Describe the functions of the anterior and posterior pituitary hormones as well as the hypothalamic hormones.
 Identify the pathophysiology and clinical manifestations of central precocious puberty, acromegaly, growth deficiency in children, and diabetes insipidus.
Identify the pathophysiology and clinical manifestations of central precocious puberty, acromegaly, growth deficiency in children, and diabetes insipidus.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the anterior pituitary hormone used to treat growth hormone deficiency in children.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the anterior pituitary hormone used to treat growth hormone deficiency in children.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the posterior pituitary hormone used to treat diabetes insipidus.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the posterior pituitary hormone used to treat diabetes insipidus.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the hypothalamic hormone drugs used to treat precocious puberty.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the hypothalamic hormone drugs used to treat precocious puberty.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the hypothalamic hormone drugs used to treat acromegaly.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the hypothalamic hormone drugs used to treat acromegaly.
 Implement the nursing process in the care of the patient receiving specific pituitary and hypothalamic hormones.
Implement the nursing process in the care of the patient receiving specific pituitary and hypothalamic hormones.
Clinical Application Case Study
Jose Rojas is a 24-year-old man who has suffered a head injury while riding his motorcycle without a helmet. He has been admitted for observation, with multiple abrasions and a blow to the head. He begins to produce massive amounts of clear, pale-yellow urine. The physician diagnoses diabetes insipidus and orders desmopressin 0.2 mL intranasally in two divided doses.
KEY TERMS
Acromegaly: a chronic disease, resulting from excessive secretion of growth hormone (GH); characterized by an abnormal pattern of bone and connective tissue growth associated with an increased incidence of diabetes mellitus and hypertension
Antidiuretic hormone: functions to regulate water balance; also known as vasopressin. This hormone is secreted when body fluids become concentrated and when blood volume is low
Corticotropin: anterior pituitary hormone obtained from animal pituitary glands; also referred to as adrenocorticotropic hormone. This hormone may be used as a diagnostic test to differentiate primary adrenal insufficiency from secondary adrenal insufficiency caused by inadequate pituitary secretion of corticotropin
Corticotropin-releasing hormone or factor: causes release of corticotropin in response to stress and threatening stimuli; most often secreted during sleep
Dwarfism: a condition marked by severely decreased linear growth and frequently severely delayed mental, emotional, dental, and sexual growth; caused by a lack of GH
Gonadotropin-releasing hormone: causes release of follicle-stimulating hormone and luteinizing hormone
Growth hormone (GH): stimulates the growth of body tissues; regulates cell division and protein synthesis required for normal growth and promotes an increase in cell size and number, including growth of muscle cells and lengthening of bone
Inhibiting hormone: produced by the hypothalamus; decreases hormone secretion that corresponds to each of the major hormones of the anterior pituitary gland
Releasing hormone: produced by the hypothalamus; accelerates the secretion of the anterior pituitary hormone
Thyroid-stimulating hormone (thyrotropin): regulates secretion of thyroid hormones
Introduction
This chapter introduces the pharmacological care of the patient experiencing increased or decreased function of the hormones secreted by the hypothalamus and pituitary gland. It focuses on drug therapy for chronic precocious puberty, acromegaly, growth hormone deficiency in children, and diabetes insipidus.
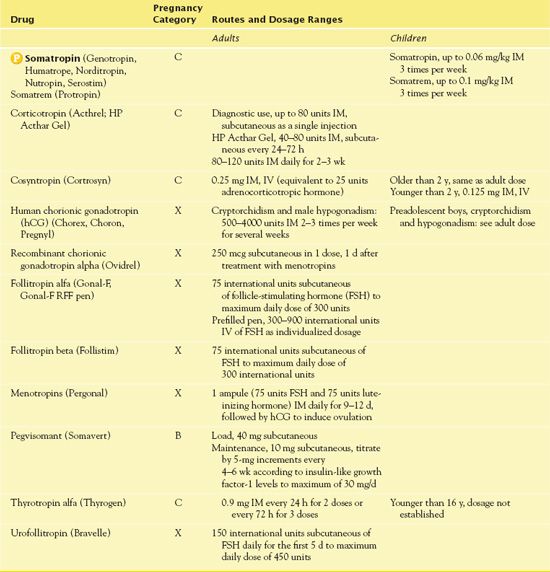
The hypothalamus of the brain and the pituitary gland interact to control most metabolic functions of the body and to maintain homeostasis (Fig. 41.1). They are anatomically connected by a funnel-shaped hypophyseal stalk. The hypothalamus controls secretions of the pituitary gland. The pituitary gland, in turn, regulates secretions or functions of other body tissues called target tissues. The pituitary gland is actually two glands, each with different structures and functions. The anterior pituitary is composed of different types of glandular cells that synthesize and secrete different hormones. The posterior pituitary is anatomically an extension of the hypothalamus and is composed mainly of nerve fibers. Although it does not manufacture any hormones itself, it stores and releases hormones synthesized in the hypothalamus. Hormones are chemical messengers with specific regulatory effect on the cells that control various bodily functions.
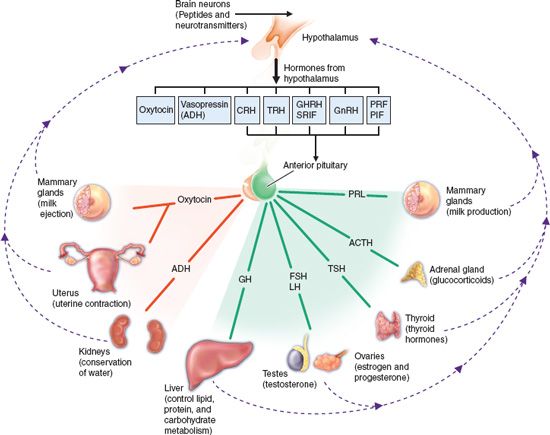
Figure 41.1 Hypothalamic and pituitary hormones and their target organs. The hypothalamus produces hormones that act on the anterior pituitary or are stored in the posterior pituitary. The anterior pituitary produces hormones that act on various body tissues and stimulate production of other hormones. T3, triiodothyronine; T4, thyroxine; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone.
Overview of Pituitary and I Hypothalamic Dysfunction
Physiology
Anterior Pituitary Hormones
The anterior pituitary gland produces seven hormones. Two of these, growth hormone and prolactin, act directly on their target tissues; the other five act indirectly by stimulating target tissues to produce other hormones.
Corticotropin, also called adrenocorticotropic hormone (ACTH), stimulates the adrenal cortex to produce corticosteroids (see Chap. 15). The hypothalamus and plasma levels of cortisol, the major corticosteroid, control secretion of corticotropin. A negative feedback mechanism inhibits the release of cortisol when plasma levels are adequate for body needs. After corticotropin administration, there is a greater release of corticotropin by way of negative feedback. Thus, the therapeutic effects are greater with corticotropin than with glucocorticoids, leading to a decrease in ACTH and changing the structure of the pituitary.
Growth hormone, or GH (also called somatotropin), stimulates growth of body tissues. It regulates cell division and protein synthesis required for normal growth and promotes an increase in cell size and number, including growth of muscle cells and lengthening of bone. These effects occur mainly via altered metabolism of carbohydrate, protein, and fat by direct and indirect effects. Authorities often consider GH an insulin antagonist because it suppresses the abilities of insulin to stimulate uptake of glucose in peripheral tissues and enhance glucose synthesis in the liver. Paradoxically, administration of GH produces hyperinsulinemia by stimulating insulin secretion. In addition, GH stimulates protein anabolism in many tissues. The hormone also enhances fat utilization by stimulating triglyceride breakdown and oxidation in fat-storing cells.
Levels of GH rise rapidly during adolescence, peak in the 20s, and then start to decline. In children, deficient GH produces dwarfism, a condition marked by severely decreased linear growth and, frequently, severely delayed mental, emotional, dental, and sexual growth. If untreated, excessive GH in preadolescents produces gigantism, resulting in heights of 8 or 9 feet.
In adults, deficient GH (less than expected for age) can cause increased fat, reduced skeletal and heart muscle mass, reduced strength, reduced ability to exercise, and worsened cholesterol levels (i.e., increased low-density lipoprotein cholesterol and decreased high-density lipoprotein cholesterol), which increases the risk of cardiovascular disease. Excessive GH in adults produces acromegaly, a chronic disease characterized by an abnormal pattern of bone and connective tissue growth associated with an increased incidence of diabetes mellitus and hypertension.
Thyroid-stimulating hormone (TSH) (also called thyrotropin) regulates secretion of thyroid hormones. Thyrotropin secretion is controlled by a negative feedback mechanism in proportion to metabolic needs. Thus, increased thyroid hormones in body fluids inhibit secretion of thyrotropin by the anterior pituitary and of thyroid-releasing hormone (TRH) by the hypothalamus.
Follicle-stimulating hormone (FSH), one of the gonadotropins, stimulates functions of sex glands. In people of both sexes, the anterior pituitary produces FSH, beginning at puberty. It acts on the ovaries in a cyclical fashion during the reproductive years, stimulating growth of ovarian follicles. These follicles then produce estrogen, which prepares the endometrium for implantation of a fertilized ovum. FSH acts on the testes to stimulate the production and growth of sperm (spermatogenesis), but it does not stimulate secretion of male sex hormones.
Luteinizing hormone (LH) (also called interstitial cell-stimulating hormone), another gonadotropin, stimulates hormone production by the gonads of both sexes. In women, LH is important in the maturation and rupture of the ovarian follicle (ovulation). After ovulation, LH acts on the cells of the collapsed follicular sac to produce the corpus luteum, which then produces progesterone during the last half of the menstrual cycle. When blood progesterone levels rise, a negative feedback effect acts on hypothalamic and anterior pituitary secretion of gonadotropins. Decreased pituitary secretion of LH causes the corpus luteum to die and stop producing progesterone. Lack of progesterone causes slough and discharge of the endometrial lining as menstrual flow. (Of course, if the ovum has been fertilized and attached to the endometrium, menstruation does not occur.) In men, LH stimulates the Leydig cells in the spaces between the seminiferous tubules to secrete androgens, mainly testosterone.
Prolactin plays a part in milk production by nursing mothers. It is not usually secreted in nonpregnant women because of the hypothalamic hormone prolactin-inhibiting factor (PIF). During late pregnancy and lactation, various stimuli, including suckling, inhibit the production of PIF, which thus allows prolactin to best synthesized and released.
Melanocyte-stimulating hormone (MSH) plays a role in skin pigmentation, and investigators have found that it also plays important roles in feeding and energy metabolism as well as in inflammation. Recently, they have explored links between MSH, particularly gamma-MSH, and cardiovascular regulation and sodium metabolism.
Posterior Pituitary Hormones
The posterior pituitary gland stores and releases two hormones that are synthesized by nerve cells in the hypothalamus.
Antidiuretic hormone (ADH), also called vasopressin, functions to regulate water balance. ADH makes renal tubules more permeable to water. This allows water in renal tubules to be reabsorbed into the plasma and thus conserves body water. In the absence of ADH, little water is reabsorbed, and large amounts are lost in the urine.
Secretion of ADH occurs when body fluids become concentrated (high amounts of electrolytes in proportion to the amount of water) and when blood volume is low. In the first instance, ADH causes reabsorption of water, dilution of extracellular fluids, and restoration of normal osmotic pressure. In the second instance, ADH raises blood volume and arterial blood pressure toward homeostatic levels.
Oxytocin functions in childbirth and lactation. It initiates uterine contractions at the end of gestation to induce childbirth, and it causes milk to move from breast glands to nipples so the infant can obtain the milk by suckling.
Hypothalamic Hormones
The hypothalamus produces releasing hormones to accelerate the secretion of a given hormone or inhibiting hormones that decreases hormone secretion that corresponds to each of the major hormones of the anterior pituitary gland.
Corticotropin-releasing hormone or factor (CRH or CRF) causes release of corticotropin in response to stress and threatening stimuli. Secretion of CRH most often occurs during sleep; it is under the influence of several neurotransmitters. Acetylcholine and serotonin stimulate its secretion, and gamma-aminobutyric acid (GABA) and norepinephrine inhibit its secretion. ADH increases the ability of CRH to stimulate corticotropin secretion, and somatostatin and elevated levels of glucocorticoids decrease or prevent this ability. Health care providers use CRH in the diagnosis of Cushing’s disease, a disorder characterized by excess cortisol.
Growth hormone-releasing hormone (GHRH) causes release of GH in response to low blood levels of GH. The neurotransmitters dopamine, norepinephrine, epinephrine, GABA, acetylcholine, and serotonin stimulate the secretion of hypothalamic GHRH. Somatostatin blocks the stimulatory effect of GHRH on secretion of GH. Uses of GHRH include testing pituitary function and stimulating growth in children with GHRH deficiency.
As previously mentioned, somatostatin (growth hormone release–inhibiting hormone) inhibits release of GH and is distributed throughout the brain and spinal cord, where it functions as a neurotransmitter. It is also found in the intestines and the pancreas, where it regulates secretion of insulin and glucagon. Several neurotransmitters, including acetylcholine, dopamine, epinephrine, GABA, and norepinephrine, increase somatostatin secretion.
Somatostatin also inhibits other functions, including secretion of corticotropin, thyroid-stimulating hormone (TSH or thyrotropin), prolactin, pancreatic secretions (e.g., insulin, glucagon), and gastrointestinal (GI) secretions (gastrin, cholecystokinin, secretin, vasoactive intestinal peptide) as well as GI motility, bile flow, and mesenteric blood flow. Hypothalamic somatostatin blocks the action of GHRH and decreases thyrotropin-releasing hormone (TRH)-induced release of TSH. GH stimulates secretion of somatostatin, and the effects of somatostatin on TSH may contribute to TSH deficiency in children being treated with GH.
TRH causes release of TSH in response to stress, such as exposure to cold. TRH is useful in diagnostic tests of pituitary function and hyperthyroidism (see Chap. 40).
Gonadotropin-releasing hormone (GnRH) causes release of FSH and LH. Several synthetic equivalents of GnRH are clinically useful.
Prolactin-releasing factor is active during lactation after childbirth. PIF is active at times other than during lactation.
NCLEX Success
1. A young woman, a graduate nurse, is scheduled to take the NCLEX examination in the morning. Which of the following hormones is released while she is asleep?
A. glucagon
B. insulin
C. corticotropin
D. antidiuretic hormone
2. A man has sustained burns over 80% of his body in a boating accident. Which of the following hormones is released to raise the arterial blood pressure?
A. antidiuretic hormone
B. oxytocin
C. melanocyte-stimulating hormone
D. luteinizing hormone
3. An adult has decreased growth hormone. Which of the following signs or symptoms is the patient likely to develop?
A. increased cholesterol
B. increased muscle strength
C. increased mental alertness
D. increased blood glucose
Pathophysiology and Clinical Manifestations
Precocious Puberty
Children diagnosed with precocious puberty develop secondary sexual characteristics prior to the age of 9 (boys) and 8 (girls). The Lawson Wilkins Pediatric Endocrine Society recommends that African American girls who develop breast and/or pubic hair prior to age 6 and Caucasian girls who develop these characteristics prior to the age of 7 be evaluated for precocious puberty. The three types of precocious puberty involve different pathological processes.
The first type, gonadotropin-dependent precocious puberty, is also known as central precocious puberty. The cause of this disorder is early maturation of the hypothalamic–pituitary–gonadal axis. Breast enlargement and pubic hair development occur in girls, and testicular enlargement and pubic hair development occur in boys. Other signs of this disorder include accelerated linear bone growth, increased bone age, and pubertal levels of some gonadal hormones (FSH and LH in girls; testosterone in boys).
The second type, gonadotropin-independent precocious puberty, is a result of excess secretion of the estrogen and androgen sex hormones from the gonads or adrenal glands. In girls, pituitary tumors are rare but do cause this type of precocious puberty. Exposure to estrogen creams used by menopausal care providers of girls may also lead to precocious puberty. In boys, Leydig cell tumors may be the cause. These tumors, which result in an asymmetric enlargement of the testes, are benign; surgical removal is appropriate.
The third type, incomplete precocious puberty, is isolated breast development in girls or hormone-mediated characteristics (e.g., acne or axillary or pubic hair) in boys. It is necessary to confirm the diagnosis using radiographic examination of the bone age—determining when the epiphyseal maturation is marginally advanced.
Acromegaly
Acromegaly results from persistent hypersecretion of GH due to the presence of a somatotroph adenoma of the anterior pituitary or a hypothalamic tumor that releases GHRH. The hypersecretion of growth hormone then results in the liver secretion of insulin-like growth factor-1 (IGF-1). This activity produces the clinical manifestations of acromegaly. The onset of these clinical manifestations is slow, as is its progression. Patients may complain of headaches with visual changes. Women have menstrual abnormalities, hot flashes, and vaginal atrophy from decreased estrogen levels. Men have erectile dysfunction, diminished libido, and decreased testicular size. Other signs and symptoms are thickening of the skin, linear bone growth, and enlargement of the liver, lungs, kidney, thyroid, and heart. High serum levels of GH and IGF-1 result in impaired glucose tolerance, hyperinsulinism, and insulin resistance.
Growth Hormone Deficiency in Children
The definition of growth deficiency in children is growth below the third percentile of the established normal values. There are several causes of GH deficiency. Mutation of a transcription factor (POUF1; also known as PIT-1) leads to variable recessive peptide hormone deficiencies that may be associated with anterior pituitary hypoplasia. Also, mutation of a transcription factor (PROP1), which causes a failure to activate POUF1/PIT-1 gene expression, results in pituitary hypoplasia and/or familial multiple pituitary hormone deficiency. In addition, mutations in the anterior pituitary cells are sources of GH deficiency.
Idiopathic short stature is present when a child’s stature falls below 2 standard deviations of the mean for age. The child has no endocrine, metabolic, or condition to account for the GH deficiency.
Diabetes Insipidus
Diabetes insipidus is a condition that results from a dysfunction in the posterior pituitary lobe. Excretion of large quantities of dilute urine results due to a deficiency in the production of ADH. With the absence of ADH, the kidneys filter the water but do not reabsorb it. When this occurs, the circulating fluid volume decreases, producing increased thirst and large amounts of urine production.
Diabetes insipidus may be idiopathic, hereditary, or acquired. Causes include lesions in the posteriorpituitary, hypothalamus, or infundibular stem that interfere with ADH synthesis, transport, and release. These lesions in the central nervous system (CNS) can be the result of a tumor, aneurysm, or thrombus. Other causes are the removal of the pituitary gland, immunological disorders, or infections.
Characteristic features include polyuria, with excretion of dilute urine ranging from 4 to as much as 30 L; extreme polydipsia, with the consumption of copious amounts of water or other fluids; and hypernatremia, with increased serum osmolality. The dehydration produces dizziness, weakness, and weight loss. Children present with enuresis, irritability, diminished sleep, and decreased weight gain along with diminished linear growth. If diabetes insipidus goes untreated, it may produce circulatory collapse and CNS depression or damage in both adults and children.
Clinical Application 41-1
 How has Mr. Rojas’ head injury contributed to the development of diabetes insipidus?
How has Mr. Rojas’ head injury contributed to the development of diabetes insipidus?
 Why is he producing large amounts of dilute urine?
Why is he producing large amounts of dilute urine?
NCLEX Success
4. A nurse is educating grandparents about child safety. Which of the following prevents precocious puberty?
A. keeping Tylenol out of a child’s reach
B. keeping estrogen cream out of a child’s reach
C. keeping Nitro-Bid paste out of a child’s reach
D. keeping hydrocortisone out of a child’s reach
5. Two weeks ago, a 4-year-old girl fell from a backyard swing and hit her head. Her mother states her potty-trained daughter has been wetting the bed for the past 3 nights. What do you suspect the bedwetting is related to?
A. regression in development due to changes in the home
B. drinking juices prior to bedtime
C. a urinary tract infection
D. possible diabetes insipidus
6. A man has received a diagnosis of acromegaly. Which of the following symptoms can cause alterations in the man’s psychosocial integrity?
A. increased libido
B. tachycardia
C. increased linear growth
D. erectile dysfunction
Drug Therapy
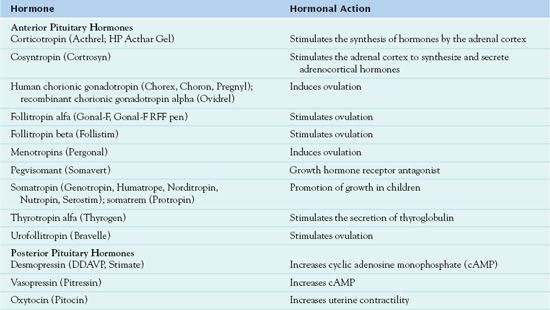
Although pituitary and hypothalamic hormones has few therapeutic uses, they have important functions when used in certain circumstances. Hypothalamic hormones are synthesized into drug formulations that are administered to treat endometriosis, metastatic breast cancer, advanced prostate cancer, uterine fibroid tumors, vasoactive intestinal tumors, and diarrhea, as well as central precocious puberty. Manufacturers have synthesized more drug formulations. Tables 41.1 and 41.2 list the exogenous hormones used in the treatment of hormonal dysfunction.
TABLE 41.1
Drugs Administered for the Treatment of Hypothalamic Hormonal Changes

TABLE 41.2
Drugs Administered for the Treatment of Pituitary Hormonal Changes
Anterior Pituitary Hormone Drugs for Growth Deficiency in Children
The prototype anterior pituitary hormone is GH. Administered therapeutically, GH is not natural but rather synthesized from bacteria using recombinant DNA technology.  Somatropin (Humatrope, others) is therapeutically equivalent to endogenous GH produced by the anterior pituitary gland.
Somatropin (Humatrope, others) is therapeutically equivalent to endogenous GH produced by the anterior pituitary gland.
Pharmacokinetics
Somatropin is well absorbed. Most of the drug is metabolized in the liver and kidneys. A small amount is excreted unchanged by the kidneys.
Action
Somatropin has the same sequence of amino acids as endogenous GH; it stimulates skeletal, linear, muscle, and organ growth. Stimulation of cartilage growth at the epiphyseal plate promotes linear growth. Lean body mass and bone mass increase, and fat mass decreases. Stimulation of erythropoietin results in an increase of red blood cells. The drug increases protein synthesis and hepatic glucose output. Absorption of nutrients from the GI tract improves.
Use
The main clinical use of somatropin is for children whose growth is impaired by a deficiency of endogenous GH. The drug is ineffective when impaired growth results from other causes or after puberty, when epiphyses of the long bones have closed. Also, it is useful in the treatment of growth failure associated with chronic kidney disease as well as short stature associated with Turner syndrome, being born small for gestational age, Prader-Willi syndrome, idiopathic short stature, certain genetic mutations, and Noonan syndrome. In addition, somatropin is useful in adults with GH deficiency. Table 41.3 gives route and dosage information for somatropin and other anterior pituitary hormone drugs.
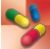 TABLE 41.3
TABLE 41.3
DRUGS AT A GLANCE: Anterior Pituitary Hormone Drugs