Understand the physiologic effects of endogenous corticosteroids.
 Identify the pathophysiology of adrenal cortex disorders.
Identify the pathophysiology of adrenal cortex disorders.
 Describe the action and the clinical indications for use of exogenous corticosteroids.
Describe the action and the clinical indications for use of exogenous corticosteroids.
 Understand the contraindications and adverse effects of corticosteroids as well as the nursing implications of their use.
Understand the contraindications and adverse effects of corticosteroids as well as the nursing implications of their use.
 Analyze how other drugs and substances as well as other factors may affect the need for corticosteroids.
Analyze how other drugs and substances as well as other factors may affect the need for corticosteroids.
 Apply the nursing process when a patient is administered a corticosteroid.
Apply the nursing process when a patient is administered a corticosteroid.
Clinical Application Case Study
Emma Mae Thompson is a 65-year-old African American woman who lives alone. Since she was 15 years old, she has smoked one pack of unfiltered cigarettes per day. When she was diagnosed with chronic obstructive pulmonary disease 5 years ago, she quit smoking. She takes albuterol sulfate 1 inhalation per nebulizer every 12 hours and beclomethasone dipropionate 160 mcg every 12 hours. Recently, she was treated for pneumonia. Mrs. Thompson’s physician prescribes prednisone orally in a tapering dose, according to the following schedule:
Day 1: 10 mg before breakfast, 5 mg before lunch, 5 mg before supper, 10 mg at bedtime
Day 2: 5 mg before breakfast, 5 mg before lunch, 5 mg before supper, 10 mg at bedtime
Day 3: 5 mg before breakfast, 5 mg before lunch, 5 mg before supper, 5 mg at bedtime
Day 4: 5 mg before breakfast, 5 mg before supper, 5 mg at bedtime
Day 5: 5 mg before breakfast, 5 mg at bedtime
Day 6: 5 mg before breakfast
KEY TERMS
Addison’s disease: primary adrenocortical insufficiency with inadequate production of Cortisol and aldosterone
Addisonian crisis: condition that mimics hypovolemic and septic shock; also known as adrenocortical insufficiency
Aldosterone: mineralocorticoid hormone secreted by the adrenal cortex to increase sodium reabsorption by the kidneys and indirectly regulate blood levels of potassium, sodium, and bicarbonate; also regulates pH, blood volume, and blood pressure
Corticosteroid: steroid hormones produced by the adrenal cortex; examples include androgens, glucocorticoids, and mineralocorticoids
Cortisol: the main glucocorticoid secreted as part of the body’s response to stress
Cushing’s disease: adrenocortical hyperfunction; may result from excessive corticotropin or primary adrenal tumor
Glucocorticoid: adrenal cortical hormone that protects the body against stress and affects protein and carbohydrate metabolism
Immunosuppression: suppression of the immune system
Mineralocorticoid: steroid hormone released by the adrenal cortex to promote sodium and water retention and potassium excretion
Negative feedback mechanism: when the output of a system affects the stimulus for the system (e.g., hormone secretion produces an effect that shuts off the stimulus for further hormone secretion)
Steroid: lipid-soluble hormone produced by the gonadal organs or the adrenal cortex
Introduction
This chapter provides an introduction to the corticosteroids, also known as the glucocorticoids or steroids. These lipid-soluble hormones are produced by the gonadal organs or the adrenal cortex, part of the adrenal glands. These hormones affect almost all body organs and are extremely important in maintaining homeostasis when secreted in normal amounts. Disease results from inadequate or excessive secretion. Exogenous corticosteroids are used as drugs in a variety of disorders. Their use must be closely monitored, because they have profound therapeutic and adverse effects. To understand the effects of corticosteroids used as drugs (exogenous corticosteroids), it is necessary to understand the physiologic effects and other characteristics of the endogenous hormones.
Physiology of Endogenous Corticosteroids
Corticosteroid secretion is controlled by the hypothalamus, the anterior pituitary, and adrenal cortex (the hypothalamic–pituitary–adrenal, or HPA, axis). Various stimuli (e.g., low plasma levels of corticosteroids, pain, anxiety, trauma, illness, anesthesia) activate the system. These stimuli cause the hypothalamus of the brain to secrete corticotropin-releasing hormone or factor (known as CRH or CRF), which stimulates the anterior pituitary gland to secrete corticotropin, and corticotropin then stimulates the adrenal cortex to secrete corticosteroids.
The rate of corticosteroid secretion is usually maintained within relatively narrow limits but changes according to need. When plasma corticosteroid levels rise to an adequate level, secretion of corticosteroids slows or stops. The mechanism by which the hypothalamus and anterior pituitary “learn” that no more corticosteroids are needed is called a negative feedback mechanism.
This negative feedback mechanism is normally very important, but it does not work during stress responses. The stress response activates the sympathetic nervous system (SNS) to produce more epinephrine and norepinephrine and the adrenal cortex to produce as much as 10 times the normal amount of cortisol. The synergistic interaction of these hormones increases the person’s ability to respond to stress. However, the increased SNS activity continues to stimulate cortisol production (the main glucocorticoid secreted as part of the body’s response to stress) and overrules the negative feedback mechanism. Excessive and prolonged corticosteroid secretion damages body tissues.
Corticosteroids are secreted directly into the bloodstream. Cortisol is approximately 90% bound to plasma proteins, and this high degree of protein binding slows cortisol movement out of the plasma, so that it has a relatively long plasma half-life of 60 to 90 minutes. The remaining 10% is unbound and biologically active. In contrast, aldosterone is only 60% bound to plasma proteins and has a short half-life of 20 minutes. In general, protein binding functions as a storage area from which the hormones are released as needed. This promotes more consistent blood levels and more uniform distribution to the tissues.
The adrenal cortex produces approximately 30 steroid hormones, which are divided into glucocorticoids, mineralocorticoids, and adrenal sex hormones. Chemically, all corticosteroids are derived from cholesterol and have similar chemical structures. However, despite their similarities, slight differences cause them to have different functions.
Glucocorticoids
Although the term “corticosteroids” actually refers to all secretions of the adrenal cortex, it is most often used to designate the glucocorticoids, which are important in metabolic, inflammatory, and immune processes. Glucocorticoids include cortisol, corticosterone, and cortisone. Cortisol accounts for at least 95% of glucocorticoid activity; corticosterone and cortisone accounts for a small amount of activity. Glucocorticoids are secreted cyclically, with the largest amount being produced in the early morning and the smallest amount during the evening hours (in people with a normal day–night schedule). At the cellular level, glucocorticoids account for most of the characteristics and physiologic effects of the corticosteroids (Box 15.1).
BOX 15.1 Effects of Glucocorticoids on Body Processes and Systems
Carbohydrate Metabolism
 ↑Formation of glucose (gluconeogenesis) by breaking down protein into amino acids. The amino acids are then transported to the liver, where they are acted on by enzymes that convert them to glucose. The glucose is then returned to the circulation for use by body tissues or storage in the liver as glycogen.
↑Formation of glucose (gluconeogenesis) by breaking down protein into amino acids. The amino acids are then transported to the liver, where they are acted on by enzymes that convert them to glucose. The glucose is then returned to the circulation for use by body tissues or storage in the liver as glycogen.
 ↓Cellular use of glucose, especially in muscle cells. This is attributed to a ↓effect of insulin on the proteins that normally transport glucose into cells and by ↓numbers and functional capacity of insulin receptors.
↓Cellular use of glucose, especially in muscle cells. This is attributed to a ↓effect of insulin on the proteins that normally transport glucose into cells and by ↓numbers and functional capacity of insulin receptors.
 Both the ↑production and ↓use of glucose promote higher levels of glucose in the blood (hyperglycemia) and may lead to diabetes mellitus. These actions also increase the amount of glucose stored as glycogen in the liver, skeletal muscles, and other tissues.
Both the ↑production and ↓use of glucose promote higher levels of glucose in the blood (hyperglycemia) and may lead to diabetes mellitus. These actions also increase the amount of glucose stored as glycogen in the liver, skeletal muscles, and other tissues.
Protein Metabolism
 Breakdown of protein into amino acids (catabolic effect); ↑rate of amino acid transport to the liver and conversion to glucose
Breakdown of protein into amino acids (catabolic effect); ↑rate of amino acid transport to the liver and conversion to glucose
 ↓Rate of new protein formation from dietary and other amino acids (antianabolic effect)
↓Rate of new protein formation from dietary and other amino acids (antianabolic effect)
 The combination of ↑breakdown of cell protein and ↓protein synthesis leads to protein depletion in virtually all body cells except those of the liver. Thus, glycogen stores in the body are ↑ and protein stores are ↓.
The combination of ↑breakdown of cell protein and ↓protein synthesis leads to protein depletion in virtually all body cells except those of the liver. Thus, glycogen stores in the body are ↑ and protein stores are ↓.
Lipid Metabolism
 ↑Breakdown of adipose tissue into fatty acids; the fatty acids are transported in the plasma and used as a source of energy by body cells.
↑Breakdown of adipose tissue into fatty acids; the fatty acids are transported in the plasma and used as a source of energy by body cells.
 ↑Oxidation of fatty acids within body cells.
↑Oxidation of fatty acids within body cells.
Inflammatory and Immune Responses
 ↓Inflammatory response. Inflammation is the normal bodily response to tissue damage and involves three stages. First, a large amount of plasma-like fluid leaks out of capillaries into the damaged area and becomes clotted. Second, leukocytes migrate into the area. Third, tissue healing occurs, largely by growth of fibrous scar tissue. Normal or physiologic amounts of glucocorticoids probably do not significantly affect inflammation and healing, but large amounts of glucocorticoids inhibit all three stages of the inflammatory process.
↓Inflammatory response. Inflammation is the normal bodily response to tissue damage and involves three stages. First, a large amount of plasma-like fluid leaks out of capillaries into the damaged area and becomes clotted. Second, leukocytes migrate into the area. Third, tissue healing occurs, largely by growth of fibrous scar tissue. Normal or physiologic amounts of glucocorticoids probably do not significantly affect inflammation and healing, but large amounts of glucocorticoids inhibit all three stages of the inflammatory process.
More specifically, corticosteroids stabilize lysosomal membranes (and thereby prevent the release of inflammatory proteolytic enzymes); ↓capillary permeability (and thereby ↓leakage of fluid and proteins into the damaged tissue); ↓the accumulation of neutrophils and macrophages at sites of inflammation (and thereby impair phagocytosis of pathogenic microorganisms and waste products of cellular metabolism); and ↓production of inflammatory chemicals, such as interleu- kin-1, prostaglandins, and leukotrienes, by injured cells.
↑, increase/increased; ↓, decrease/decreased.
 ↓Immune response. The immune system normally protects the body from foreign invaders, and several immune responses overlap inflammatory responses, including phagocytosis. In addition, the immune response stimulates the production of antibodies and activated lymphocytes to destroy the foreign substance. Glucocorticoids impair protein synthesis, including the production of antibodies; ↓the numbers of circulating lymphocytes, eosinophils, and macrophages; and ↓amounts of lymphoid tissue. These effects help account for the immunosuppressive and antiallergic actions of the glucocorticoids.
↓Immune response. The immune system normally protects the body from foreign invaders, and several immune responses overlap inflammatory responses, including phagocytosis. In addition, the immune response stimulates the production of antibodies and activated lymphocytes to destroy the foreign substance. Glucocorticoids impair protein synthesis, including the production of antibodies; ↓the numbers of circulating lymphocytes, eosinophils, and macrophages; and ↓amounts of lymphoid tissue. These effects help account for the immunosuppressive and antiallergic actions of the glucocorticoids.
Cardiovascular System
 Help regulate arterial blood pressure by modifying vascular smooth muscle tone by modifying myocardial contractility and by stimulating renal mineralocorticoid and glucocorticoid receptors
Help regulate arterial blood pressure by modifying vascular smooth muscle tone by modifying myocardial contractility and by stimulating renal mineralocorticoid and glucocorticoid receptors
 ↑The response of vascular smooth muscle to the pressor effects of catecholamines and other vasoconstrictive agents
↑The response of vascular smooth muscle to the pressor effects of catecholamines and other vasoconstrictive agents
Nervous System
 Physiologic amounts help to maintain normal nerve excitability; pharmacologic amounts ↓ nerve excitability, slow activity in the cerebral cortex, and alter brain wave patterns.
Physiologic amounts help to maintain normal nerve excitability; pharmacologic amounts ↓ nerve excitability, slow activity in the cerebral cortex, and alter brain wave patterns.
 ↓Secretion of CRH by the hypothalamus and of corticotropin by the anterior pituitary gland. This results in suppression of further glucocorticoid secretion by the adrenal cortex (negative feedback system).
↓Secretion of CRH by the hypothalamus and of corticotropin by the anterior pituitary gland. This results in suppression of further glucocorticoid secretion by the adrenal cortex (negative feedback system).
Musculoskeletal System
 Maintain muscle strength when present in physiologic amounts but cause muscle atrophy (from protein breakdown) when present in excessive amounts.
Maintain muscle strength when present in physiologic amounts but cause muscle atrophy (from protein breakdown) when present in excessive amounts.
 ↓Bone formation and growth and ↑bone breakdown. Glucocorticoids also ↓ intestinal absorption and ↑ renal excretion of calcium. These effects contribute to bone demineralization (osteoporosis) in adults and to ↓linear growth in children.
↓Bone formation and growth and ↑bone breakdown. Glucocorticoids also ↓ intestinal absorption and ↑ renal excretion of calcium. These effects contribute to bone demineralization (osteoporosis) in adults and to ↓linear growth in children.
Respiratory System
 Maintain open airways. Glucocorticoids do not have direct bronchodilating effects, but help maintain and restore responsiveness to the bronchodilating effects of endogenous catecholamines, such as epinephrine.
Maintain open airways. Glucocorticoids do not have direct bronchodilating effects, but help maintain and restore responsiveness to the bronchodilating effects of endogenous catecholamines, such as epinephrine.
 Stabilize mast cells and other cells to inhibit the release of bronchoconstrictive and inflammatory substances, such as histamine.
Stabilize mast cells and other cells to inhibit the release of bronchoconstrictive and inflammatory substances, such as histamine.
Gastrointestinal System
 ↓Viscosity of gastric mucus. This effect may ↓ protective properties of the mucus and contribute to the development of peptic ulcer disease.
↓Viscosity of gastric mucus. This effect may ↓ protective properties of the mucus and contribute to the development of peptic ulcer disease.
Mineralocorticoids
Mineralocorticoids are a class of steroids that play a vital role in the maintenance of fluid and electrolyte balance through their influence on salt and water metabolism. Aldosterone is the main mineralocorticoid and is responsible for approximately 90% of mineralocorticoid activity. Characteristics and physiologic effects of mineralocorticoids are summarized in Box 15.2.
BOX 15.2 Effects of Mineralocorticoids on Body Processes and Systems
 The overall physiologic effects are to conserve sodium and water and eliminate potassium. Aldosterone increases sodium reabsorption from kidney tubules, and water is reabsorbed along with the sodium. When sodium is conserved, another cation must be excreted to maintain electrical neutrality of body fluids; thus, potassium is excreted. This is the only potent mechanism for controlling the concentration of potassium ions in extracellular fluids.
The overall physiologic effects are to conserve sodium and water and eliminate potassium. Aldosterone increases sodium reabsorption from kidney tubules, and water is reabsorbed along with the sodium. When sodium is conserved, another cation must be excreted to maintain electrical neutrality of body fluids; thus, potassium is excreted. This is the only potent mechanism for controlling the concentration of potassium ions in extracellular fluids.
 Secretion of aldosterone is controlled by several factors, most of which are related to kidney function. In general, secretion is increased when the potassium level of extracellular fluid is high, the sodium level of extracellular fluid is low, the renin-angiotensin system of the kidneys is activated, or the anterior pituitary gland secretes corticotropin.
Secretion of aldosterone is controlled by several factors, most of which are related to kidney function. In general, secretion is increased when the potassium level of extracellular fluid is high, the sodium level of extracellular fluid is low, the renin-angiotensin system of the kidneys is activated, or the anterior pituitary gland secretes corticotropin.
 Inadequate secretion of aldosterone causes hyperkalemia, hyponatremia, and extracellular fluid volume deficit (dehydration). Hypotension and shock may result from decreased cardiac output. Absence of mineralocorticoids causes death.
Inadequate secretion of aldosterone causes hyperkalemia, hyponatremia, and extracellular fluid volume deficit (dehydration). Hypotension and shock may result from decreased cardiac output. Absence of mineralocorticoids causes death.
 Excessive secretion of aldosterone produces hypokalemia, hypernatremia, and extracellular fluid volume excess (water intoxication). Edema and hypertension may result.
Excessive secretion of aldosterone produces hypokalemia, hypernatremia, and extracellular fluid volume excess (water intoxication). Edema and hypertension may result.
Adrenal Sex Hormones
The adrenal cortex secretes male (androgens) and female (estrogens and progesterone) sex hormones. Compared with the effect of hormones produced by the testes and ovaries, the adrenal sex hormones have an insignificant effect on normal body function. Adrenal androgens, secreted continuously in small quantities by both sexes, are responsible for most of the physiologic effects exerted by the adrenal sex hormones. They increase protein synthesis (anabolism), which increases the mass and strength of muscle and bone tissue; they affect development of male secondary sex characteristics; and they increase hair growth and libido in women. Excessive secretion of adrenal androgens in women causes masculinizing effects (e.g., hirsutism, acne, breast atrophy, deepening of the voice, amenorrhea). Female sex hormones are secreted in small amounts and normally exert few physiologic effects. Excessive secretion may produce feminizing effects in men (e.g., breast enlargement, decreased hair growth, voice changes).
Pathophysiology of Adrenal Cortex Disorders
Disorders of the adrenal cortex involve increased or decreased production of corticosteroids, especially cortisol as the primary glucocorticoid and aldosterone as the primary mineralocorticoid. These disorders include the following:
• Primary adrenocortical insufficiency (Addison’s disease) is associated with destruction of the adrenal cortex by disorders such as tuberculosis, cancer, or hemorrhage; with atrophy of the adrenal cortex caused by autoimmune disease or prolonged administration of exogenous corticosteroids; and with surgical excision of the adrenal glands. In primary adrenocortical insufficiency, there is inadequate production of both cortisol and aldosterone.
• Secondary adrenocortical insufficiency, produced by inadequate secretion of corticotropin, is most often caused by prolonged administration of corticosteroids. This condition is largely a glucocorticoid deficiency; mineralocorticoid secretion is not significantly impaired.
• Congenital adrenogenital syndromes and adrenal hyperplasia result from deficiencies in one or more enzymes required for cortisol production. Low plasma levels of cortisol lead to excessive corticotropin secretion, which then leads to excessive adrenal secretion of androgens and hyperplasia (abnormal increase in number of cells).
• Androgen-producing tumors of the adrenal cortex, which are usually benign, produce masculinizing effects.
• Adrenocortical hyperfunction (Cushing’s disease)may result from excessive corticotropin or a primary adrenal tumor. Adrenal tumors may be benign or malignant. Benign tumors often produce one corticosteroid normally secreted by the adrenal cortex, but malignant tumors often secrete several corticosteroids.
• Hyperaldosteronism is a rare disorder caused by adenoma (a benign tissue from glandular tissue) or hyperplasia of the adrenal cortex cells that produce aldosterone. It is characterized by hypokalemia, hypernatremia, hypertension, thirst, and polyuria.
Drug Therapy with Exogenous Corticosteroids
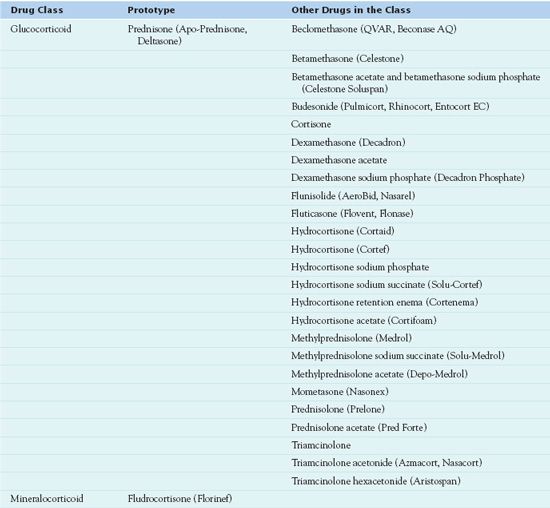
Exogenous corticosteroids, or glucocorticoids, are administered to treat disorders of the adrenal cortex or endocrine system. The administration of corticosteroids decreases the inflammatory symptoms and alters the immune response produced by nonendocrine disorders. People once viewed hydrocortisone, a short-acting corticosteroid and an exogenous equivalent of endogenous cortisol, as the prototype corticosteroid drug. Now they consider  prednisone, an intermediate acting corticosteroid, to be the prototype corticosteroid. Table 15.1 lists the glucocorticoids and mineralocorticoids.
prednisone, an intermediate acting corticosteroid, to be the prototype corticosteroid. Table 15.1 lists the glucocorticoids and mineralocorticoids.
Pharmacokinetics
The rate of absorption of corticosteroids depends on the route of administration. Oral administration results in rapid absorption by the gastrointestinal (GI) tract, with rapid distribution to the intestines, muscles, liver, and kidneys. The corticosteroid is metabolized by the liver by cytochrome P450 3A4 enzymes, and the medication is conjugated to inactive metabolites. About 25% of the metabolites are excreted in the bile and then in the feces. The other 75%, which enter the circulation, are excreted in the kidneys. Plasma binding affects metabolism of corticosteroids, which means that patients with serum albumin levels less than 3.5 g/dL are prone to increased effects of corticosteroids and symptoms of hypercorticism.
The administration of exogenous corticosteroids suppresses the HPA axis. As a result, secretion of corticotropin decreases, causing atrophy of the adrenal cortex and decreased production of endogenous adrenal corticosteroids.
Action
Like endogenous glucocorticoids, exogenous corticosteroids act at the cellular level by binding to drug receptors in target tissues. The lipid-soluble drugs easily diffuse through the cell membranes of target cells. Inside the cell, they bind with receptors in intracellular cytoplasm. The drug–receptor complex then moves to the cell nucleus, where it interacts with DNA to stimulate or suppress gene transcription.
Corticosteroids can increase or decrease the transcription of many genes to alter the synthesis of proteins. These proteins regulate many physiologic effects, such as the transportation of proteins. Metabolic effects do not occur for at least 45 to 60 minutes because of the time required for protein synthesis. Several hours or days may be needed for full production of proteins.
Because the genes vary in different types of body cells, corticosteroid effects also vary, depending on the specific cells being targeted. For example, supraphysiologic concentrations of glucocorticoids induce the synthesis of lipolytic and proteolytic enzymes and other specific proteins in various tissues. Overall, corticosteroids have multiple mechanisms of action and effects (Fig. 15.1), including the following:
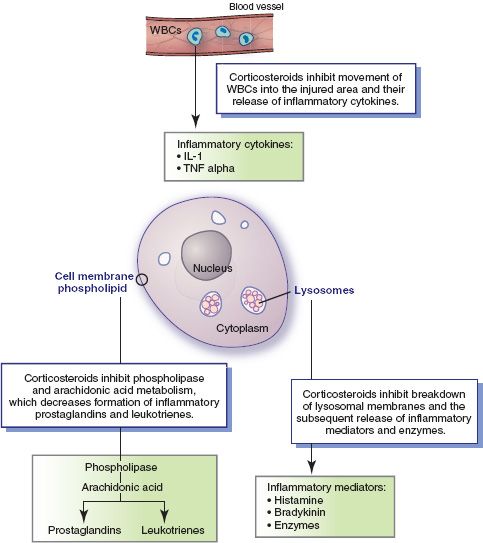
Figure 15.1 Inflammatory processes and anti-inflammatory actions of corticosteroids. Cellular responses to injury include the following: phospholipid in the cell membrane is acted on by phospholipase to release arachidonic acid, metabolism of arachidonic acid produces the inflammatory mediators prostaglandins and leukotrienes, lysosomal membrane breaks down and releases inflammatory chemicals (e.g., histamine, bradykinin, intracellular digestive enzymes), and white blood cells (WBCs) are drawn to the area and release inflammatory cytokines (e.g., interleukin-1 [IL-1] alpha). Overall, corticosteroid drugs act to inhibit the release, formation, or activation of various inflammatory mediators.
• Inhibiting arachidonic acid metabolism. Normally, when a body cell is injured or activated by various stimuli, the enzyme phospholipase A2 causes the phospholipids in cell membranes to release arachidonic acid. Free arachidonic acid is then metabolized to produce proinflammatory prostaglandins (see Chap. 14) and leukotrienes. At sites of tissue injury or inflammation, corticosteroids induce the synthesis of proteins that suppress the activation of phospholipase A2. This action, in turn, decreases the release of arachidonic acid and the formation of prostaglandins and leukotrienes.
• Strengthening or stabilizing biologic membranes. Two biologic membranes are especially important in inflammatory processes. Stabilization of cell membranes inhibits the release of arachidonic acid and production of prostaglandins and leukotrienes, as described above. Stabilization of lysosomal membranes inhibits release of bradykinin, histamine, enzymes, and perhaps other substances from lysosomes. (Lysosomes are intracellular structures that contain inflammatory chemical mediators and enzymes that destroy cellular debris and phagocytized pathogens.) This reduces capillary permeability and thus prevents leakage of fluid into the injured area and development of edema. It also reduces the chemicals that normally cause vasodilation and tissue irritation.
• Inhibiting the production of interleukin-1, tumor necrosis factor, and other cytokines. This action also contributes to the anti-inflammatory and immunosuppressant effects of corticosteroids.
• Impairing phagocytosis. The drugs inhibit the ability of phagocytic cells to leave the bloodstream and move into the injured or inflamed tissue.
• Impairing lymphocytes. The drugs inhibit the ability of these immune cells to increase in number and perform their functions.
• Inhibiting tissue repair. The drugs inhibit the growth of new capillaries, fibroblasts, and collagen needed for tissue repair.
Use
Corticosteroids are administered to control symptoms but do not cure underlying disease processes. They are extensively used to treat many different disorders. Except for replacement therapy in deficiency states, the use of corticosteroids is largely empiric. Because the drugs affect virtually every aspect of inflammatory and immune responses, they are used in the treatment of a broad spectrum of diseases with an inflammatory or immunologic component.
Table 15.2 presents information about routes of administration and dosage ranges.
 TABLE 15.2
TABLE 15.2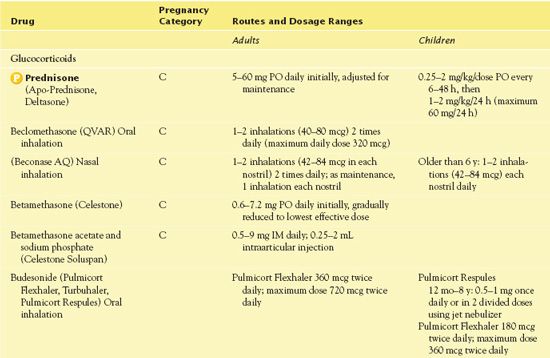


General Indications
The corticosteroids discussed in this chapter are used to treat potentially serious or disabling disorders, including the following:
• Allergic or hypersensitivity disorders, such as allergic reactions to drugs, serum and blood transfusions, and dermatoses with an allergic component
• Collagen disorders, such as systemic lupus erythematosus, scleroderma, and periarteritis nodosa. Collagen is the basic structural protein of connective tissue, tendons, cartilage, and bone, and it is therefore present in almost all body tissues and organ systems. The collagen disorders are characterized by inflammation of various body tissues. Signs and symptoms depend on which body tissues or organs are affected and the severity of the inflammatory process.
• Dermatologic disorders that may be treated with systemic corticosteroids include acute contact dermatitis, erythema multiforme, herpes zoster (prophylaxis of postherpetic neuralgia), lichen planus, pemphigus, skin rashes caused by drugs, and toxic epidermal necrolysis. Corticosteroid preparations that are applied topically in dermatologic disorders are discussed in Chapter 60.
• Endocrine disorders, such as adrenocortical insufficiency and congenital adrenal hyperplasia (see Chap. 43 for additional information). Corticosteroids are given to replace or substitute for the natural hormones (both glucocorticoids and mineralocorticoids) in cases of insufficiency and to suppress corticotropin when excess secretion causes adrenal hyperplasia. These conditions are rare and account for a small percentage of corticosteroid use.
• GI disorders, such as ulcerative colitis and regional enteritis (Crohn’s disease)
• Hematologic disorders, such as idiopathic thrombocytopenic purpura or acquired hemolytic anemia
• Hepatic disorders characterized by edema, such as cirrhosis and ascites
• Neoplastic disease, such as acute and chronic leukemias, Hodgkin’s disease, other lymphomas, and multiple myeloma (see later discussion)
• Neurologic conditions, such as cerebral edema, brain tumor, acute spinal cord injury (see later discussion), and myasthenia gravis
• Ophthalmic disorders, such as optic neuritis, sympathetic ophthalmia, and chorioretinitis. Corticosteroid preparations that are applied topically in ophthalmologic disorders are discussed in Chapter 58.
• Organ or tissue transplants and grafts (e.g., kidney, heart, bone marrow). Corticosteroids suppress cellular and humoral immune responses (see Chap. 11) and help prevent rejection of transplanted tissue. Drug therapy is usually continued as long as the transplanted tissue is in place.
• Renal disorders characterized by edema, such as the nephrotic syndrome
• Respiratory disorders, such as asthma, status asthmaticus, chronic obstructive pulmonary disease (COPD), and inflammatory disorders of nasal mucosa (rhinitis)
• Rheumatic disorders, such as ankylosing spondylitis, acute and chronic bursitis, acute gouty arthritis, rheumatoid arthritis, and osteoarthritis
• Shock. Corticosteroids are clearly indicated only for shock resulting from Addisonian crisis (also known as adrenal or adrenocortical insufficiency), which may mimic hypovolemic or septic shock. The use of corticosteroids in septic shock has been highly controversial, and randomized studies and meta-analyses have indicated that corticosteroids are not beneficial in treating septic shock. However, more recent small studies indicate possible clinical usefulness in septic shock, because this form of shock may be associated with relative adrenal insufficiency. In anaphylactic shock resulting from an allergic reaction, corticosteroids may increase or restore cardiovascular responsiveness to adrenergic drugs.
Clinical Application 15-1
 What is the purpose of administering prednisone to Mrs. Thompson?
What is the purpose of administering prednisone to Mrs. Thompson?
 What is the purpose of tapering the dose?
What is the purpose of tapering the dose?
 Could prednisone be discontinued without tapering the dose?
Could prednisone be discontinued without tapering the dose?
NCLEX Success
1. A woman is experiencing periods of increased anxiety and panic attacks over her illness and that of her family members. Epinephrine and norepinephrine are released. What glucocorticoid is released by the adrenal cortex in response to stress?
A. insulin
B. glucose
C. thyroid hormone
D. cortisol
2. A boy has contracted a rash caused by poison ivy over a large portion of his arms and legs following a camping trip. His health care provider has prescribed oral prednisone, which is to be administered in a tapering dose over the next 10 days. What effect does the medication have on the rash?
A. It decreases the accumulation of neutrophils and macrophages at the site, thus reducing inflammation.
B. It eliminates the itching associated with the allergy.
C. It increases the white blood cell count to assist in healing.
D. It increases the protein metabolism to allow for the rejuvenation of tissue.
3. A patient is receiving prednisone 10 mg orally every day to reduce symptoms of Crohn’s disease. Which of the following effects is associated with daily administration of prednisone?
A. atrophy of the adrenal cortex
B. decreased serum glucose
C. weight loss
D. fluid volume deficit
4. A patient with chronic obstructive pulmonary disease is being administered a corticosteroid by nebulizer. What effect does the corticosteroid have?
A. decrease in blood pressure
B. decrease in mucus secretion
C. decrease in edema
D. increase in immunity
5. A patient is admitted to the emergency department in anaphylactic shock following numerous bee stings. The prescriber orders parenteral administration of corticosteroids. What effect is expected from the administration of these agents?
A. decreased heart rate and blood pressure
B. increased circulation to the lower extremities
C. increased or restored cardiovascular responsiveness
D. decreased production of aldosterone



