Describe general characteristics of beta-lactam antibiotics.
 Discuss the penicillins in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
Discuss the penicillins in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
 Recognize the importance of questioning patients about allergies before the initial dose of all drugs, especially penicillins.
Recognize the importance of questioning patients about allergies before the initial dose of all drugs, especially penicillins.
 Describe characteristics of beta-lactamase inhibitor drugs.
Describe characteristics of beta-lactamase inhibitor drugs.
 Give the rationale for combining a penicillin and a beta-lactamase inhibitor drug.
Give the rationale for combining a penicillin and a beta-lactamase inhibitor drug.
 Discuss the cephalosporins in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
Discuss the cephalosporins in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
 Discuss the carbapenems in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
Discuss the carbapenems in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
 Discuss the one monobactam drug in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
Discuss the one monobactam drug in relation to effectiveness, safety, spectrum of antibacterial activity, mechanism of action, indications for use, administration, observation of patient response, and teaching of patients.
 Use the nursing process in the care of patients receiving beta-lactam antibacterials.
Use the nursing process in the care of patients receiving beta-lactam antibacterials.
Clinical Application Case Study
Paul O’Brian, a 55-year-old professional musician who travels frequently, has no known drug allergies. He is being scheduled for the placement of a new cardiac pacemaker tomorrow. The physician orders that cefazolin be administered “on call” for the procedure (1 g IV).
KEY TERMS
Beta-lactamase: enzyme produced by some bacteria that attacks the beta-lactam ring, rendering the drug ineffective and leading to a resistance to beta-lactam antibiotics
Cross-allergenicity: allergy to a drug of another class with a similar chemical structure
Extended-spectrum: bactericidal activity against a wide range of bacteria
Superinfection: infection after a previous infection; typically caused by microorganisms that are resistant to the antibiotics used previously
Introduction
This chapter discusses the pharmacological care of the patient who is receiving a drug in the antibiotic class of beta-lactam antibacterials, specifically the penicillins, cephalosporins, carbapenems, and monobactams. Beta-lactam antibacterial drugs inhibit synthesis of bacterial cell walls by binding to proteins (penicillin-binding proteins; see Chap. 13) in bacterial cell membranes. This binding produces a defective cell wall that allows leakage of the intracellular contents, destroying the microorganisms. Beta-lactam antibacterial drugs are typically considered bactericidal.
Beta-lactam antibacterials derive their name from the beta-lactam ring that is part of their chemical structure. An intact beta-lactam ring is essential for the antibacterial activity of these drugs. Several gram-positive and gram-negative bacteria produce beta-lactamases, which are enzymes that disrupt the beta-lactam ring and inactivate the beta-lactam antibacterial drugs. This is the major mechanism by which microorganisms acquire resistance to these drugs. Penicillinase and cephalosporinase are beta-lactamase enzymes that act on penicillins and cephalosporins to render them resistant and ineffective. Patients receive beta-lactamase inhibitors concurrently with the beta-lactam antibacterial drugs to overcome this resistance.
Although a beta-lactam ring is common to all beta-lactam antibiotics, the characteristics of these drugs vary widely because of differences in their chemical structures. Because of these differences, the range of activity to particular bacteria is also different. The drugs may also differ in the routes of administration, susceptibility to beta-lactamase enzymes, and adverse effects. Table 16.1 lists the various types of beta-lactam antibiotics.
Penicillins
The penicillins are effective and safe, and they are among the most commonly prescribed antibacterials. The first antibiotic developed was penicillin, which was discovered from the Penicillium mold. When penicillin was introduced, it was effective against many organisms. It was once necessary to give the drug parenterally because it was destroyed by gastric acid, and injections were painful. With extensive use, strains of drug-resistant staphylococci appeared. Scientists developed semisynthetic derivatives, formed by adding side chains to the penicillin nucleus, to increase gastric acid stability, beta-lactamase stability, and antimicrobial spectrum of activity, especially against gram-negative microorganisms. As a class, penicillins usually are more effective in treating infections caused by gram-positive bacteria than those caused by gram-negative bacteria. However, their clinical usefulness varies significantly according to the subgroup or individual drug and microbial patterns of resistance.
 Ampicillin (Principen) is the prototype penicillin.
Ampicillin (Principen) is the prototype penicillin.
Pharmacokinetics
After absorption, ampicillin is widely distributed. Penetration into the cerebrospinal fluid (CSF) occurs only with inflamed meninges. The kidneys rapidly excrete ampicillin, largely as unchanged drug, and it produces high drug concentrations in the urine. It is present in breast milk, and the volume of distribution increases during pregnancy, when the half-life, generally 1 to 2 hours, is decreased.
Action
Ampicillin, like all penicillins, inhibits bacterial cell wall synthesis by binding to one or multiple penicillin-binding proteins.
Use
Clinical indications for use of ampicillin include bacterial infections caused by susceptible microorganisms. Health care providers use the drug in the treatment or prophylaxis of infective endocarditis. The drug’s broad spectrum is often useful in skin, soft tissue, respiratory, gastrointestinal (GI), and genitourinary infections. The broad-spectrum coverage of ampicillin extends its activity against gram-negative bacilli. Table 16.2 provides route and dosage information for the individual penicillins.
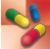 TABLE 16.2
TABLE 16.2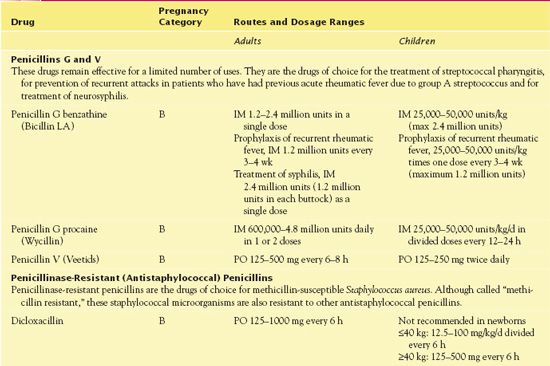


The incidence of resistance among streptococci, staphylococci, and other microorganisms continues to increase.
QSEN Safety Alert 
Before prescribing ampicillin or other penicillins for streptococcal infections, clinicians should perform culture and susceptibility studies and be aware of local patterns of streptococcal susceptibility or resistance.
Use in Children
Ampicillin and other penicillins are widely used to treat infections in children and are generally safe. However, caution is necessary in neonates because immature kidney function slows drug elimination. Dosage should be based on age, weight, severity of the infection being treated, and renal function. Specialized pediatric dosing references provide guidance to dosing based on age and weight.
Use in Older Adults
Ampicillin and other penicillins are relatively safe in older adults. However, decreased renal function, other disease processes, and concurrent drug therapies increase the risks of adverse effects.
Use in Patients With Renal Impairment
Because ampicillin is excreted primarily by the kidneys, it is important to use caution with renal impairment.
Use in Patients With Hepatic Impairment
Ampicillin can be used in patients with hepatic impairment, as can almost all the penicillins. Caution is necessary when using amoxicillin–clavulanate (Augmentin) in patients with hepatic injury or destruction. No specific recommendations for dosage adjustment are available. Development of cholestatic jaundice and hepatic dysfunction with previous use of the drug are contraindications. Cholestatic liver impairment usually subsides when the drug is stopped.
Use in Patients With Critical Illness
Use of ampicillin in the people who are critically ill is not nearly as great as it once was, primarily because resistance to the drug has emerged and other broad-spectrum agents are available. Patients frequently take other beta-lactam agents concomitantly with other antimicrobial drugs because critically ill patients often have multiple organisms that cause infections. The extended-spectrum drugs (e.g., piperacillin, which is a derivative of ampicillin) and penicillin–beta-lactamase inhibitor combinations (e.g., Zosyn), which have bactericidal activity against a wide range of bacteria, are most likely to be used in critical care units for the treatment of respiratory diseases such as pneumonia, blood, or other infections. Through chemical modification, these extended-spectrum drugs affect additional types of bacteria, typically gram-negative bacteria. With cephalosporins, third-and fourth-generation drugs are commonly used and are usually given by intermittent intravenous (IV) infusions every 8 or 12 hours. Blood levels of penicillins need to be maintained above the minimum inhibitory concentration (MIC) of the microorganisms causing the infection. Thus, continuous or extended infusions may be of benefit with serious infections, especially those caused by relatively resistant organisms such as Pseudomonas or Acinetobacter. It is necessary to monitor function of the kidneys, liver, and other organs in critically ill patients and to reduce drug dosages when indicated.
Use in Patients Receiving Home Care
When using oral beta-lactam antibiotics such as ampicillin, the home care nurse mainly needs to teach patients about accurate administration of the drug and observation for therapeutic and adverse effects. With using liquid suspensions for children, shaking to resuspend the medication and measuring with a measuring spoon or calibrated device to ensure safe dosing are required. Household spoons should not be used because they vary widely in capacity and may lead to incorrect dosing.
Adverse Effects
The most common adverse effects of ampicillin are hypersensitivity reactions, including rash and/or anaphylactoid reactions. Commonly reported GI adverse effects include abdominal pain, diarrhea, gastritis, and nausea and vomiting. Nephropathy, such as interstitial nephritis, although infrequent, has occurred with all penicillins. It is most often associated with high doses of parenteral penicillins and is attributed to hypersensitivity reactions.
The U.S. Food and Drug Administration (FDA) has issued a BLACK BOX WARNING ♦ to alert health care providers that inadvertent IV administration of penicillin G benzathine may result in cardiopulmonary arrest and death. Long-acting repository forms have additives that decrease their solubility in tissue fluids and delay their absorption.
Contraindications
Contraindications include hypersensitivity or allergic reactions to any penicillin formulation. An allergic reaction to one penicillin means the patient is allergic to all drugs of the penicillin class. The potential for cross-allergenicity (allergy to a drug of another class with similar chemical structure) with cephalosporins and carbapenems exists; each has the characteristic bicyclic core structure, the chemical unit thought to be most responsible for beta-lactam hypersensitivity. Recent data suggest that the incidence is less than 1%, lower than previously thought.
Nursing Implications
Preventing Interactions
Many medications and herbs interact with ampicillin, increasing or decreasing its effects (Boxes 16.1 and 16.2). In addition, ampicillin inhibits the renal tubular secretion of methotrexate, which may lead to prolonged and higher drug concentrations of methotrexate.
BOX 16.1  Drug Interactions: Ampicillin
Drug Interactions: Ampicillin
Drugs That Increase the Effects of Ampicillin
 Allopurinol
Allopurinol
Increases the incidence of skin rash
 Clavulanic acid
Clavulanic acid
Overcomes resistance in bacteria that secrete beta-lactamase
 Probenecid
Probenecid
Inhibits the renal tubular secretion
 Uricosuric drugs
Uricosuric drugs
Block renal excretion
Drugs That Decrease the Effects of Ampicillin
 Chloroquine
Chloroquine
Decreases the serum concentration
 Fusidic acid
Fusidic acid
Diminishes the therapeutic effect
 Tetracycline derivatives:
Tetracycline derivatives:
Diminish the therapeutic effect
BOX 16.2  Herb and Dietary Interactions: Ampicillin
Herb and Dietary Interactions: Ampicillin
Herbs and Foods That Decrease the Effects of Ampicillin
 Food
Food
Decreases absorption
 Khat
Khat
Decreases absorption
Penicillins are often given concomitantly with aminoglycosides for serious infections, such as those caused by Pseudomonas aeruginosa. These drugs should not be admixed in a syringe, given in an IV solution, or administered via Y-site, because the penicillin inactivates the aminoglycoside. If feasible, dose separation is ideal.
Administering the Medication
It is necessary to give oral ampicillin, like most oral penicillins, on an empty stomach, approximately 1 hour before or 2 hours after a meal. Patients should take the oral drug with a full glass of water, preferably to promote absorption and decrease inactivation, which may occur in an acidic environment. If need be, they may take it with food; however, the absorption rate decreases with food. Oral suspensions of the drug are stable for 7 days at room temperature and 14 days when refrigerated.
When diluted with 0.9% sodium chloride, ampicillin is stable for 8 hours for concentrations up to 30 mg/mL. It is stable for only 1 hour when diluted with dextrose-containing solutions for concentrations of 10 to 20 mg/mL. It is necessary to give IV penicillins for the full prescribed course of treatment to prevent complications such as rheumatic fever, endocarditis, and glomerulonephritis. IV concentrations should not exceed 30 mg/mL.
Assessing for Therapeutic Effects
It is not necessary to obtain drug levels when administering ampicillin or any of the antimicrobials in the penicillin class. When administering ampicillin, it is recommended that serum creatinine and blood urea nitrogen (BUN) be monitored.
Assessing for Adverse Effects
The nurse carefully assesses the characteristics of a rash, if present. It is necessary to distinguish, if possible, a hypersensitivity reaction from a nonallergic ampicillin rash.
Patient Teaching
The nurse instructs patients to take oral penicillins for the full prescribed course of treatment to prevent complications. Box 16.3 outlines patient teaching guidelines for ampicillin and other oral penicillins.
BOX 16.3  Patient Teaching Guidelines for Oral Penicillins
Patient Teaching Guidelines for Oral Penicillins
General Considerations
 Do not take any penicillin if you have ever had an allergic reaction to penicillin in which you had difficulty breathing, swelling, or skin rash. However, some people who call a minor stomach upset an allergic reaction, which is incorrect, are not given penicillin when that is the best antibiotic in a given situation.
Do not take any penicillin if you have ever had an allergic reaction to penicillin in which you had difficulty breathing, swelling, or skin rash. However, some people who call a minor stomach upset an allergic reaction, which is incorrect, are not given penicillin when that is the best antibiotic in a given situation.
 Complete the full course of drug treatment for greater effectiveness and prevention of secondary infection with drug-resistant bacteria.
Complete the full course of drug treatment for greater effectiveness and prevention of secondary infection with drug-resistant bacteria.
 Follow instructions carefully about the dose and how often it is taken. Drug effectiveness depends on maintaining adequate blood levels. Penicillins often need more frequent administration than some other antibiotics, because they are rapidly excreted by the kidneys.
Follow instructions carefully about the dose and how often it is taken. Drug effectiveness depends on maintaining adequate blood levels. Penicillins often need more frequent administration than some other antibiotics, because they are rapidly excreted by the kidneys.
Self- or Caregiver Administration
 Take most penicillins on an empty stomach, 1 hour before or 2 hours after a meal. Penicillin V, amoxicillin, and Augmentin can be taken with food. (Take Augmentin with meals to increase absorption and decrease gastrointestinal upset.)
Take most penicillins on an empty stomach, 1 hour before or 2 hours after a meal. Penicillin V, amoxicillin, and Augmentin can be taken with food. (Take Augmentin with meals to increase absorption and decrease gastrointestinal upset.)
 Take each dose with a full glass of water; do not take with orange juice or with other acidic fluids (they may destroy the drug).
Take each dose with a full glass of water; do not take with orange juice or with other acidic fluids (they may destroy the drug).
 Take at even intervals, preferably around the clock.
Take at even intervals, preferably around the clock.
 Shake liquid penicillins well, to mix thoroughly and measure the dose accurately.
Shake liquid penicillins well, to mix thoroughly and measure the dose accurately.
 Discard liquid penicillin after 1 week if stored at room temperature or after 2 weeks if refrigerated. Liquid forms deteriorate and should not be taken after their expiration dates.
Discard liquid penicillin after 1 week if stored at room temperature or after 2 weeks if refrigerated. Liquid forms deteriorate and should not be taken after their expiration dates.
 Report skin rash, hives, itching, severe diarrhea, shortness of breath, fever, sore throat, black tongue, or any unusual bleeding to your health care provider. These symptoms may indicate an allergy to penicillin.
Report skin rash, hives, itching, severe diarrhea, shortness of breath, fever, sore throat, black tongue, or any unusual bleeding to your health care provider. These symptoms may indicate an allergy to penicillin.
Other Drugs in the Class
Antistaphylococcal Penicillins and Aminopenicillins
Choice of a beta-lactam antibacterial depends on the organism causing the infection, severity of the infection, and other factors. Penicillin G or the aminopenicillin amoxicillin is the drug of choice in many infections. Other aminopenicillins are indicated in Pseudomonas infections. Antistaphylococcal penicillin is indicated in staphylococcal infections; the antistaphylococcal drugs of choice are nafcillin for IV use and dicloxacillin for oral use.
Proper administration of penicillins is important. With intramuscular (IM) penicillins, it is necessary to inject them deep into large muscle masses to decrease tissue irritation. With IV penicillins, it is necessary to usually first dilute reconstituted penicillins in 50 to 100 mL of 5% dextrose or 0.9% sodium chloride injection and infuse them over 30 to 60 minutes to minimize vascular irritation and phlebitis. It should be noted that ticarcillin may cause decreased platelet aggregation.
Penicillin-Beta-Lactamase Inhibitor Combinations
Beta-lactamase inhibitors are drugs with a beta-lactam structure but minimal antibacterial activity. They bind with and inactivate the beta-lactamase enzymes produced by many bacteria (e.g., Escherichia coli; Klebsiella, Enterobacter, and Bacteroides species; Staphylococcus aureus). When combined with a penicillin, the beta-lactamase inhibitor protects the penicillin from destruction by the enzymes and extends the penicillin’s spectrum of antimicrobial activity. Thus, the combination drug may be effective in infections caused by bacteria that are resistant to a beta-lactam antibiotic alone.
Clavulanate, sulbactam, and tazobactam are the beta-lactamase inhibitors available in combinations with penicillins.
• Ampicillin and sulbactam is available as Unasyn, in vials with 1 g of ampicillin and 0.5 g of sulbactam, or 2 g of ampicillin and 1 g of sulbactam.
• Amoxicillin and clavulanate is marketed as Augmentin, in 250-, 500-, and 875-mg tablets, each of which contains 125 mg of clavulanate.
QSEN Safety Alert 
Thus, two 250-mg tablets are not equivalent to one 500-mg tablet.
• Augmentin is also available as 1000-mg extended release tablets containing 62.5 mg of clavulanate. In addition, a chewable formulation is available as 200 mg of amoxicillin and 28.5 mg of clavulanate, as well as 400 mg of amoxicillin and 57 mg of clavulanate.
• Ticarcillin and clavulanate is available as Timentin, an IV formulation containing 3 g of ticarcillin and 100 mg of clavulanate.
• Piperacillin and tazobactam is marketed as Zosyn, an IV formulation. Three dosage strengths are available, with 2 g piperacillin and 0.25 g tazobactam, 3 g piperacillin and 0.375 g tazobactam, or 4 g piperacillin and 0.5 g tazobactam.
NCLEX Success
1. A nurse is preparing to administer the first dose of piperacillin–tazobactam (Zosyn) to a patient in an infusion clinic. The nurse should take which of the following precautions?
A. Ask the patient about past allergic reactions to penicillins.
B. Ask the patient about past allergic reactions to aminoglycosides.
C. Mix the piperacillin–tazobactam with lidocaine to reduce pain of infusion.
D. Instruct the patient to eat a snack to decrease stomach upset from piperacillin–tazobactam.
2. A woman is to receive amoxicillin–clavulanate (Augmentin) 500 mg PO every 8 hours for bronchitis. The nurse retrieves two 250-mg tablets from the medication cart. This is incorrect for which of the following reasons?
A. The amount of sulbactam in amoxicillin-clavulanate 250 mg is 62.5 mg per tablet, twice the intended amount.
B. This provides twice the intended dose of clavulanate.
C. The 250-mg tablets have less absorption than the 500-mg tablets.
D. Administration of amoxicillin-clavulanate is only intravenous, so selecting tablets means that the wrong drug is being administered.
Cephalosporins
Cephalosporins are a widely used group of drugs derived from a fungus and closely related chemically to the penicillins. Although technically cefoxitin and cefotetan are not cephalosporins, they are categorized with the cephalosporins because of their similarities to the group. Cephalosporins are broad-spectrum agents with activity against both gram-positive and gram-negative bacteria. Compared with penicillins, these drugs are generally less active against gram-positive organisms but more active against gram-negative ones.
Classification
Cephalosporins are classified into five subgroups, or “generations,” based on their pharmacology and spectrum of activity.
First-Generation Cephalosporins
The first cephalosporin, cephalothin, is no longer available for clinical use. However, it may be used to determine susceptibility to first-generation cephalosporins, which have essentially the same spectrum of antimicrobial activity. In general, first-generation cephalosporins have strong activity against gram-positive bacteria and poor activity against gram-negative bacteria. Therefore, these drugs are effective against streptococci, staphylococci (except methicillin-resistant S. aureus [MRSA]), Shigella, E. coli, Proteus mirabilis, and Bacteroides species (except Bacteroides fragilis). They are not effective against Enterobacter, Pseudomonas, and Serratia species.
Often, health care providers use first-generation cephalosporins for surgical prophylaxis, especially with prosthetic implants, because gram-positive organisms such as staphylococci cause most infections of surgical sites. Prescribers may also order them for treatment of infections caused by susceptible organisms in body sites where drug penetration and host defenses are adequate.
 Cefazolin, the prototype cephalosporin, is the drug of choice for surgical prophylaxis in most surgical procedures. Some advantages of cefazolin over other first-generation cephalosporins include less frequent dosing, higher blood levels after parenteral administration, and increased gram-positive coverage.
Cefazolin, the prototype cephalosporin, is the drug of choice for surgical prophylaxis in most surgical procedures. Some advantages of cefazolin over other first-generation cephalosporins include less frequent dosing, higher blood levels after parenteral administration, and increased gram-positive coverage.
Second-Generation Cephalosporins
Second-generation cephalosporins are more active against some gram-negative organisms and somewhat less active against gram-positive cocci than the first-generation agents. Thus, they may be effective in infections resistant to other antibiotics, including infections caused by Haemophilus influenzae, Klebsiella species, E. coli, and some strains of Proteus. Because each of these drugs has a different antimicrobial spectrum, susceptibility tests must be performed for each drug rather than for the entire group, as may be done with first-generation drugs. Cefoxitin (Mefoxin), for example, is active against B. fragilis, an anaerobic organism resistant to most drugs.
Often, other uses for second-generation cephalosporins also include surgical prophylaxis, especially for gynecologic and colorectal surgery. Prescribers also order the drugs for treatment of intra-abdominal infections such as pelvic inflammatory disease, diverticulitis, and other infections caused by organisms inhabiting pelvic and colorectal areas (perhaps caused by penetrating wounds of the abdomen).
Third-Generation Cephalosporins
Third-generation cephalosporins further extend the spectrum of activity against gram-negative organisms. In addition to activity against the usual enteric pathogens (e.g., E. coli, Proteus and Klebsiella species), they are also active against several strains resistant to other antibiotics and to first-and second-generation cephalosporins. Thus, they may be useful in infections caused by unusual strains of enteric organisms such as the group Enterobacteriaceae (which includes Citrobacter, Serratia, Enterobacter, P. mirabilis, and E. coli). Another difference is the ability of third-generation cephalosporins to penetrate inflamed meninges to reach therapeutic concentrations in CSF. Thus, they may be useful in meningeal infections caused by common pathogens, including H. influenzae, Neisseria meningitidis, and Streptococcus pneumoniae. Although some of the drugs are active against Pseudomonas organisms, drug-resistant strains may emerge when a cephalosporin is used alone for treatment of pseudomonal infection.
Overall, cephalosporins gain gram-negative activity and lose gram-positive activity as they move from the first to the third generation. The second-and third-generation drugs are more active against gram-negative organisms because they are more resistant to the beta-lactamase enzymes (cephalosporinases) produced by some bacteria to inactivate cephalosporins.
Fourth-Generation Cephalosporins
Fourth-generation cephalosporins have a greater spectrum of antimicrobial activity and greater stability against breakdown by beta-lactamase enzymes compared with third-generation drugs. Cefepime, the first fourth-generation cephalosporin to be developed, is active against both gram-positive and gram-negative organisms. With gram-positive organisms, it is active against streptococci and staphylococci (except for methicillin-resistant staphylococci). With gram-negative organisms, its activity against P. aeruginosa is similar to that of ceftazidime, and its activity against Enterobacteriaceae is greater than that of third-generation cephalosporins. Moreover, cefepime retains activity against strains of Enterobacteriaceae and P. aeruginosa that have acquired resistance to third-generation agents.
Fifth-Generation Cephalosporins
Ceftaroline (Teflaro) is an IV cephalosporin for the treatment of community-acquired pneumonia and skin infections. It is the first cephalosporin to be considered active against resistant gram-positive organisms, such as MRSA, vancomycin-resistant S. aureus (VRSA), vancomycin-insensitive S. aureus (VISA), and heteroresistant VISA.
Pharmacokinetics
After the cephalosporins are absorbed, they achieve therapeutic concentrations in most body fluids and tissues, with maximum concentrations in the liver and kidneys. However, many cephalosporins do not reach therapeutic levels in the CSF. Most third-generation cephalosporins achieve more consistent CSF penetration in those patients with inflamed meninges.
Cefazolin is distributed into most body tissues and crosses the placenta. The onset of action is rapid with both IV and IM administration. The drug peaks at the end of IV infusion and within 1 to 2 hours with IM injection. The duration of action is 6 to 12 hours. The drug, which is largely excreted unchanged via the kidneys, concentrates in the urine. Thus, metabolism is not hepatic. Therefore, no dosage adjustments are necessary with hepatic impairment.
Action
Cefazolin inhibits the third and last step of bacterial wall synthesis by binding to one or more penicillin-binding proteins.
Use
Cefazolin is a frequently used parenteral agent. It reaches a higher serum concentration, is more protein bound, and has a slower rate of elimination than other first-generation drugs. These factors prolong serum half-life, which means that cefazolin can be given less frequently.
Clinical indications for the use of the cephalosporins include surgical prophylaxis and treatment of infections of the respiratory tract, skin and soft tissues, bones and joints, urinary tract, brain and spinal cord, and bloodstream (septicemia). In infections caused by MRSA, cephalosporins are not clinically effective even if in vitro testing indicates susceptibility (except for the newest cephalosporin, ceftaroline). Infections caused by Neisseria gonorrhoeae, at one time susceptible to penicillin, are now treated with a third-generation cephalosporin such as ceftriaxone.
Tables 16.3 and 16.4 provide important information about parenteral and oral cephalosporins, respectively.
 TABLE 16.3
TABLE 16.3Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


