Outline the etiology of cancer development.
 Describe the major types of antineoplastic drugs in terms of mechanism of action, indications for use, and the nursing process.
Describe the major types of antineoplastic drugs in terms of mechanism of action, indications for use, and the nursing process.
 Discuss adverse effects of cytotoxic antineoplastic drugs and their prevention or management.
Discuss adverse effects of cytotoxic antineoplastic drugs and their prevention or management.
 Be able to teach patients about the administration of some anticancer drugs.
Be able to teach patients about the administration of some anticancer drugs.
 Understand how to implement the nursing process in the care of patients undergoing drug therapy for cancer, including how to teach and promote efforts to prevent cancer.
Understand how to implement the nursing process in the care of patients undergoing drug therapy for cancer, including how to teach and promote efforts to prevent cancer.
Clinical Application Case Study
Julia Gardner, a 60-year-old school secretary, has stage 2b breast cancer and is receiving adjuvant chemotherapy after a mastectomy. Mrs. Gardner has finished her fourth cycle of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) treatment. She will be receiving subsequent chemotherapy in 3 weeks with paclitaxel (Taxol) and trastuzumab (Herceptin).
KEY TERMS
Alopecia: hair loss
Cell cycle: series of intracellular events occurring from one cell division to the next
Chemotherapy: in oncology, the treatment of cancer with an antineoplastic drug or with a combination of such drugs typically given in cycles
Mucositis: inflammation of mucous membranes
Mutation: structural change in the genetic material of a cell
Myelosuppression: bone marrow depression
Neutropenia: low neutrophil count
Oncogenes: genes that have the potential to cause a normal cell to become cancerous
Palliation: alleviation of pain and symptoms without expecting to eliminate the cause
Proto-oncogenes: genes that have the potential to change into active oncogenes
Remission: period when symptoms of a disease have subsided (“remitted”)
Thrombocytopenia: low platelet count
Tumor lysis syndrome: life-threatening condition that occurs when large numbers of cancer cells are killed or damaged simultaneously and release their intracellular contents into the bloodstream
Tumor suppressor genes: genes that inhibit unrestrained cell growth; when inactivated, mutant abnormal cells may be allowed to proliferate; also called antioncogenes
Introduction
The term cancer is used to describe many disease processes with the common characteristics of uncontrolled cell growth, invasiveness, and metastasis as a result of intracellular genetic changes that allow new cells to grow in an unregulated manner. Oncology is the study of cancer and its treatment. Drugs used in oncologic disorders include cytotoxic medications that kill, damage, or slow the growth of cancer cells as well as those that prevent or treat adverse drug effects. Chemotherapy is a major treatment modality for cancer, along with surgery and radiation therapy. Major groups of anticancer drugs include traditional cytotoxic agents (e.g., alkylating agents, antimetabolites, antitumor antibiotics, plant alkaloids); newer cytotoxic “biologic targeted therapies” (e.g., monoclonal antibodies, growth factor inhibitors); and hormone inhibitors, which are not cytotoxic. In addition, several drugs play a role in ameliorating the adverse effects of cytotoxic drugs (e.g., cytoprotectants), including some immunostimulants. This chapter describes the characteristics of cancer etiology, pathophysiology, clinical manifestations, and the drugs used in the treatment of this disease. A nurse who has additional training in chemotherapy use administers these drugs. The focus of this chapter is on the nursing management of patients receiving the medication and not on dosing and administration of individual drugs. The general considerations for chemotherapy management are outlined for each major drug group.
Overview of Cancer
Etiology
Normal cells reproduce in response to a need for tissue growth or repair and stop reproduction in response to growth regulation signals. The normal cell cycle is the interval between the “birth” of a cell and its subsequent division into two daughter cells. This cycle involves the orderly stages of growth as well as protein, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) synthesis (Fig. 12.1). Newly formed daughter cells may then enter the resting phase (G0) or proceed through the reproductive cycle to form more new cells. Normal cells regulated by normal growth genes are well differentiated in appearance and function, and they have a characteristic lifespan.
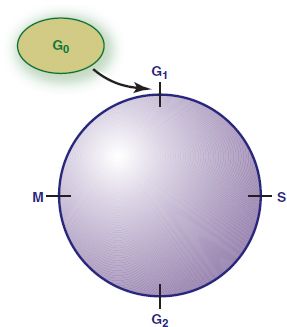
Figure 12.1 Normal cell cycle. The normal cell cycle (the interval between the birth of a cell and its division into two daughter cells) involves several phases. During the resting phase (G0), cells perform all usual functions except replication; that is, they are not dividing but are capable of doing so when stimulated. Different types of cells spend different lengths of time in this phase, after which they either reenter the cell cycle and differentiate or die. During the first active phase (G1), ribonucleic acid (RNA) and enzymes required for production of deoxyribonucleic acid (DNA) are developed. During the next phase (S), DNA is synthesized for chromosomes. During G2, RNA is synthesized, and the mitotic spindle is formed. Mitosis occurs in the final phase (M). The resulting two daughter cells may then enter the resting phase (G0) or proceed through the reproductive cycle.
In contrast, malignant cells have lost the normal genetic regulation that controls cell growth, invading normal tissues and taking blood and nutrients away from these tissues. They grow in an uncontrolled fashion without regard to growth regulation signals (e.g., contact with other cells) that stop the growth of normal cells. Also, they are undifferentiated, having lost the structural and functional characteristics of the cells from which they originated. In addition, they lack cell adhesion; hence, as well as invading normal tissue, malignant cells enter blood and lymph vessels, circulate through the body, and can produce additional neoplasms at sites distant from the primary tumor.
A malignant cell develops from a damaged normal cell, beginning with a random mutation (abnormal structural change in the genetic material of a cell) that occurs in conjunction with acquired damage to genes that regulate normal cell growth. Usually, body defenses (e.g., an immune response) destroy a mutated cell if the DNA damage cannot be repaired by normal cell enzymes. However, if the mutated cell eludes destruction by the immune system and additional mutations develop without repair or apoptosis (programmed cell death) during succeeding cell divisions, malignant transformation may occur. Underlying the failure to repair or destroy these abnormal cells is damage to genes that regulate cell growth; the cells have lost their ability to effectively regulate cell growth. Additional changes allow cells with progressively more malignant characteristics to survive despite their abnormalities. It often takes years for malignant cells to be able to grow into a clinically detectable neoplasm.
Cancer is a heterogeneous disease and has multiple causes, such as environmental factors (tobacco) and genetic factors that may combine to contribute to the progression of malignancy. Genetic causes include mutation of genes, abnormal activation of genes that regulate cell growth and mitosis, and damage to tumor suppressor genes.
Pathophysiology
Abnormal genes, called oncogenes, which are involved in cancer growth, are mutations of normal growth-regulating genes, called proto-oncogenes, which are present in all body cells. When proto-oncogenes are exposed to carcinogens and genetically altered to become oncogenes, they may stimulate cell growth continuously, allowing abnormal, disordered, and unregulated cell replication. The unregulated cell growth and proliferation promoted by oncogenes contributes to neoplastic transformation of the cell. Tumors of the breast, colon, lung, and bone have all been linked to activation of oncogenes.
Tumor suppressor genes (antioncogenes) normally function to inhibit inappropriate cellular growth and proliferation. Abnormal tumor suppressor genes (i.e., absent, damaged, mutated, or inactivated) may be inherited or result from exposure to carcinogens. When a tumor suppressor gene inside a cell is inactivated, abnormal cells can begin unregulated cell growth. One tumor suppressor gene, p53, is present in virtually all normal tissues. When cellular DNA is damaged, the p53 gene allows time for DNA repair and restricts proliferation of cells with abnormal DNA. Mutations of the p53 gene, a common genetic change in cancer, are associated with more than 90% of small cell lung cancers and more than 50% of breast and colon cancers. Mutant p53 proteins can also form complexes with normal p53 proteins and inactivate the function of the normal suppressor gene.
Thus, activation of oncogenes and inactivation of antioncogenes probably both play roles in cancer development. Multiple genetic abnormalities are characteristic of cancer cells and may occur concurrently or sequentially. Overall, evidence indicates that neoplastic transformation is a progressive process involving both a series of cellular mutations and damage to growth-regulating genes, which allows abnormal cells to replicate without normal immune destruction. Malignancy probably results from a combination of genetic and environmental factors experienced over a person’s lifetime, including random cell mutations, exposure to carcinogens, and host genetic or tissue characteristics, that increase susceptibility to cancer development.
Once a cancer develops, factors influencing its continued growth include blood and nutrient supply, immune response, and hormonal stimulation (e.g., in tumors of the breast, uterus, ovary, and prostate). Malignant tumors are able to form new blood vessels, a process called angiogenesis, to support their growth and escape immune destruction as a result of their similarity to other normal cells.
Types of Malignant Neoplasms
Malignant neoplasms are classified according to the type of tissue involved and other characteristics. With the exception of the acute leukemias, they are considered chronic diseases.
Hematologic malignancies involve the bone marrow and lymphoid tissues; they include leukemias, lymphomas, and multiple myeloma. Leukemias are cancers of the bone marrow characterized by overproduction of abnormal white blood cells. The four main types are acute lymphocytic, acute myelogenous, chronic lymphocytic, and chronic myelogenous. Lymphomas are tumors of lymphoid tissue characterized by abnormal proliferation of the white blood cells normally found in lymphoid tissue. They usually develop within lymph nodes and may occur anywhere, because virtually all body tissues contain lymphoid structures. The two main types are Hodgkin’s disease and non-Hodgkin’s lymphoma. Multiple myeloma is a tumor of the bone marrow in which abnormal plasma cells proliferate. Because normal plasma cells produce antibodies and abnormal plasma cells cannot fulfill this function, the body’s immune system is impaired. As the malignant cells expand, they crowd out normal cells, interfere with other bone marrow functions, infiltrate and destroy bone, and eventually metastasize to other tissues, such as the spleen, liver, and lymph nodes.
Solid neoplasms are composed of a mass of malignant cells (parenchyma) and a supporting structure of connective tissue, blood vessels, and lymphatics (stroma). The two major classifications are carcinomas and sarcomas. Carcinomas are derived from epithelial tissues (skin, mucous membrane, linings and coverings of viscera) and are the most common type of malignant tumors. They are further classified by cell type, such as adenocarcinoma or basal cell carcinoma. Sarcomas are derived from connective tissue (muscle, bone, cartilage, fibrous tissue, fat, blood vessels). They are subclassified by cell type (e.g., osteogenic sarcoma, angiosarcoma).
Grading and Staging of Malignant Neoplasms
When a malignant neoplasm is identified, it is further “graded” according to the degree of malignancy and “staged” according to tissue involvement. Grades 1 and 2 are similar to the normal tissue of origin and show cellular differentiation. Grades 3 and 4 are unlike the normal tissue of origin, less differentiated, and more malignant. Staging indicates whether the neoplasm is localized or metastasized and which organs are involved. These characteristics assist in treatment decision-making (e.g., localized tumors are usually amenable to surgery, radiation, or chemotherapy combined with radiation [concurrent] therapy, whereas metastatic disease requires systemic chemotherapy).
Clinical Manifestations
Clinical manifestations vary according to the location and extent of the disease process. There are few effects initially. However, local effects occur as the tumor grows; it becomes large enough to cause pressure, distort or affect blood supply in surrounding tissues, interfere with organ function, or obstruct ducts and organ outlets. Systemic conditions such as cachexia, anorexia, and weight loss occur from the effects of the growing tumor increasing overall metabolic demands and altering glucose utilization at the cellular level. Other symptoms and signs may include anemia, malnutrition, pain, immunosuppression, infection, hemorrhagic tendencies, thromboembolism, and hypercalcemia, as well as various symptoms related to impaired function of affected organs and tissues.
Drug Therapy
Chemotherapy is most often used to indicate the use of traditional cytotoxic antineoplastic drugs for the treatment of cancer. Except for hormone inhibitors that slow the growth of cancer cells stimulated by hormones, the purpose of all antineoplastic drugs is to damage or kill cancer cells (i.e., be cytotoxic). The goal of treatment with cytotoxic antineoplastic drugs is cure, remission (period when symptoms of a disease have subsided) or palliation (alleviation of pain and symptoms without expecting to eliminate the cause). In hematologic malignancies such as leukemias, drug therapy is the treatment of choice because the disease is disseminated and must be treated systemically rather than locally, with surgery or radiation. In solid tumors, drug therapy may be used, before (neoadjuvant) or after (adjuvant) surgery or radiation therapy and when there is metastasis that is not surgically resectable.
Antineoplastic drugs are also sometimes used in the treatment of nonmalignant conditions. For example, smaller doses of methotrexate (MTX) are used as an immunosuppressant for treating the inflammation of rheumatoid arthritis and psoriasis.
Traditional chemotherapeutic drugs share common general characteristics (Box 12.1). Most chemotherapy regimens contain a combination of drugs with actions at different places in the cell cycle process of cell growth and replication (Fig. 12.2), destroying a greater number of cancer cells and reducing the risk of the emergence of drug resistance. Drug resistance may emerge when (1) cancer cells overexpress target genes that prevent the drug’s being absorbed by the malignant cells, (2) tumor cells are able to inactivate the drug, or (3) apoptosis occurs in tumor cells is defective. Oncologists order many newer drugs for use after initial cytotoxic drug therapy to prevent drug resistance from affecting the tumor response.
BOX 12.1 General Characteristics of Cytotoxic Antineoplastic Drugs
 Most of these drugs kill malignant cells by interfering with cell replication, with the supply and use of nutrients (e.g., amino acids, purines, pyrimidines), or with the genetic materials in the cell nucleus (DNA or RNA).
Most of these drugs kill malignant cells by interfering with cell replication, with the supply and use of nutrients (e.g., amino acids, purines, pyrimidines), or with the genetic materials in the cell nucleus (DNA or RNA).
 The drugs act during the cell’s reproductive cycle. Some, called cell cycle-specific, act mainly during specific phases such as DNA synthesis or formation of the mitotic spindle. Others act during any phase of the cell cycle and are called cell cycle-nonspecific.
The drugs act during the cell’s reproductive cycle. Some, called cell cycle-specific, act mainly during specific phases such as DNA synthesis or formation of the mitotic spindle. Others act during any phase of the cell cycle and are called cell cycle-nonspecific.
 The drugs are most active against rapidly dividing cells, both normal and malignant. Commonly damaged normal cells are those of the bone marrow, the lining of the gastrointestinal tract, and the hair follicles. Healthy cells usually recover fairly soon. However, malignant cells have lost normal genetic growth regulation and continue to actively divide in an unregulated way; thus, more of them are susceptible to the effects of cytotoxic drugs.
The drugs are most active against rapidly dividing cells, both normal and malignant. Commonly damaged normal cells are those of the bone marrow, the lining of the gastrointestinal tract, and the hair follicles. Healthy cells usually recover fairly soon. However, malignant cells have lost normal genetic growth regulation and continue to actively divide in an unregulated way; thus, more of them are susceptible to the effects of cytotoxic drugs.
 Each drug dose kills a specific percentage of cells. To achieve a cure, all malignant cells must be killed or reduced to a small number that can be killed by the person’s immune system.
Each drug dose kills a specific percentage of cells. To achieve a cure, all malignant cells must be killed or reduced to a small number that can be killed by the person’s immune system.
 Most cytotoxic antineoplastic drugs are potential teratogens, and women should not become pregnant during drug therapy and for several months after the therapy stops.
Most cytotoxic antineoplastic drugs are potential teratogens, and women should not become pregnant during drug therapy and for several months after the therapy stops.
 Weight-based dosing is used to individualize dosing with all cytotoxic drugs due to their potential to cause significant toxicities to normal cells as well as cancer cells.
Weight-based dosing is used to individualize dosing with all cytotoxic drugs due to their potential to cause significant toxicities to normal cells as well as cancer cells.
 Oncologists combine various cytotoxic drugs with different cell cycle activities and different organ toxicities in treatment plans called “regimens” to more effectively kill the cancer cells while minimizing damage to normal tissues.
Oncologists combine various cytotoxic drugs with different cell cycle activities and different organ toxicities in treatment plans called “regimens” to more effectively kill the cancer cells while minimizing damage to normal tissues.
 Most cytotoxic drugs are water soluble rather than lipid soluble; thus, they do not cross cell membranes readily and must be administered by intravenous injection or infusion.
Most cytotoxic drugs are water soluble rather than lipid soluble; thus, they do not cross cell membranes readily and must be administered by intravenous injection or infusion.
 Many of the cytotoxic drugs are prodrugs that are metabolized by liver enzymes to active metabolites, which then have cytotoxic activity affecting protein, DNA, or RNA synthesis or cell division.
Many of the cytotoxic drugs are prodrugs that are metabolized by liver enzymes to active metabolites, which then have cytotoxic activity affecting protein, DNA, or RNA synthesis or cell division.
 Because cytotoxic drugs are primarily metabolized in the liver and then excreted in urine, they can also have toxic effects on the liver and kidney, especially at higher doses.
Because cytotoxic drugs are primarily metabolized in the liver and then excreted in urine, they can also have toxic effects on the liver and kidney, especially at higher doses.
 Many cytotoxic antineoplastic drugs have U.S. Food and Drug Administration (FDA)-issued BLACK BOX WARNINGS ♦ about potentially serious adverse effects, and all have special precautions for safe usage.
Many cytotoxic antineoplastic drugs have U.S. Food and Drug Administration (FDA)-issued BLACK BOX WARNINGS ♦ about potentially serious adverse effects, and all have special precautions for safe usage.
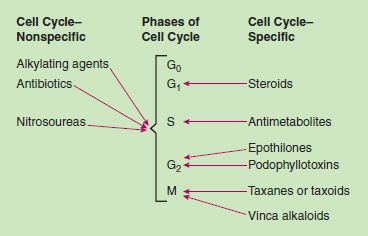
Figure 12.2 Cell cycle effects of cytotoxic antineoplastic drugs.
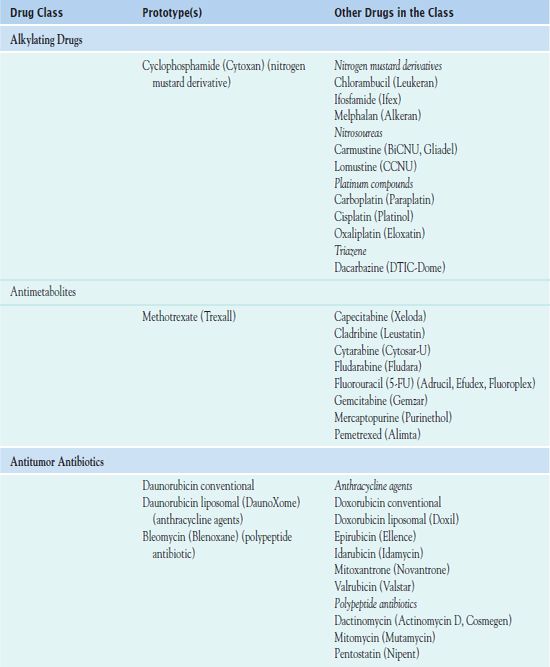
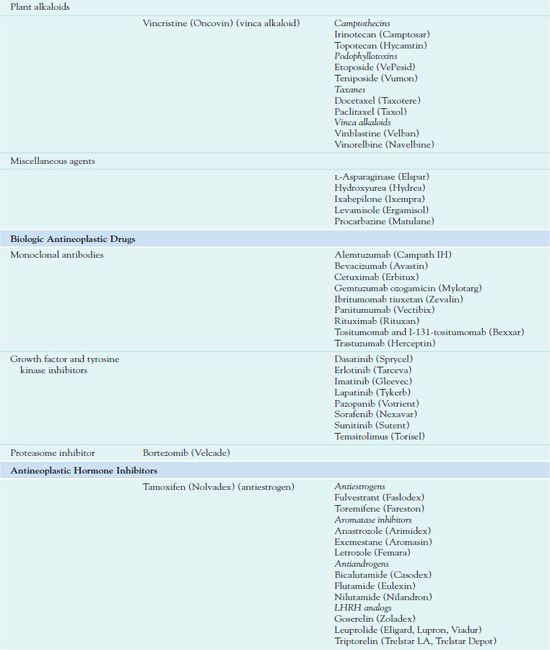
Chemotherapy administration usually occurs in cycles, depending on the type of cancer and which drugs are used. Cyclic administration involves taking the drugs for a specific period, with a recovery period following each treatment cycle. The recovery period allows time for the patient to produce new, healthy cells to replace the normal rapidly dividing cells that have been affected by the drugs. Chemotherapy is often continued as long as it is effective and does not produce unacceptable toxicity or until there is no longer any evidence of malignancy. Table 12.1 outlines the drugs used in cancer treatment.

LHRH, luteinizing hormone—releasing hormone.
Cytotoxic Antineoplastic Drugs Used I to Treat Cancer
The consequences of inappropriate or erroneous chemotherapy may be fatal for patients (from the disease or from the treatment); thus, medical oncologists experienced in use of cancer drugs manage chemotherapy regimens.
QSEN Safety Alert 
Because of the toxicity of these drugs, nurses who administer intravenous (IV) cytotoxic chemotherapy receive special training and are certified in handling and administering the chemotherapy drugs safely and accurately.
This chapter focuses on the overall nursing considerations for caring for patients receiving chemotherapy, not the administration of particular drugs. Therefore, information is not presented in a prototype format.
Pharmacokinetics
Most cytotoxic drugs are water soluble rather than lipid soluble and require administration by IV injection or infusion. Some, such as cyclophosphamide, are better absorbed and are available as oral formulations. A few, such as MTX, are also given intrathecally. The nitrosoureas (e.g., carmustine, lomustine) are unique in being lipid soluble and able to cross the blood–brain barrier; hence, they are widely used in the treatment of brain tumors. Carmustine is also formulated as a dissolving wafer, which can be implanted in the brain tissue at the time of surgical tumor resection.
Liposomal preparations of some cytotoxic drugs (e.g., doxorubicin) use a lipid membrane to encase the drug molecules. Liposomal preparations can increase drug concentration in malignant tissues that are more permeable, allowing the lipid vesicle to more easily concentrate in the tumor, while lowering the concentration in normal tissues that are less permeable, thereby increasing effectiveness and decreasing toxicity (e.g., cardiotoxicity).
Action
Cytotoxic antineoplastic drugs are usually classified in terms of their mechanisms of action (alkylating agents, antimetabolites) or their sources (plant alkaloids, antibiotics).
Alkylating Drugs
Alkylating drugs include nitrogen mustard derivatives, nitrosoureas, platinum compounds, and triazenes.
• Nitrogen mustard derivatives are cell cycle–nonspecific agents, causing cross-linking of DNA and RNA and interfering with subsequent cell division. The most widely used alkylating drug, a nitrogen mustard derivative, is  cyclophosphamide (Cytoxan, Neosar).
cyclophosphamide (Cytoxan, Neosar).
• Nitrosoureas also interfere with DNA replication and RNA synthesis and may inhibit essential enzymatic reactions in cancer cells. Carmustine and lomustine are both cell cycle–nonspecific, and they are lipid soluble. These features allow them to enter the brain and cerebrospinal fluid more readily than other antineoplastic drugs, which make them useful for treating lymphomas and brain tumors.
• Platinum compounds are cell cycle–nonspecific agents that cross-link DNA inhibiting DNA, RNA, and protein synthesis. Cisplatin (Platinol) is the most widely used of the platinum compounds.
• Triazene compounds are cell cycle–nonspecific agents with antitumor and mutagenic properties. The drugs are effective for metastatic malignant melanoma, Hodgkin’s disease, and various sarcomas. The most widely used drug in the class is dacarbazine (DTIC-Dome).
Antimetabolites
Antimetabolites are drugs that are similar to metabolites or nutrients needed by cells for reproduction. These drugs replace normal metabolites or inhibit essential enzymes, inside the cell, depriving the cell of substances needed for DNA formation or causing abnormal DNA formation. The drugs are cell cycle–specific because they exert their cytotoxic effects only during the S phase of the cell’s reproductive cycle, when DNA is being synthesized. Antimetabolites that are used in cancer treatment are folate antagonists, such as  methotrexate (Trexall), or MTX; purine antagonists (e.g., mercaptopurine, cladribine, fludarabine); or pyrimidine antagonists (e.g., fluorouracil, capecitabine, cytarabine). These drugs are most effective against rapidly growing tumors, and individual drugs vary in their effectiveness with different kinds of cancer.
methotrexate (Trexall), or MTX; purine antagonists (e.g., mercaptopurine, cladribine, fludarabine); or pyrimidine antagonists (e.g., fluorouracil, capecitabine, cytarabine). These drugs are most effective against rapidly growing tumors, and individual drugs vary in their effectiveness with different kinds of cancer.
Antitumor Antibiotics
 Bleomycin (Blenoxane) inhibits DNA, RNA, and protein synthesis in susceptible cells, preventing cell division. The anthracycline agents (e.g., doxorubicin, daunorubicin, epirubicin) bind to DNA so that DNA and RNA transcription is blocked. They are active in all phases of the cell cycle, and their cytotoxic effects are similar to those of the alkylating agents. Mitomycin causes DNA cross-linking and inhibits DNA synthesis.
Bleomycin (Blenoxane) inhibits DNA, RNA, and protein synthesis in susceptible cells, preventing cell division. The anthracycline agents (e.g., doxorubicin, daunorubicin, epirubicin) bind to DNA so that DNA and RNA transcription is blocked. They are active in all phases of the cell cycle, and their cytotoxic effects are similar to those of the alkylating agents. Mitomycin causes DNA cross-linking and inhibits DNA synthesis.
Plant Alkaloids
Plant alkaloids include several types of drugs.
• Camptothecins (also called DNA topoisomerase inhibitors) inhibit an enzyme required for DNA replication and repair.
• Podophyllotoxins act mainly in the G2 phase of the cell cycle and prevent mitosis.
• Taxanes (e.g., paclitaxel, docetaxel) interfere with the ability of the chromosomes to separate during cell division.
• Vinca alkaloids (e.g.,  vincristine [Oncovin], vinblastine (Velban), vinorelbine (Navelbine)) are cell cycle–specific agents that interfere with cell mitosis. Despite having similar structures, they have different antineoplastic activities and adverse effects.
vincristine [Oncovin], vinblastine (Velban), vinorelbine (Navelbine)) are cell cycle–specific agents that interfere with cell mitosis. Despite having similar structures, they have different antineoplastic activities and adverse effects.
Miscellaneous Drugs
Miscellaneous agents vary in their sources, mechanisms of action, indications for use, and toxic effects. L-Asparaginase (Elspar) is an enzyme that inhibits protein synthesis and reproduction by depriving cells of required amino acids. Pegaspargase (Oncaspar) is a modified formulation for people who are hypersensitive to L-asparaginase. Hydroxyurea (Hydrea) acts in the S phase of the cell cycle to impair DNA synthesis. Ixabepilone (Ixempra) is the first of a new class of antineoplastic drugs called epothilones. Obtained from bacteria, epothilones stop growth of tumor cells by preventing cell division (antimitotic effects). Ixabepilone is a semisynthetic analog of epothilone. Procarbazine (Matulane), a monoamine oxidase inhibitor, inhibits DNA, RNA, and protein synthesis.
Use
Researchers have found that certain combinations of cytotoxic drugs with different cytotoxic activities in the cell cycle and different organ toxicities are most effective in certain types of cancer. Hence, concurrent administration of multiple drugs based on the patient’s type of malignancy may be necessary. When both cytotoxic and hormone inhibitor drug therapies are required, however, concurrent administration is not appropriate, because hormone antagonists decrease malignant cell growth and cytotoxic agents are most effective when the cells are actively dividing. Patients with breast cancer usually receive a hormone-inhibiting drug before a cytotoxic drug in metastatic disease and after chemotherapy when used for adjuvant treatment.
It is important to use weight-based dosing to individualize dosing of all cytotoxic drugs to minimize toxicity of normal cells. In addition, some cytotoxic drugs have a cumulative maximum dose that can be given for cancer treatment without risk of irreversible vital organ damage (e.g., heart failure with doxorubicin, pulmonary fibrosis with carmustine and bleomycin). Table 12.2 provides route and dosage information for some cytotoxic antineoplastic drugs.
 TABLE 12.2
TABLE 12.2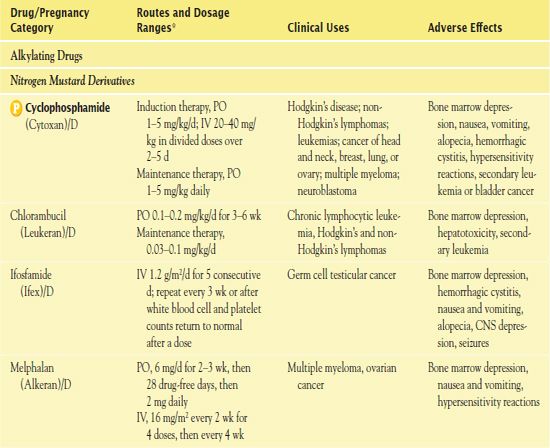
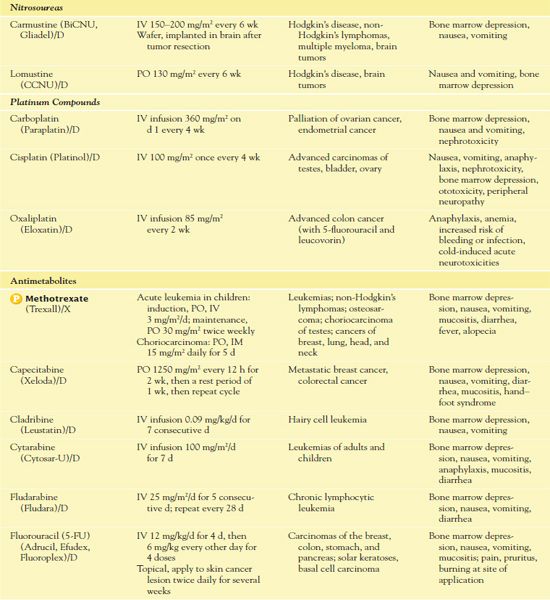
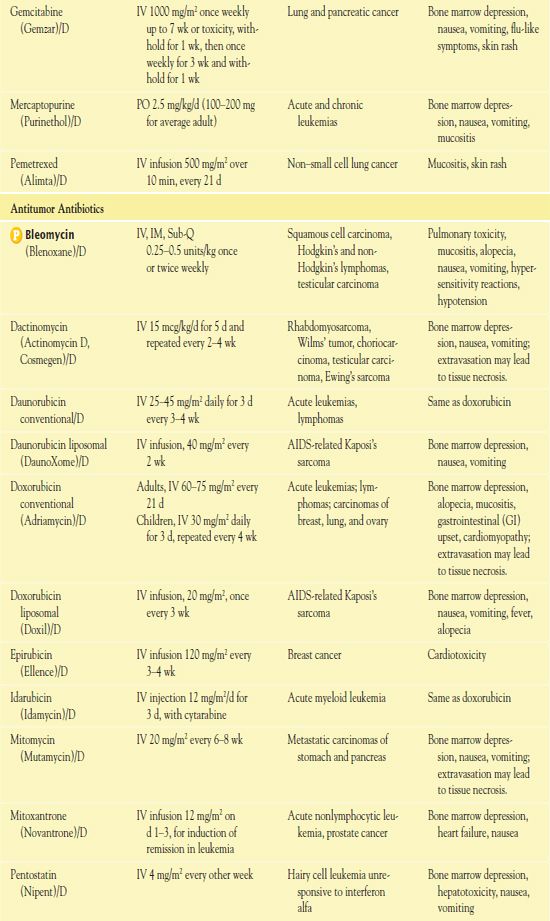
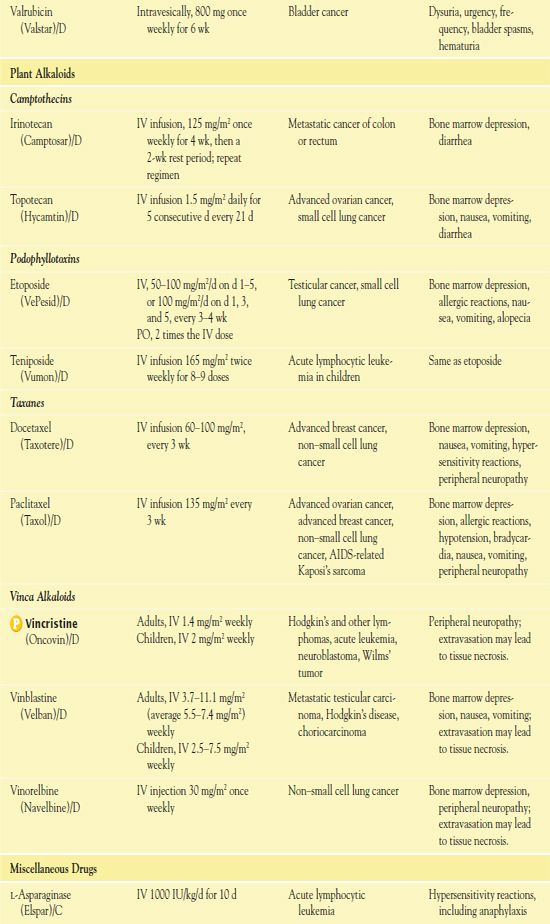
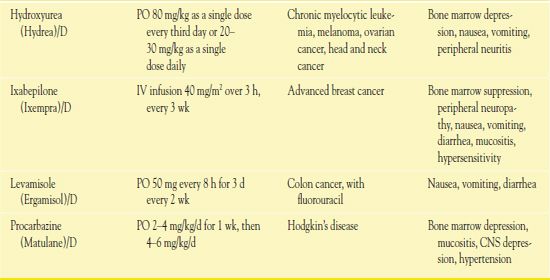
*Dosages may vary significantly or change often, according to use in different types of cancer and in different combinations.
AIDS, acquired immunodeficiency syndrome; CNS, central nervous system.
Use in Children
Children are at risk for certain types of malignancies, including acute leukemias, lymphomas, brain tumors, Wilms’ tumor, and sarcomas of muscle and bone. In recent years, there have been rapid advances in cancer care for children and high cure rates for many pediatric malignancies. Many of the advances have resulted from the systematic enrollment of children in well-designed clinical trials.
Pediatric oncologists should design, order, and supervise chemotherapy for children. To determine dosage of cytotoxic drugs, prescribers should use body surface area, because this takes overall size into account. Special efforts are necessary to maintain nutrition, organ function, psychological support, growth and development, and other aspects of health status during and after therapy. Long-term follow-up care is important for childhood cancer survivors because they have an increased risk of health problems. For example, children who receive an anthracycline drug (e.g., doxorubicin) are at increased risk for cardiotoxic effects (e.g., heart failure) during treatment or after receiving the drug. Efforts to reduce cardiotoxicity include using alternative drugs (if effective), giving smaller cumulative doses of the anthracycline, and observing patients closely so that early manifestations can be recognized and treated before heart problems occur. In addition, after successful cytotoxic chemotherapy, children are at increased risk for developing cancers in later life (e.g., leukemia). Children treated for Hodgkin’s disease seem to have the highest risk of developing a new cancer.
Use in Older Adults
Increasing age is a risk factor for development of cancer, and the number of new cancer cases is about 10 times greater in people 65 years of age and older. Although elderly people are more likely to have chronic cardiovascular, renal, and other disorders (comorbidities) that increase their risks of serious adverse effects, age alone should not be the reason for denying them the potential benefits of traditional cytotoxic chemotherapy. However, it is important to consider several factors when treating older adults for cancer. Physiologic age-related changes that may add to the challenge of managing cancer include decreased renal function, reduced hepatic blood flow, and diminished cardiac reserve. Psychosocial issues that may affect treatment include access to care, financial and transportation issues, functional status, need for independence, and social support.
For example, older adults are more sensitive to the neurotoxic effects of vincristine and need reduced dosages of some drugs (e.g., cyclophosphamide, MTX) if they have impaired renal function. It is necessary to monitor creatinine clearance (CrCl), because serum creatinine is not a reliable indicator of renal function in older adults due to decreased muscle mass. Most antineoplastic drugs are metabolized by the liver; thus, decreased liver blood flow and decreased liver size can lead to accumulation of drug due to decreased clearance, resulting in increased toxicity in the elderly adult.
Use in Patients With Renal Impairment
Some antineoplastic drugs are nephrotoxic (e.g., cisplatin, MTX), and the kidneys play a large role in their excretion. The presence of impaired renal function increases the risk of further impairment or accumulation of toxic drug levels. Thus, it is necessary to monitor renal function carefully during therapy and reduce drug dosages according to CrCl levels. The following paragraphs describe the renal effects of selected cytotoxic chemotherapy drugs and precautions to reduce nephrotoxicity.
The alkylating drugs may be nephrotoxic. These effects usually subside when the drug is stopped. For example, cisplatin, an alkylating agent, has multiple active metabolites that are renally excreted, causing renal tubular damage. When a patient already has significant renal dysfunction, oncologists avoid using this drug.
The antimetabolites may also be nephrotoxic. MTX use in patients with impaired renal function may lead to accumulation of toxic amounts or additional renal damage. Evaluation of the patient’s renal status should take place before and during MTX therapy. If significant renal impairment occurs, it is necessary to discontinue the drug or to reduce the dosage until renal function improves. In patients who receive high doses of MTX for treatment of osteosarcoma, the drug may cause renal damage resulting in acute renal failure. Nephrotoxicity is attributed to precipitation of MTX and a metabolite in renal tubules. Reducing renal impairment involves monitoring renal function closely, ensuring adequate hydration, alkalinizing the urine, and measuring serum drug levels. With mercaptopurine, dosage reduction is necessary to prevent nephrotoxicity. With irinotecan, dosage reduction is necessary in patients with moderate renal impairment (CrCl, 20–39 mL/min).
Caution is warranted with many other drugs when used in patients with renal impairment. Cytarabine is detoxified mainly by the liver. However, patients with renal impairment may have more central nervous system–related adverse effects, and dosage reduction may be necessary. Gemcitabine has resulted in mild proteinuria and hematuria during clinical trials, and hemolytic–uremic syndrome has been reported in a few patients. Signs of this syndrome may include anemia, elevated bilirubin and reticulocyte counts, and renal failure. If this condition occurs, it is essential to stop gemcitabine immediately; hemodialysis may be required. Bleomycin is rarely associated with nephrotoxicity, but its elimination half-life is prolonged in patients with a CrCl of less than 35 mL perminute. L-Asparaginase often increases blood urea nitrogen; acute renal failure and fatal renal insufficiency have been reported. Procarbazine may cause more severe adverse effects if given to patients with impaired renal function. Hospitalization is recommended for the first course of treatment.
Use in Patients With Hepatic Impairment
Some antineoplastic drugs are hepatotoxic, and the liver is the site of the metabolism of many. Patients with metastatic cancer often have impaired liver function, and their risk of further impairment or accumulation of toxic drug levels increase. It is necessary to monitor hepatic function with most drugs. However, abnormal values for the usual liver function tests (e.g., serum aspartate aminotransferase [AST] and alanine aminotransferase [ALT], bilirubin, alkaline phosphatase) may indicate liver injury but do not indicate decreased ability to metabolize drugs. Dosage reduction may be necessary in some cases. The following paragraphs describe the hepatic effects of selected cytotoxic chemotherapy drugs and precautions to reduce hepatotoxicity.
Alkylating drugs may lead to hepatotoxicity.
Antimetabolites also have hepatotoxic effects. MTX may cause acute hepatotoxicity (increased serum ALT and AST, hepatitis) as well as chronic hepatotoxicity (fibrosis and cirrhosis). Chronic toxicity is potentially fatal. It is more likely to occur after prolonged use (e.g., 2 years or longer) and after a total dose of at least 1.5 g. It is essential that liver function tests be closely monitored in patients with preexisting liver damage or impaired hepatic function. Gemcitabine has increased serum ALT and AST in most patients during clinical trials. Mercaptopurine causes hepatotoxicity, especially with higher doses (above 2.5 mg/kg/d) and in combination with doxorubicin. Encephalopathy and fatal liver necrosis have occurred. It is essential to stop the drug if signs of hepatotoxicity (e.g., jaundice, hepatomegaly) occur. Serum aminotransferases, alkaline phosphatase, and bilirubin should be monitored weekly initially, then monthly.
Antitumor antibiotics may be hepatotoxic as well. Daunorubicin (liposomal formulation) requires dosage reduction according to serum bilirubin (e.g., bilirubin 1.2–3 mg/dL, three fourths the normal dose; bilirubin above 3 mg/dL, one half the normal dose). Doxorubicin toxicity increases with impaired hepatic function. It is necessary to perform liver function tests before drug administration and to reduce the dosage of both regular and liposomal formulations according to the serum bilirubin. Idarubicin is not appropriate for patients with a serum bilirubin above 5 mg/dL.
Some plant alkaloids have hepatic effects. Irinotecan may cause abnormal liver function tests in patients with liver metastases. Paclitaxel may cause more toxicity in patients with impaired hepatic function. Hospitalization is recommended for the first course of therapy. Topotecan is cleared from plasma more slowly in patients with hepatic impairment. Vinblastine and vincristine may cause more toxicity with hepatic impairment, and dosage should be reduced 50% for patients with a direct serum bilirubin value above 3 mg/dL.
L-Asparaginase is hepatotoxic in most patients; it may increase preexisting hepatic impairment and hepatotoxicity of other medications. Signs of liver impairment, which usually subside when the drug is discontinued, include increased AST, ALT, alkaline phosphatase, and bilirubin and decreased serum albumin, cholesterol, and plasma fibrinogen. Procarbazine may cause increased hepatotoxicity in cases of impaired hepatic function.
Use in Patients With Critical Illness
Cytotoxic drug treatment is often significantly dose reduced or discontinued in the setting of critical illness when there is compromised perfusion to the liver and kidneys, which can increase toxicity. However, palliative chemotherapy, which can alleviate pain or obstruction in critically ill patients with advanced cancer, is used on a case-by-case basis with consideration of therapeutic benefits versus the risks of treatment toxicities.
Use in Home Care
Although administration of cytotoxic drug therapy occurs most frequently in a clinic or hospital setting, people often self-administer oral cytotoxic drugs, and some give themselves IV infusions at home. All cytotoxic drugs are hazardous substances and require special handling and disposal, according to safety guidelines, in the home. The home care nurse may be involved in a wide range of activities associated with antineoplastic drug therapy, including administering the anticancer drugs, administering other drugs to prevent or manage adverse effects, and assessing patient and family responses to therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


