Describe the etiology and pathophysiology of tuberculosis and Mycobacterium avium complex.
 Describe the characteristics of latent, active, and drug-resistant tuberculosis.
Describe the characteristics of latent, active, and drug-resistant tuberculosis.
 Describe drug therapy for tuberculosis, including the rationale for multiple-drug therapy.
Describe drug therapy for tuberculosis, including the rationale for multiple-drug therapy.
 List the action, uses, adverse effects, and nursing implications of first-line antitubercular drugs.
List the action, uses, adverse effects, and nursing implications of first-line antitubercular drugs.
 Describe how second-line antitubercular drugs are added to drug regimens to treat multidrug-resistant tuberculosis.
Describe how second-line antitubercular drugs are added to drug regimens to treat multidrug-resistant tuberculosis.
 Describe the drugs used to prevent or treat Mycobacterium avium complex.
Describe the drugs used to prevent or treat Mycobacterium avium complex.
 Discuss ways to increase adherence to antitubercular drug therapy regimens.
Discuss ways to increase adherence to antitubercular drug therapy regimens.
 Understand how to implement the nursing process in the care of patients undergoing drug therapy for tuberculosis.
Understand how to implement the nursing process in the care of patients undergoing drug therapy for tuberculosis.
Clinical Application Case Study
Ramon Diaz is a 58-year-old homeless man. He is spending the night at a shelter where one of your coworkers volunteers one evening a month. Mr. Diaz has a productive cough, and your coworker asks him how he feels; he has visibly lost weight since he was in the shelter 1 month ago. He says he sweats a lot at night and thinks he has a fever. At a free clinic, his sputum test is positive for Mycobacterium tuberculosis. From the clinic, Mr. Diaz is sent to the county jail, where he will stay until his sputum test is negative. He is started on a regimen, once daily, of isoniazid 300 mg for 6 months, rifampin 600 mg for 6 months, pyrazinamide 2000 mg for 2 months, and ethambutol 1200 mg for 2 months. The health department supplies his medications and is responsible for overseeing his course of therapy. You are a nurse working for the health department.
KEY TERMS
Directly observed therapy: method of medication administration where a nurse (or responsible adult) observes a patient taking a dose of antitubercular drug; highly recommended when using intermittent regimens and for high-risk patients
Extensively drug-resistant tuberculosis: relatively rare type of multidrug-resistant tuberculosis that is resistant to isoniazid and rifampin plus resistant to any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).
Hepatitis: inflammation of the liver; usually caused by an infectious agent, toxin, or drug
Jaundice: yellow discoloration of the skin and of body tissues and fluids resulting from abnormally high levels of bilirubin in the blood; common symptom when drugs cause liver damage.
Multidrug-resistant tuberculosis: tuberculosis that is resistant to isoniazid and rifampin
Introduction
Tuberculosis (TB) is an infectious disease that usually affects the lungs but may involve the lymph nodes, pleurae, bones, joints, kidneys, and the gastrointestinal (GI) tract. TB, which commonly occurs in many parts of the world, infects one third of the world’s population. In 2010, a total of 8.8 million people worldwide became sick with TB. In the United States, active disease has decreased to a low level in U.S.-born people; most new cases occur in foreign-born immigrants. Large numbers of people have inactive or latent TB infection. Contributing factors include increased exposure during a resurgence of active disease between 1985 and 1992, immigration from countries where the disease commonly occurs (e.g., the Philippines, Vietnam, India, and China), and increasing numbers of people with conditions or medications that depress the immune system. TB is a leading killer of people living with human immunodeficiency virus (HIV).
Overview of Tuberculosis and Mycobacterium avium Complex Disease
Etiology
Mycobacterium tuberculosis, the tubercle bacillus, is the cause of TB. In general, these bacilli multiply slowly, and they may lie dormant in the body for many years. Mycobacterium avium and Mycobacterium intracellulare, which may also cause lung disease, are different types of mycobacteria that resemble each other so closely that they are usually grouped together as Mycobacterium avium complex (MAC). These atypical mycobacteria are found in water (including natural water sources, indoor water systems, pools, and hot tubs) and soil throughout the United States as well as in animals.
Pathophysiology
There are four phases in the initiation and progression of TB:
1. Transmission. This occurs when an uninfected person inhales infected airborne droplets that are exhaled by an infected person. Major factors affecting transmission are the number of bacteria expelled by the infected person and the closeness and duration of the contact between the infected and the uninfected person.
2. Primary infection. Authorities estimate that 30% of people who are exposed to TB bacilli become infected and develop a mild, pneumonia-like illness that is often undiagnosed. The initial infection occurs about 2 to 10 weeks after exposure. Within approximately 6 months of exposure, macrophages encapsulate the bacilli in calcified tubercles. The macrophages are unable to eliminate the bacteria completely. In the center of the calcified tubercle lies a caseous (cheesy) mass that contains small numbers of viable but dormant TB bacilli. The calcified tubercles, most commonly located in the upper lobes of the lungs, are visible on a chest radiograph.
3. Latent tuberculosis infection. The immune system is able to stop bacterial growth in most people who become infected with TB bacteria. The bacteria become inactive, although they remain alive in the body and can become active later. People with inactive or latent TB infection have no symptoms, do not feel sick, and do not spread TB to others. Active TB can develop years later if the latent infection is not effectively treated. In many people with latent TB, the infection remains inactive throughout their lives. In others, the TB bacteria become active and cause disease, usually when a person’s immune system becomes weak as a result of disease, immunosuppressive drugs, or aging.
4. Active tuberculosis. About 5% to 10% of people develop active TB when they are first infected. People with latent TB develop active disease in two ways: further exposure to infected airborne droplets or reactivation of the latent TB because of weakened immune status. Although the lungs are the most common site for an active TB infection, the disease can spread to other parts of the body. Disseminated TB can infect the musculoskeletal system; the spine is the most common site, followed by the knees and hips. Other sites include the brain, liver, and kidneys.
Both new and reactivated infections of TB are more likely in people whose immune system is depressed by diseases such as HIV, diabetes mellitus, or cancer. Immunosuppression results from drugs used during cancer treatment and after organ transplantation. In people with both TB and HIV, TB progresses more rapidly, often involves extrapulmonary sites, is more severe, and is often fatal.
In addition to latent TB, a major concern among public health and infectious disease authorities is an increase in drug-resistant infections. Drug-resistant mutants of M. tuberculosis microorganisms may be present in any infected person. When infected people receive anti-TB drugs, the drugs do not kill or weaken the drug-resistant mutants. Instead, the resistant bacteria are able to reproduce in the presence of the drugs and to transmit the property of drug resistance to newly produced bacteria. Eventually, the majority of TB bacilli in the body are drug resistant. Once a drug-resistant strain of TB emerges, it can be transmitted to other people just like a drug-susceptible strain.
The emergence of drug-resistant TB organisms has long been attributed mainly to poor patient adherence to prescribed anti-TB drug therapy—that is, when previously infected patients do not take the drugs and doses prescribed for the length of time prescribed. However, drug-resistant strains can spread from one person to another, and there is increasing evidence that many drug-resistant infections are new infections, especially in people whose immune system is suppressed. Drug-resistant TB has been identified in many parts of the world, especially in Africa and Asia, and is a major concern in HIV-infected people. Most cases in the United States occur in foreign-born people. Factors contributing to the development of drug-resistant disease include delayed diagnosis and delayed determination of drug susceptibility (which can take several weeks). In addition, some countries lack adequate laboratory facilities or do not test TB bacteria for susceptibility to second-line anti-TB drugs. These delays in effective treatment allow rapid disease progression and rapid transmission to others, especially to those with impaired immune systems. Important risk factors for drug-resistant TB include history of previous TB treatment; contact with patients infected with drug-resistant TB; HIV co-infection; and being a member of a population group with a high prevalence of drug-resistant TB (Albanna & Menzies, 2011).
Drug-resistant TB is TB that is resistant to one first-line drug. Multidrug-resistant tuberculosis (MDR-TB) is TB that is resistant to isoniazid (INH) and rifampin, two of the most effective anti-TB drugs available (with or without resistance to other anti-TB drugs). MDR-TB is associated with rapid progression, with 4 to 16 weeks from diagnosis to death, and a high death rate (50% to 80%). It is also difficult and expensive to treat; most experts recommend 24 months of drug therapy. The cure rate is only about 50% or less for MDR-TB compared with a cure rate of 90% or more for drug-susceptible strains of TB. Authorities now describe some MDR-TB cases as extensively drug-resistant TB (XDR-TB), an even more worrisome threat to public health. XDR-TB is resistance to the second-line drugs used to treat MDR-TB. Some cases of TB are resistant to six or seven drugs, and there are essentially no effective drugs for their treatment.
Experts believe that the organisms that cause MAC are transmitted by inhalation of droplets of contaminated water; there is no evidence of spread to humans from animals or other humans. MAC rarely causes significant disease in immunocompetent people but causes an opportunistic pulmonary infection in approximately 50% of patients with advanced HIV infection.
Clinical Manifestations
The initial symptoms of TB are a low-grade temperature, weight loss, cough, fatigue, and night sweats. The cough may be nonproductive or productive. Pulmonary and systemic symptoms may be present for weeks to months. Hemoptysis may occur. Dyspnea and orthopnea become progressively worse as TB becomes more advanced. Symptoms are less pronounced in the elderly. Disseminated TB occurs in 16% of U.S. cases; it is more prevalent in patients with HIV disease (Smeltzer, Bare, Hinkle & Cheever, 2010).
Symptoms of MAC include a productive cough, weight loss, hemoptysis, and fever. As the disease becomes disseminated through the body, chronic lung disease develops, and the bacteria are found in the blood, bone marrow, liver, lymph nodes, and other body tissues.
Box 20.1 gives information about the tuberculin skin test reactions and TB blood tests, which may be useful in diagnosis.
BOX 20.1 Guidelines for Interpretation of Tests for Tuberculosis
Mantoux Skin Test
Intradermal injection of 5 tuberculin units of purified protein derived from Mycobacterium tuberculosis
When to Use
(general)
 For people who are at high risk for acquiring tuberculosis (TB) because of exposure to someone with TB
For people who are at high risk for acquiring tuberculosis (TB) because of exposure to someone with TB
 For people who are at high risk to progression from latent to active TB because of other medical conditions
For people who are at high risk to progression from latent to active TB because of other medical conditions
How to Interpret Results
Induration: 5 or more mm
 Consider positive in:
Consider positive in:
 HIV-infected persons
HIV-infected persons
 Recent contact with a person with TB
Recent contact with a person with TB
 People with changes on chest radiograph consistent with prior TB
People with changes on chest radiograph consistent with prior TB
 People with organ transplants
People with organ transplants
 People who are immunosuppressed for other reasons (e.g., prednisone use)
People who are immunosuppressed for other reasons (e.g., prednisone use)
Induration: 10 or more mm
 Consider positive in:
Consider positive in:
 Recent immigrants from high-prevalence countries
Recent immigrants from high-prevalence countries
 Injectable drug users
Injectable drug users
 Residents and employees of high-risk congregate settings (e.g., long-term care facilities or prisons)
Residents and employees of high-risk congregate settings (e.g., long-term care facilities or prisons)
 Mycobacteriology laboratory personnel
Mycobacteriology laboratory personnel
 People in high-risk clinical positions
People in high-risk clinical positions
 Children younger than 4 years of age
Children younger than 4 years of age
 Infants, children, and adolescents exposed to high-risk adults
Infants, children, and adolescents exposed to high-risk adults
Induration: 15 or more mm
 Consider positive in any person, including people with no known risk of TB
Consider positive in any person, including people with no known risk of TB
False-Positive Reactions Possible
 When a non-TB mycobacterial infection is present or when the patient has had previous bacillus Calmette- Guérin (BCG) vaccination (BCG, derived from Mycobacterium bovis, is used to vaccinate children against TB in many parts of the world.)
When a non-TB mycobacterial infection is present or when the patient has had previous bacillus Calmette- Guérin (BCG) vaccination (BCG, derived from Mycobacterium bovis, is used to vaccinate children against TB in many parts of the world.)
False-Negative Reactions Possible
 When the patient’s weakened immune system is unable to react to the skin test
When the patient’s weakened immune system is unable to react to the skin test
 There has been a very recent TB infection (within 8 to 10 weeks of exposure)
There has been a very recent TB infection (within 8 to 10 weeks of exposure)
 When live-virus vaccinations (e.g., measles or smallpox) have been given recently
When live-virus vaccinations (e.g., measles or smallpox) have been given recently
Blood Test
Interferon-gamma release assay (IGRA) measures the concentration of a substance (interferon-gamma), which is released from white blood cells when the blood of a person infected with M. tuberculosis is mixed with antigens derived from M. tuberculosis (e.g., QuantiFERON-TB Gold)
When to Use
 The Centers for Disease Control and Prevention (CDC) states that this test can be used in place of tuberculin skin testing in all situations in which a tuberculin skin test is recommended.
The Centers for Disease Control and Prevention (CDC) states that this test can be used in place of tuberculin skin testing in all situations in which a tuberculin skin test is recommended.
How to Interpret Results
 Positive: indicates a patient has been infected with TB
Positive: indicates a patient has been infected with TB
 Negative: indicates that infection with TB is unlikely
Negative: indicates that infection with TB is unlikely
 Indeterminate: also possible
Indeterminate: also possible
Major Advantages
 People need only a single visit for the test; results are available in 24 hours.
People need only a single visit for the test; results are available in 24 hours.
 People who have received previous BCG vaccinations do not have false-positive results.
People who have received previous BCG vaccinations do not have false-positive results.
*The IGRA does not determine whether there is latent TB or active disease.
Clinical Application 20-1
 Identify the signs and symptoms of TB with which Mr. Diaz presents. Does he have latent TB or active TB disease?
Identify the signs and symptoms of TB with which Mr. Diaz presents. Does he have latent TB or active TB disease?
Drug Therapy
Drugs are used to treat both latent TB and active TB. Patients with latent TB cannot spread the disease to others, but treatment of the latent disease prevents progression of the disease to an active state. It is particularly important to treat latent TB in those patients who are at high risk for progression to active TB. Treatment of active TB prevents worsening of the disease in the individual patient and prevents spread of the disease to others. It is essential to initiate drug therapy promptly and to complete the entire course of treatment.
Four treatment regimens have been approved for latent TB. Fewer drugs are necessary to treat latent TB because fewer mycobacterial organisms are present. The drugs used in the four treatment regimens are INH, rifampin, and rifapentine (Table 20.1).
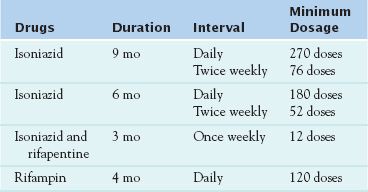
The U.S. Food and Drug Administration (FDA) has approved 10 drugs for the treatment of active TB. However, the following discussion considers only the first-line anti-TB drugs, which are INH, rifampin, and other rifamycins, ethambutol, and pyrazinamide. (Note that more drugs are necessary to treat active TB, because more mycobacterial organisms are present.) People with active TB need to take several drugs for 6 to 9 months.
Use of multiple drugs to treat TB is necessary to prevent the development of drug-resistant TB. TB regimens are modified for use with HIV, drug resistance, and pregnancy, as well as in children. Because drug susceptibility testing results can be delayed, treatment always begins empirically; it may be necessary to adjust the regimen when results are known (Table 20.2).
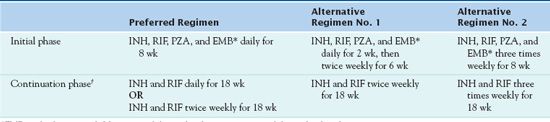
*EMB can be discontinued if drug susceptibility studies demonstrate susceptibility to first-line drugs.
†A continuation phase of weekly INH/rifapentine can be used for HIV-negative patients who do not have cavities on a chest radiograph and who have negative acid-fast bacilli smears at the completion of the initial phase of treatment.
INH, isoniazid; RIF, rifampin; PZA, pyrazinamide; EMB, ethambutol.
Second-line anti-TB drugs are also used in combination with other drugs when there is drug resistance to one of the first-line drugs or the patient is unable to tolerate use of a first-line drug. The second-line drugs are certain aminoglycoside antibiotics (amikacin [Amikin], capreomycin sulfate [Capastat], kanamycin [Kantrex], and streptomycin); cycloserine (Seromycin); ethionamide (Trecator); aminosalicylic acid (Paser); and certain fluoroquinolones (most commonly used are moxifloxacin [Avelox], levofloxacin [Levaquin], ofloxacin [Floxin], and ciprofloxacin [Cipro]).
Adequate drug therapy of patients with active TB usually produces improvement within 2 to 3 weeks, with decreased fever and cough, weight gain, improved well-being, and improved chest radiographs. Most patients have negative sputum cultures within 3 to 6 months. If the patient is symptomatic or if the culture is positive after 3 months, nonadherence or drug resistance must be considered. Cultures that are positive after 6 months often include drug-resistant organisms.
If the initial drug regimen seems to be ineffective, the health care practitioner must suspect drug resistance. The patient undergoes repeated sensitivity testing and begins to take at least three new anti-TB drugs (Albanna & Menzies, 2011). When the results of the second sensitivity testing are available, the prescriber removes drugs to which the mycobacteria are resistant and adds additional drugs. Table 20.3 lists the drugs used for MDR-TB. The regimen for treating drug-resistant TB (resistant to single first-line agent) or MDR-TB (resistant to both INH and rifampin) may include four to six drugs, including an injectable agent to which the organism is sensitive. Treatment includes second-line drugs that have many adverse effects and are more expensive and less effective than first-line drugs. A regimen for drug-resistant TB or MDR-TB includes whichever first-line agents are effective against the organism, an injectable agent, and a fluoroquinolone; if more drugs are necessary, second-line oral anti-TB drugs are useful (see Table 20.3) (Sia & Wieland, 2011).
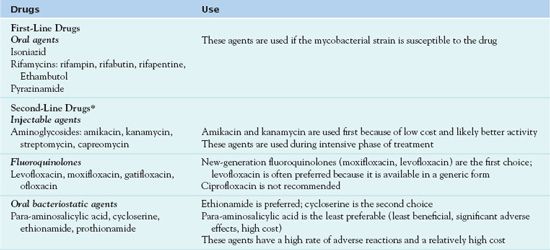
*Agents with unclear anti-TB activity include linezolid, amoxicillin/clavulanate, imipenem/cilastatin, clarithromycin, and others; these drugs have many adverse effects. These may be used when other treatments are inadequate, but routine use is not recommended.
Adapted from Albanna, A. S. & Menzies, D. (2011). Drug-resistant tuberculosis: What are the treatment options? Drugs, 71, 817—818.
Albanna and Menzies (2011) describe considerations that are taken into account when designing treatment regimens for resistant TB. Administration daily, rather than at intervals, is best. Treatment lasts 24 months after repeat sputum cultures are negative. XDR-TB, along with resistance to first-line agents, is also typically resistant to an injectable agent and a fluoroquinolone. Treatment for XDR-TB is similar to that for MDR-TB but with use of even more drugs; prescribers often include high-dose INH and new-generation fluoroquinolones. Therapy is extremely difficult, and there are no specific guidelines. Compared with treatment of MDR-TB, treatment of XDR-TB has a lower success rate and a higher mortality rate.
Clinical Application 20-2
 Mr. Diaz is taking INH, rifampin, pyrazinamide, and ethambutol for his TB. Why is he taking these four drugs? For what length of time does he take each drug?
Mr. Diaz is taking INH, rifampin, pyrazinamide, and ethambutol for his TB. Why is he taking these four drugs? For what length of time does he take each drug?
Isoniazid
 Isoniazid (INH), the most commonly used anti-TB drug and the prototype, is bactericidal, relatively inexpensive, and nontoxic. Although use by itself for treatment of latent TB is appropriate, use with other anti-TB drugs is essential for treatment of active TB.
Isoniazid (INH), the most commonly used anti-TB drug and the prototype, is bactericidal, relatively inexpensive, and nontoxic. Although use by itself for treatment of latent TB is appropriate, use with other anti-TB drugs is essential for treatment of active TB.
Pharmacokinetics
INH is well absorbed from the GI tract, with peak serum concentrations occurring 1 to 2 hours after a 300-mg dose. Food slows absorption. The drug penetrates and reaches therapeutic concentrations in essentially all body fluids and cavities, including the cerebrospinal fluid (CSF). Its half-life is 1 to 4 hours. It is acetylated in the liver to acetylisoniazid, which is excreted by the kidneys. Metabolism of INH is genetically determined; some people are “slow acetylators” and others are “rapid acetylators.” A person’s rate of acetylation affects response to INH. If the rate is slow, INH is more likely to accumulate to toxic concentrations, and the development of peripheral neuropathy is more likely. However, there is no significant difference in the clinical effectiveness of INH. Liver or kidney impairment may slow elimination.
Action
INH penetrates body cells and mycobacteria, inhibiting formation of bacterial cell walls. The drug not only kills actively growing intracellular and extracellular organisms but it inhibits the growth of dormant organisms in macrophages and tuberculous lesions.
Use
It is appropriate to use INH alone or in combination with other anti-TB drugs in the treatment of latent TB. However, it is essential that it always be given in combination with other anti-TB drugs in the treatment of active TB. Table 20.4 gives route of administration and dosage information for INH.
 TABLE 20.4
TABLE 20.4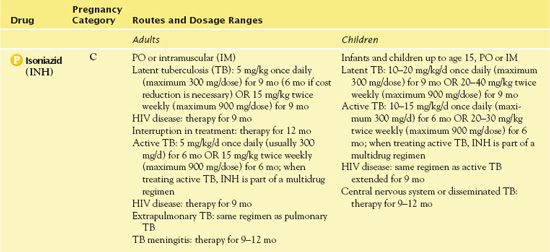
HIV, human immunodeficiency virus.
For the treatment of TB in pregnant women, the initial regimen should be INH, rifampin, and ethambutol for at least 9 months (Sia & Wieland, 2011). Box 20.2 contains more information about the use of anti-TB drugs in pregnancy.
BOX 20.2 Treatment of Tuberculosis in Pregnancy



