors, increase the sensitivity of pain receptors, or stimulate the release of inflammatory substances.
For a person to feel pain, the signal from the nociceptors in peripheral tissues must be transmitted to the spinal cord, then to the hypothalamus and cerebral cortex in the brain. The signal is carried to the spinal cord by two types of nerve cells, A-delta fibers and C fibers. A-delta fibers, which are myelinated and found mainly in skin and muscle, transmit fast, sharp, well-localized pain signals. These fibers release glutamate and aspartate (excitatory amino acid neurotransmitters) at synapses in the spinal cord. C fibers, which are unmyelinated and found in muscle, abdominal viscera, and periosteum, conduct the pain signal slowly and produce a poorly localized, dull, or burning type of pain. Tissue damage resulting from an acute injury often produces an initial sharp pain transmitted by A-delta fibers followed by a dull ache or burning sensation transmitted by C fibers. C fibers release somatostatin and substance P at synapses in the spinal cord. Glutamate, aspartate, substance P, and perhaps other chemical mediators enhance transmission of the pain signal.
The dorsal horn of the spinal cord is the control center or relay station for information from the A-delta and C nerve fibers, for local modulation of the pain impulse, and for descending influences from higher centers in the central nervous system (CNS) (e.g., attention, emotion, memory). Here, nociceptive nerve fibers synapse with nonnociceptive nerve fibers (neurons that carry information other than pain signals). The brain also contains descending pathways that inhibit nociceptive input. Some brain nuclei are serotonergic and project to the dorsal horn of the spinal cord, where they suppress nociceptive transmission. Another inhibitory pathway is noradrenergic and originates in the pons. Thus, increasing the concentration of norepinephrine and serotonin in the synapse inhibits transmission of nerve impulses that carry pain signals to the brain and spinal cord.
In the brain, the thalamus is a relay station for incoming sensory stimuli, including pain. Perception of pain is a primitive awareness in the thalamus, and sensation is not well localized. From the thalamus, pain messages are relayed to the cerebral cortex, where they are perceived more specifically and analyzed to determine actions needed.
The CNS has its own system, the endogenous analgesia system, for relieving pain by suppressing the transmission of pain signals from peripheral nerves. Nerve signals entering the brain or morphine-like drugs can activate the system. Important elements include receptors and endogenous peptides with actions similar to those of morphine. Receptors are highly concentrated in some regions of the CNS, including the ascending and descending pain pathways and portions of the brain essential to the endogenous analgesia system. The peptides (i.e., endorphins, enkephalins, and dynorphins) interact with receptors to inhibit perception and transmission of pain signals. Endorphin release can be triggered by excitement, stress, or aerobic exercise. Enkephalins are believed to interrupt the transmission of pain signals at the spinal cord level by inhibiting the release of substance P from C nerve fibers. The endogenous analgesia system may also inhibit pain signals at other points in the pain pathway.
Clinical Manifestations
Pain is a subjective experience, and patients’ self-reporting of pain is considered the gold standard of pain assessment measurements because it offers the most valid measurement of pain. However, numerous factors, including mood, sleep disturbances, fatigue, and medications, may influence self-reporting. Cultural, gender, age, and other psychosocial factors can play a role in manifestations of pain.
A variety of pain measurement tools exists, including visual analogue scales, verbal or numerical rating scales, or picture scales. The pain measurement tool chosen should be appropriate to the individual patient, considering developmental, cognitive, emotional, language, and cultural factors. A mnemonic device (SOCRATES) can be used to assess the clinical manifestations of pain. Its meaning appears below:
• Site
• Onset
• Character
• Radiation
• Associations with other symptoms
• Time course (pattern)
• Exacerbating/relieving factors
• Severity
Nurses can use observational tools for patients who cannot communicate their pain for various reasons (e.g., unconsciousness, cognitive impairment). These tools involve facial expressions, limb movements, vocalization, restlessness, and guarding as indicators of pain. In patients with pain, vital signs may demonstrate tachycardia, tachypnea, and hypertension.
Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain
by CHOU, R, FANCIULLO, G. J., FINE, P. C, ADLER, J. A., BALLANTYNE, J. C, DAVIES, P., DONOVAN, M. I., FISHBAIN, D A., FOLEY, K. M., FUDIN, J., GILSON, A. M., KELTER, A., MIASKOWSKI, C, MAUSKOP, A., O’CONNOR, P. C, PASSIK, S. D., PASTERNAK, G. W., PORTENOY, R K, RICH, B. A., ROBERTS, R. G, TODD, K H.
The Journal of Pain 2009,10(2), 113-130
The use of long-term opioid therapy for chronic non-cancer pain has escalated. A systematic review of the evidence regarding chronic opioid use for chronic cancer pain demonstrates that chronic opioid therapy can be effectively and safely administered in carefully selected and monitored patients. Clinical practice guidelines by the American Pain Society and the American Academy of Pain Medicine present recommendations to minimize adverse effects and the potential for abuse of opioids. The comprehensive recommendations provide guidance on the myriad of issues that must be considered to ensure patient safety while providing effective pain control.
IMPLICATIONS FOR NURSING PRACTICE: The safe and effective use of opioids in chronic noncancer pain involves knowledge and clinical skills. Recommendations from the clinical guidelines take into consideration the most effective plan of care, incorporating the best available evidence with clinician judgment and patient preferences to assess and manage adverse effects, abuse, and addiction.
Drug Therapy
Opioid analgesics relieve moderate to severe pain by inhibiting the transmission of pain signals from peripheral tissues to the brain, reducing the perception of pain sensation in the brain, producing sedation, and decreasing the emotional upsets often associated with pain. They also inhibit the production of pain and inflammation by prostaglandins and leukotrienes in peripheral tissues. Most of these drugs are Schedule II drugs under federal narcotics laws and may lead to drug abuse and dependence (see Chap. 1).
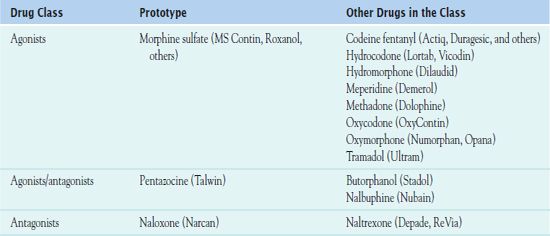
Table 48.1 outlines the subgroups of opioid analgesics: the opioid agonists, agonists/antagonists, and antagonists. The larger group of agonists includes morphine and morphine-like drugs. These agents have activity at mu and kappa receptors and thus produce prototypical effects. As their name indicates, the agonists/antagonists have both agonist and antagonist activity. Antagonists are antidote drugs that reverse the effects of agonists. In addition, numerous combinations of opioid and nonopioid analgesics (see Chap. 14) are available and commonly used in most health care settings. In addition, preemptive analgesia is used to reduce postsurgical pain by simultaneously administering medications from different drug classes to suppress pain by blocking multiple pain pathways (Box 48.2).
BOX 48.2 Preemptive Analgesia
To lessen postsurgical pain, simultaneous administration of medications from different drug classes creates a multimodal approach to suppress pain by blocking multiple pain pathways. The drugs are commonly given before the start of surgery and continue through the maintenance phase. Many of these are considered opioid sparing, which allows a clinically significant reduction in dose of opioid during and after surgery. This reduces opioid adverse effects without sacrificing the control of pain. Rather than treating pain after it has occurred, the goal is to significantly suppress both short- and long-term pain. In addition, properly managed pain reduces health care costs and improves postoperative outcome. This therapeutic intervention is called preemptive analgesia.
Drugs that can be used in preemptive analgesia include the following.
 Parenteral drugs include
Parenteral drugs include
 Ketamine, primarily used for the induction and maintenance of general anesthesia, usually in combination with a sedative
Ketamine, primarily used for the induction and maintenance of general anesthesia, usually in combination with a sedative
 Dexmedetomidine (Precedex) that has sedative and hypnotic effects similar to natural sleep without respiratory depression
Dexmedetomidine (Precedex) that has sedative and hypnotic effects similar to natural sleep without respiratory depression
 Intravenous (IV) nonsteroidal anti-inflammatory drugs (NSAIDs) such as ketorolac tromethamine (Toradol), ibuprofen (Caldolor), and the IV form of acetaminophen (Ofirmev) for short-term management of moderate to severe pain. Caution must be exercised when administering NSAIDs (see Chap. 14) as there is an elevated risk of postoperative bleeding.
Intravenous (IV) nonsteroidal anti-inflammatory drugs (NSAIDs) such as ketorolac tromethamine (Toradol), ibuprofen (Caldolor), and the IV form of acetaminophen (Ofirmev) for short-term management of moderate to severe pain. Caution must be exercised when administering NSAIDs (see Chap. 14) as there is an elevated risk of postoperative bleeding.
 Oral medications include
Oral medications include
 Gabapentin (Neurontin) and pregabalin (Lyrica), both GABA analogues and anticonvulsants (Chap. 52)
Gabapentin (Neurontin) and pregabalin (Lyrica), both GABA analogues and anticonvulsants (Chap. 52)
 Celecoxib (Celebrex), an NSAID and selective
Celecoxib (Celebrex), an NSAID and selective
 COX-2 inhibitor
COX-2 inhibitor
 Clonidine (Catapres), a direct acting alpha-2 adrenergic receptor agonist
Clonidine (Catapres), a direct acting alpha-2 adrenergic receptor agonist
 Neuroaxial blocks such as epidural and spinal anesthesia, peripheral regional anesthetic techniques, and local wound infiltration using long-acting local anesthetics to provide superior analgesia. Regional techniques can be combined with general anesthesia or sedation.
Neuroaxial blocks such as epidural and spinal anesthesia, peripheral regional anesthetic techniques, and local wound infiltration using long-acting local anesthetics to provide superior analgesia. Regional techniques can be combined with general anesthesia or sedation.
Opioid Agonists
 Morphine sulfate, the prototype, is an opium alkaloid used mainly to relieve moderate to severe pain. A Schedule II-controlled drug, administration is most often oral or parenteral. Patient response depends on route of administration and dosage.
Morphine sulfate, the prototype, is an opium alkaloid used mainly to relieve moderate to severe pain. A Schedule II-controlled drug, administration is most often oral or parenteral. Patient response depends on route of administration and dosage.
Pharmacokinetics
Morphine is well absorbed after oral (PO), intramuscular (IM), subcutaneous, and intravenous (IV) administration. PO formulations undergo significant first-pass metabolism in the liver, which means that PO doses must be larger than injected doses to have equivalent therapeutic effects. After PO administration of fast-acting (e.g., immediate-release) formulations, peak activity occurs in about 60 minutes. After IV injection, maximal analgesia and respiratory depression usually occur within 10 to 20 minutes. After IM injection, these effects occur in about 30 minutes. After subcutaneous injection, morphine effects may be delayed up to 60 to 90 minutes. Morphine is extensively metabolized in the liver, and metabolites are excreted in urine. Morphine is about 30% bound to plasma proteins, and its half-life is 2 to 4 hours. The duration of action is 5 to 7 hours.
Action
Morphine relieves pain by binding to receptors in the brain, spinal cord, and peripheral tissues. When bound to the drug, receptors function like gates that close and thereby block or decrease transmission of pain impulses from one nerve cell to the next. The receptors also activate the endogenous analgesia system. The major types of receptors are mu, kappa, and delta.
Most of the effects of morphine (analgesia; CNS depression, with respiratory depression and sedation; euphoria; decreased gastrointestinal [GI] motility; and physical dependence) are attributed to activation of the mu receptors. Analgesia, sedation, and decreased GI motility occur with activation of kappa receptors. The endogenous analgesia system involves the delta receptors, which may not bind with opioid drugs.
Use
The main indication for morphine is to prevent or relieve acute or chronic pain. Specific conditions for morphine include acute myocardial infarction, biliary colic, renal colic, burns and other traumatic injuries, postoperative states, and cancer. Health care providers usually give morphine for chronic pain, such as that associated with terminal cancer, only when other measures and milder drugs are ineffective. Other clinical uses of morphine include the following:
• Before and during surgery to promote sedation, decrease anxiety, facilitate induction of anesthesia, and decrease the amount of anesthesia required
• During labor and delivery (obstetric analgesia)
• Treatment of GI disorders, such as abdominal cramping and diarrhea
• Treatment of acute pulmonary edema
• Treatment of severe, unproductive cough (codeine may be used)
• Unlabeled use: relief of dyspnea associated with acute left ventricular failure and pulmonary edema
Table 48.2 gives dosages of morphine sulfate and other opioid agonists.
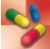 TABLE 48.2
TABLE 48.2
*Actiq, Fentora, and Ionsys are special preparations for specific uses. (Check manufacturers’ instructions for dosage and administration instructions.)
Use in Children
In general, experts do not completely understand the physiology, pathology, assessment, and management of pain in children. Many authorities indicate that in children pain is often ignored or undertreated, including children having surgical and other painful procedures for which adults routinely receive an anesthetic, a strong analgesic, or both. This is especially true in preterm and full-term neonates. In the past, one reason for inadequate prevention and management of pain in newborns was a common belief that they did not experience pain because of immature nervous systems. However, research indicates that neonates have abundant C fibers and that A-delta fibers are developing during the first few months of life. These nerve fibers carry pain signals from peripheral tissues to the spinal cord. In addition, brain pain centers and the endogenous analgesia system are developed and functional.
Older infants and children may experience pain even when analgesics are readily available. For example, children may be unable to communicate their discomfort, or they may fear injections. Health care providers or parents may fear adverse effects of morphine, including excessive sedation, respiratory depression, and addiction.
To make the situation more complex, formulations of morphine and other opioids for children are not generally available. Rectal suppositories, which are used more often in children than in adults, are useful when PO or parenteral routes are not indicated.
QSEN Safety Alert 
When children’s doses are calculated based on adult doses, fractions and decimals often result, which greatly increases the risk of a dosage error. It is advisable to have two people do the calculations independently and compare results.
With all forms of opioids, it is important to note that the effects of the drugs in children may differ from those expected in adults because of physiologic and pharmacokinetic differences. Even with suppositories, the dose of medication received by the child is unknown because drug absorption is erratic and because adult suppositories are sometimes cut in half or otherwise altered. The nurse assesses children regularly and is alert for unusual signs and symptoms.
Use in Older Adults
It is necessary to use morphine cautiously in older adults, especially if they are debilitated; have hepatic, renal, or respiratory impairment; or are receiving other drugs that depress the CNS. Older adults are especially sensitive to respiratory depression, excessive sedation, confusion, and other adverse effects. However, they should receive adequate analgesia, along with vigilant monitoring. Specific recommendations for use in older adults include the following:
• Start with low doses and increase doses gradually, if necessary.
• Give morphine less frequency than for younger adults because the duration of action may be longer.
• Monitor carefully for sedation or confusion. In addition, monitor voiding and urine output because acute urinary retention is more likely to occur in older adults.
Use in Patients With Renal Impairment
Patients with renal impairment should take minimal doses of morphine for the shortest effective time because usual doses may produce profound sedation and a prolonged duration of action. Morphine produces an active metabolite that may accumulate in patients with renal impairment.
Use in Patients With Hepatic Impairment
Morphine is extensively metabolized by the liver; therefore, morphine may accumulate and cause increased adverse effects in the presence of hepatic impairment. Drug accumulation and increased adverse effects may occur if dosage is not reduced, especially with chronic use.
Use in Patients With Critical Illness
Morphine is commonly used to manage pain associated with disease processes and invasive diagnostic and therapeutic procedures. In intensive care units, morphine and other opioid agonists are also used concurrently with sedatives and neuromuscular blocking agents, which increase the risks of adverse drug reactions and interactions. In patients with critical illness, morphine is usually given by IV bolus injection or continuous infusion. Guidelines include the following:
• Assume that all critically ill patients are in pain or at high risk for development of pain.
• When pain is thought to be present, identify and treat the underlying cause when possible.
• Prevent pain when possible. Some specific interventions include being very gentle when performing nursing care to avoid tissue trauma and positioning patients to prevent ischemia, edema, and misalignment. In addition, give analgesics before painful procedures, when indicated.
• Consider patient-controlled analgesia (PCA), which is any method that allows a person in pain to administer his or her own pain relief. Commonly, an IV device regulated by programmable settings delivers morphine at a preset bolus dosage when the patient presses a button. It is possible to program a lockout period into the pump so that the frequency of administration is controlled, as well as the basal amount of drug to be delivered. When starting a continuous IV infusion of pain medication, a health care professional gives a loading dose to attain therapeutic blood levels quickly. The nurse assesses pain scores after initiation, after any change in pump setting, and periodically using a standardized pain rating scale to assess pain relief response to the PCA medication based on hospital protocol. Box 48.3 outlines specific assessment considerations prior to and throughout the administration of opioids using PCA.



