Describe the asthma and bronchoconstriction in terms of its pathophysiology.
 Compare and contrast the short-acting (rescue) and the long-term maintenance inhaled beta2-adrenergic agonists.
Compare and contrast the short-acting (rescue) and the long-term maintenance inhaled beta2-adrenergic agonists.
 Identify the prototype drug from each drug class used to treat asthma and bronchoconstriction.
Identify the prototype drug from each drug class used to treat asthma and bronchoconstriction.
 Describe drugs used to treat asthma and bronchoconstriction in terms of mechanism of action, indications for use, major adverse effects, and nursing implications.
Describe drugs used to treat asthma and bronchoconstriction in terms of mechanism of action, indications for use, major adverse effects, and nursing implications.
 Understand how to use the nursing process in the care of patients with asthma and bronchoconstriction.
Understand how to use the nursing process in the care of patients with asthma and bronchoconstriction.
Clinical Application Case Study
Terry Lee, a 38-year-old man, presents to the emergency department in severe respiratory distress, with profound dyspnea, wheezing, and circumoral cyanosis. He received a diagnosis of asthma at age 10. Mr. Lee is admitted to the intensive care unit. His treatment regimen includes albuterol via inhalation every 20 minutes for four doses and intravenous corticosteroids every 6 hours.
KEY TERMS
Airway hyperresponsiveness: exaggerated bronchoconstrictive response to stimuli
Bronchospasm (also called bronchoconstriction): constriction of the air passages of the lung (as in asthma) by spasmodic contraction of the bronchial muscles
Leukotrienes: strong chemical mediators of bronchoconstriction and inflammation, the major pathologic features of asthma
Maintenance inhalant medications: long-term control beta2-agonists used to achieve and maintain prophylactic control of persistent asthma
Mast cells: cells releasing substances that produce bronchoconstriction and inflammation in response to causative stimuli
Rescue inhalant medications: quick-relief, short-acting beta2-agonists used during periods of acute symptoms and exacerbations
Status asthmaticus: acute, severe asthma
Triggers: factors that initiate asthma symptoms
Work-exacerbated asthma: adverse respiratory outcome resulting from work-related conditions
Introduction
This chapter describes drugs used to treat asthma and other respiratory disorders characterized by bronchoconstriction, inflammation, airway hyperresponsiveness, mucosal edema, and excessive mucus production. These signs and symptoms characterize such features as asthma, bronchitis, and emphysema. Here, the emphasis is on asthma because of its widespread prevalence, especially in urban populations. To understand the use of drugs in the management of asthma, it is necessary to provide an overview of the condition and its prevalence, etiology, pathophysiology, and clinical manifestations.
Overview of Asthma
Etiology
Although all the causes of asthma are not known, the condition appears to involve the relationship between host factors (predominantly genetic) and environmental exposures that occur at a critical time in immune system development. The strongest identifiable predisposing factor for developing asthma seems to be the genetic predisposition for the development of an IgE-mediated response to common allergens. Asthma may occur at any age but is especially common in children and older adults. Numerous stimuli may precipitate acute symptoms of asthma, and hyperresponsiveness to such stimuli may initiate both inflammation and bronchoconstriction. Viral infections of the respiratory tract are often the causative agents, especially in infants and young children, whose airways are small and easily obstructed. Asthma symptoms may persist for days or weeks after the viral infection resolves. Common environmental factors that cause asthma symptoms (triggers) vary from person to person and include outdoor allergens such as pollens and molds; indoor allergens, including dust mites, dander, and molds; and respiratory irritants, such as smoke from cigarettes and occupational environments. Children who are exposed to allergens and airway irritants such as tobacco smoke during infancy are at high risk for development of asthma. Authorities have suggested that strenuous physical activities; certain drugs; stress and emotion; and changes in season, weather, and temperature are asthma triggers.
Asthma is more prevalent in African Americans and Hispanics than in Caucasians. It results in death more often in African Americans. However, the differences are usually attributed to urban living and lesser access to health care rather than race or ethnicity.
Work-exacerbated asthma (WEA) (i.e., adverse respiratory outcomes resulting from repeated and prolonged conditions at work) is also a major health problem. People with WEA often have symptoms while in the work environment, with improvement on days off and during vacations. Symptoms sometimes persist after termination of exposure.
Many people with asthma have sensitivities to certain drugs that can precipitate acute symptoms of asthma. In about 25% of patients with asthma, aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) can precipitate an exacerbation. Research has also demonstrated an increased risk of asthma and its exacerbation with the use of acetaminophen. Asthma symptoms may also worsen in some people who take beta-adrenergic-blocking agents and angiotensin-converting enzyme inhibitors.
An official American Thoracic Society statement: Work-Exacerbated Asthma
by HENNEBERGER, P. K., REDLICH, C. A., CALLAHAN, D. B., HARBER, P., LEMIÈRE, C., MARTIN, J., TARLO, S. M., VANDENPLAS, O. & TORÉN, K.; ATS AD HOC COMMITTEE ON WORK-EXACERBATED ASTHMA.
American Journal of Respiratory Critical Care Medicine, 2011, 184(3), 368–378
This systematic review of the current literature identifies adverse respiratory outcomes resulting from conditions at work, or work-exacerbated asthma (WEA). This condition differs from occupational asthma that is caused by work. Occupational exposures can trigger the onset of asthma and worsen the existing condition. The purpose of this review is to
• Summarize current knowledge about the descriptive epidemiology, clinical characteristics, and management and treatment of WEA
• Propose a case definition for WEA
• Discuss needs for prevention and research (statements about prevention and research needs were reached by consensus)
Different types of agents or conditions at work may exacerbate asthma. Cases of WEA with persistent work-related symptoms can have clinical characteristics (level of severity, medication needs) and adverse socioeconomic outcomes (unemployment, reduction in income) similar to those of cases of occupational asthma. Compared with adults with asthma unrelated to work, people with WEA report more days with symptoms, seek more medical care, and have a lower quality of life.
IMPLICATIONS FOR NURSING PRACTICE: WEA is a common and underrecognized adverse outcome resulting from conditions at work. The nurse should consider WEA in any patient with asthma that is becoming worse or who has work-related symptoms. Management of WEA should focus on reducing work exposures and optimizing standard medical management, with a change in jobs only if these measures are not successful. Additional research is needed (1) to improve the understanding of the risk factors for WEA and its mechanisms and outcomes, and (2) to inform and evaluate preventive interventions.
Some patients are allergic to sulfites and may experience life-threatening asthma attacks if they ingest foods processed with these preservatives (e.g., beer, wine, dried fruit). The U.S. Food and Drug Administration (FDA) has banned the use of sulfites on foods meant to be served raw, such as salads in salad bars. Patients with severe asthma should be cautioned against ingesting food and drug products containing sulfites or metabisulfites.
Gastroesophageal reflux disease (GERD), a common disorder characterized by heartburn and esophagitis, is also associated with asthma. Asthma that worsens at night may be associated with nighttime acid reflux. Although the precise mechanism of pulmonary symptoms of GERD in asthma is not known, there are suggestions that microaspirations or a vagally mediated, reflex type of bronchoconstriction may be involved. Asthma may also aggravate GERD, because antiasthma medications that dilate the airways also relax muscle tone in the gastroesophageal sphincter and may increase acid reflux. It has been suggested that in people with inadequate control of their asthma symptoms, a trial of an H2-blocker or proton pump inhibitor therapy may be of benefit.
Pathophysiology
Asthma
Bronchospasm (also called bronchoconstriction) involves strong muscle contractions that narrow the airways. Airway smooth muscle extends from the trachea through the bronchioles. The muscle wraps around the airways in a spiral pattern, and contraction causes a sphincter type of action that can completely occlude the airway lumen. Inflammation, mucosal edema, and excessive mucus aggravate bronchoconstriction, and numerous stimuli described previously (in Etiology) may precipitate it. In some people, airway hyperresponsiveness occurs, and the bronchoconstrictive response is exaggerated.
When lung tissues are exposed to causative stimuli, mast cells release substances that cause bronchoconstriction and inflammation. Mast cells are found throughout the body in connective tissues and are abundant in tissues surrounding capillaries in the lungs. When sensitized mast cells in the lungs or eosinophils in the blood are exposed to allergens or irritants, multiple cytokines and other chemical mediators (e.g., acetylcholine, cyclic guanosine monophosphate [GMP], histamine, interleukins, leukotrienes, prostaglandins, serotonin) are synthesized and released. These chemicals act directly on target tissues of the airways, causing smooth muscle constriction, increased capillary permeability and fluid leakage, and changes in the mucus-secreting properties of the airway epithelium.
Bronchoconstrictive substances are antagonized by cyclic adenosine monophosphate (cyclic AMP). Cyclic AMP is an intracellular substance that initiates various intracellular activities, depending on the type of cell. In lung cells, cyclic AMP inhibits release of bronchoconstrictive substances and thus indirectly promotes bronchodilation. In mild to moderate asthma, bronchoconstriction is usually recurrent and reversible, either spontaneously or with drug therapy.
In advanced or severe asthma, airway obstruction becomes less reversible and worsens because chronically inflamed airways undergo structural changes (e.g., fibrosis, enlarged smooth muscle cells, enlarged mucous glands), called “airway remodeling,” that inhibit their function.
Other Bronchoconstrictive Disorders
Box 31.1 summarizes the pathophysiology of chronic bronchitis and emphysema.
BOX 31.1 Other Bronchoconstrictive Disorders: Chronic Bronchitis and Emphysema
Chronic bronchitis and emphysema, commonly called chronic obstructive pulmonary disease (COPD), usually develop after long-standing exposure to airway irritants such as cigarette smoke. The conditions are also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), or chronic airflow limitation (CAL). In both conditions, bronchoconstriction and inflammation are more constant and less reversible than with asthma. Anatomic and physiologic changes occur over several years and lead to increasing dyspnea, activity intolerance, and reduced quality of life. Chronic bronchitis often leads to emphysema.
Chronic Bronchitis
Chronic bronchitis is defined in clinical terms as frequent cough with sputum production for 3 months per year, for two consecutive years. The hallmark of chronic bronchitis is an increase in the number and size of the goblet cells and mucous glands of the airway. This leads to increased mucus in the airways, a factor in narrowing of the airways and causing a cough with sputum. At the cellular level, inflammation infiltrates the airway walls leading to scarring and remodeling that thickens the walls; this further narrows the airways. As chronic bronchitis progresses, there is further thickening and scarring of the wall limitation of airflow.
Emphysema
Emphysema is an enlargement and destruction of the alveoli distal to the terminal bronchioles from long-term lung damage and inflammation. The changes to the alveolar walls decrease the surface area available for gas exchange. The elasticity of the lung also decreases leading to loss of supporting structures for the alveoli. Additionally, there is destruction of capillaries feeding the alveoli. The small airways that collapse early during expiration cause trapping of carbon dioxide and ultimate reduction of oxygen exchange at the alveoli.
Clinical Manifestations
Asthma is an airway disorder characterized by bronchoconstriction, inflammation, and airway hyperresponsiveness to various stimuli. Resultant symptoms include dyspnea, wheezing, chest tightness, cough, and sputum production. Wheezing is a high-pitched, whistling sound caused by turbulent airflow through an obstructed airway. Thus, any condition that produces significant airway occlusion can cause wheezing. However, a chronic cough may be the only symptom for some people with asthma. The presence of wheezing or coughing is not a dependable standard for evaluating the severity of an asthma attack.
Patients who use peak expiratory flow rate (PEFR) monitoring can assess the severity of airway constriction through trending of peak flow measurements. In general, a PEFR value 80% or greater of the patient’s best PEFR (established when breathing is normal) is considered within a safe range; from 50% to 80% of the PEFR value is a moderate drop; and less than 50% of the patient’s best PEFR value is considered a severe reduction in breathing function that requires urgent intervention.
QSEN Safety Alert 
The nurse encourages patients to develop an action plan with their health care provider to identify the correct action for acute episodes of asthma when the PEFR value is less than 50% of the best PEFR.
Symptoms vary in incidence and severity from occasional episodes of mild respiratory distress, with normal functioning between exacerbations, to persistent, daily, or continual respiratory distress if not adequately controlled. Inflammation and damaged airway mucosa are chronically present, even when patients appear symptom free. Acute episodes of asthma may last minutes to hours.
Acute, severe asthma that does not respond to the usual use of bronchodilators and is characterized by severe respiratory distress is called status asthmaticus. This life-threatening condition requires emergency treatment due to the high probability of respiratory failure. Patients may show signs of acute breathlessness, chest tightness, agitation, confusion, or an inability to concentrate. In status asthmaticus, the lack of wheezing sound or coughing may indicate severe bronchoconstriction, impaired gas exchange in the lungs, and a worsening of the condition.
Drug Therapy
Management of asthma involves prevention of airway inflammation and avoidance of triggers for better symptom control. Because of asthma’s significance as a public health problem, the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) assembled a group of experts who established “Guidelines for the Diagnosis and Management of Asthma.” These current guidelines (Box 31.2), updated in 2007, include an expanded portion on childhood asthma. They emphasize the importance of classifying asthma severity and the assessment of asthma control. The guidelines, which are the current “standard of care” for adults and children with asthma, center around four essential components of asthma care: assessment and monitoring, patient education, control of factors contributing to asthma severity, and pharmacologic treatment.
BOX 31.2 National Asthma Education and Prevention Program (NAEPP) Expert Panel Guidelines
Definition
Asthma is “a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role: in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells. In susceptible people, this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning.”
Goals of Therapy
 Minimal or no chronic symptoms day or night
Minimal or no chronic symptoms day or night
 Minimal or no exacerbations
Minimal or no exacerbations
 No limitations on activities; for children, no school/parent’s work missed
No limitations on activities; for children, no school/parent’s work missed
 Minimal use of short-acting inhaled beta2-agonist (less than 1 time per day, less than 1 canister per month)
Minimal use of short-acting inhaled beta2-agonist (less than 1 time per day, less than 1 canister per month)
 Minimal or no adverse effects from medications
Minimal or no adverse effects from medications
General Recommendations
 Establish and teach patients/parents/caregivers about quick-relief measures and long-term control measures. Assist to identify and control environmental factors that aggravate asthma. For acute attacks, gain control as quickly as possible (a short course of systemic corticosteroids may be needed); then step down to the least medication necessary to maintain control.
Establish and teach patients/parents/caregivers about quick-relief measures and long-term control measures. Assist to identify and control environmental factors that aggravate asthma. For acute attacks, gain control as quickly as possible (a short course of systemic corticosteroids may be needed); then step down to the least medication necessary to maintain control.
 Review the treatment regimen every 1 to 6 months. If control is adequate and goals are being met, a gradual stepwise reduction in medication may be possible. If control is inadequate, the treatment regimen may need to be changed. For example, frequent or increasing use of a short-acting beta2-agonist (greater than two times a week with intermittent asthma; daily or increasing use with persistent asthma) may indicate the need to initiate or increase long-term control therapy. However, first reassess patients’ medication techniques (e.g., correct use of inhalers), adherence, and environmental control measures.
Review the treatment regimen every 1 to 6 months. If control is adequate and goals are being met, a gradual stepwise reduction in medication may be possible. If control is inadequate, the treatment regimen may need to be changed. For example, frequent or increasing use of a short-acting beta2-agonist (greater than two times a week with intermittent asthma; daily or increasing use with persistent asthma) may indicate the need to initiate or increase long-term control therapy. However, first reassess patients’ medication techniques (e.g., correct use of inhalers), adherence, and environmental control measures.
Quick Relief for Acute Exacerbations
 Adults and children > 5 years: short-acting, inhaled, beta2-agonist, two to four puffs as needed. If symptoms are severe, patients may need up to three treatments at 20-minute intervals or a nebulizer treatment. A short course of a systemic corticosteroid may also be needed.
Adults and children > 5 years: short-acting, inhaled, beta2-agonist, two to four puffs as needed. If symptoms are severe, patients may need up to three treatments at 20-minute intervals or a nebulizer treatment. A short course of a systemic corticosteroid may also be needed.
 Children ≤ 5 years: short-acting beta2-agonist by nebulizer or face mask and spacer or holding chamber. Alternative: oral beta2-agonist. With viral respiratory infections, the beta2-agonist may be needed every 4 to 6 hours up to 24 hours or longer and a systemic corticosteroid may be needed.
Children ≤ 5 years: short-acting beta2-agonist by nebulizer or face mask and spacer or holding chamber. Alternative: oral beta2-agonist. With viral respiratory infections, the beta2-agonist may be needed every 4 to 6 hours up to 24 hours or longer and a systemic corticosteroid may be needed.
Long-Term Control
 Step 1 Mild Intermittent (symptoms ≤ 2 days per week or ≤ 2 nights per month): no daily medication needed; treat acute exacerbations with an inhaled beta2-agonist and possibly a short course of a systemic corticosteroid.
Step 1 Mild Intermittent (symptoms ≤ 2 days per week or ≤ 2 nights per month): no daily medication needed; treat acute exacerbations with an inhaled beta2-agonist and possibly a short course of a systemic corticosteroid.
 Step 2 Mild Persistent (symptoms greater than 2 per week but less than 1 time per day or greater than 2 nights per month):
Step 2 Mild Persistent (symptoms greater than 2 per week but less than 1 time per day or greater than 2 nights per month):
 Adults and children > 5 years: low-dose inhaled corticosteroid. Alternatives: cromolyn or nedocromil, a leukotriene modifier, or sustained- release theophylline to maintain a serum theophylline drug level of 5 to 15 mcg/mL.
Adults and children > 5 years: low-dose inhaled corticosteroid. Alternatives: cromolyn or nedocromil, a leukotriene modifier, or sustained- release theophylline to maintain a serum theophylline drug level of 5 to 15 mcg/mL.
 Children ≤ 5 years: administration of the inhaled corticosteroid by a nebulizer or metered-dose inhaler (MDI) with a holding chamber. Alternatives: cromolyn (via nebulizer or MDI with holding chamber) or a leukotriene modifier.
Children ≤ 5 years: administration of the inhaled corticosteroid by a nebulizer or metered-dose inhaler (MDI) with a holding chamber. Alternatives: cromolyn (via nebulizer or MDI with holding chamber) or a leukotriene modifier.
 Step 3 Moderate Persistent (symptoms daily and greater than 1 night per week):
Step 3 Moderate Persistent (symptoms daily and greater than 1 night per week):
 Adults and children > 5 years: low- to mediumdose inhaled corticosteroid and a long-acting beta2– agonist. Alternatives: increase corticosteroid dose or continue low to medium dose of corticosteroid and add a leukotriene modifier or theophylline.
Adults and children > 5 years: low- to mediumdose inhaled corticosteroid and a long-acting beta2– agonist. Alternatives: increase corticosteroid dose or continue low to medium dose of corticosteroid and add a leukotriene modifier or theophylline.
 Children < 5 years: low-dose inhaled corticosteroid and a long-acting beta2-agonist or mediumdose inhaled corticosteroid.
Children < 5 years: low-dose inhaled corticosteroid and a long-acting beta2-agonist or mediumdose inhaled corticosteroid.
 Step 4 Severe Persistent (symptoms continual during daytime hours and frequent at night):
Step 4 Severe Persistent (symptoms continual during daytime hours and frequent at night):
 Adults and children > 5 years: high-dose inhaled corticosteroid and long-acting beta2-agonist and, if necessary, a systemic corticosteroid (2 mg/kg/d, not to exceed 60 mg/d). Reduce systemic corticosteroid when possible.
Adults and children > 5 years: high-dose inhaled corticosteroid and long-acting beta2-agonist and, if necessary, a systemic corticosteroid (2 mg/kg/d, not to exceed 60 mg/d). Reduce systemic corticosteroid when possible.
 Children < 5 years: same as for adults and older children.
Children < 5 years: same as for adults and older children.
Low (L), Medium (M), and High (H) Doses of Inhaled Corticosteroids
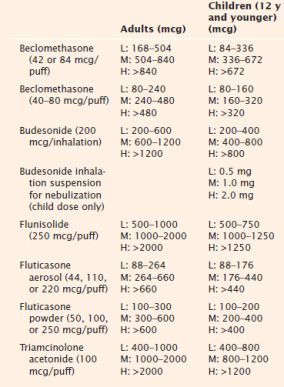
Adapted from National Asthma Education and Prevention Program. (2007, August). Expert panel report 3: Guidelines for the diagnosis and management of asthma (NIH Publication No. 08-4051). Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute. Retrieved January 4, 2012, from http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf
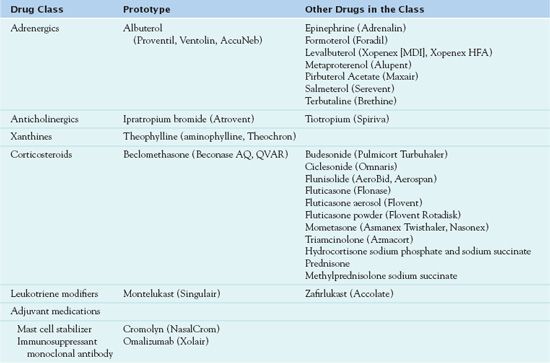
Table 31.1 lists the drugs used for the treatment of asthma. Bronchodilators and anti-inflammatory drugs are the pharmacologic cornerstones of asthma management. Bronchodilators used to prevent and treat bronchoconstriction include adrenergics, anticholinergics, and xanthines. Anti-inflammatory drugs, such as corticosteroids, leukotriene modifiers, mast cell stabilizers, and immunosuppressant monoclonal antibodies, are used to prevent and treat inflammation of the airways. Reducing inflammation also reduces bronchoconstriction by decreasing mucosal edema and mucous secretions that narrow airways and by decreasing airway hyperresponsiveness to various stimuli.
Overall, the severity of the disease process and the patient’s response to therapy largely determine the choice of drug and route of administration. An aerosol, which is given by inhalation, acts directly on the airways. Therefore, people can usually take it in smaller doses, and it produces fewer adverse effects than oral or parenteral drugs. A selective, short-acting, inhaled beta2-adrenergic agonist (e.g., albuterol) is the initial drug of choice for acute bronchospasm. Inflammation is a major component of asthma; thus, health care providers may give an inhaled corticosteroid early in the disease process, often with a bronchodilator or mast cell stabilizer. In acute episodes of bronchoconstriction, prescribers often order that a corticosteroid be taken orally or intravenously for several days.
A common regimen for treatment of moderate asthma is an inhaled corticosteroid on a regular schedule, two to four times daily, and a short-acting, inhaled beta2-adrenergic agonist as needed for prevention or treatment of bronchoconstriction. For more severe asthma, therapy may involve continuation of the inhaled corticosteroid with both a short-acting and a long-acting beta2-agonist. The possible addition of a leukotriene modifier may also further control symptoms and reduce the need for corticosteroids and inhaled bronchodilators.
Management of status asthmaticus entails beta2-agonists in high doses and as often as every 20 minutes for 1 to 2 hours (by metered-dose inhalers [MDIs] with spacer devices or by compressed-air nebulization). However, high doses of nebulized albuterol have been associated with tachycardia, hypokalemia, and hyperglycemia. After symptoms have subsided, dosage reduction usually may occur, with extended dosage intervals. People may take high doses of systemic corticosteroids for several days, intravenously or orally. If the patient is able to take an oral drug, there is no therapeutic advantage to intravenous (IV) administration.
In general, researchers have not studied herbal and dietary therapies in asthma, in controlled clinical trials. Thus, people should avoid such treatments.
QSEN Safety Alert 
Because asthma can result in death in a matter of minutes, health care providers should counsel patients not to use dietary or herbal supplements in place of prescribed bronchodilating and antiinflammatory medications. Delays in appropriate treatment can have serious, even fatal, consequences.
Adrenergics
Specific effects of adrenergic medications depend mainly on the type of adrenergic receptor activated by the drug. Adrenergic receptors are those responses that are activated by adrenaline-like compounds. The drugs discussed in this chapter are more selective for specific adrenergic receptors or are primarily administered by inhalation to produce more localized therapeutic effects in the management of asthma and bronchoconstriction and thus have fewer systemic adverse effects. Other adrenergic drugs affect multiple adrenergic receptors and have many clinical uses; other chapters discuss these drugs more extensively (see Chaps. 29 and 58).
Administering bronchodilators by inhalation is most effective and the treatment of first choice to relieve acute asthma. Two general types of inhaled beta2-adrenergic agonists are used for asthma management: rescue inhalant medications (quick-relief, short-acting drugs) used during periods of acute symptoms and exacerbations and maintenance inhalant medications (long-term control drugs used to achieve and maintain prophylactic control of persistent asthma). Box 31.3 lists the rescue and maintenance medications used in asthma therapy.  Albuterol (Proventil, Ventolin, AccuNeb), the prototype adrenergic bronchodilator, is a rescue medication.
Albuterol (Proventil, Ventolin, AccuNeb), the prototype adrenergic bronchodilator, is a rescue medication.
BOX 31.3 Rescue and Maintenance Beta2-Agonist Inhaled Medications
Short-acting rescue medications
 Albuterol
Albuterol
 Metaproterenol
Metaproterenol
 Levalbuterol
Levalbuterol
 Pirbuterol
Pirbuterol
 Albuterol/ipratropium (combines a beta2-agonist and an inhaled anticholinergic)
Albuterol/ipratropium (combines a beta2-agonist and an inhaled anticholinergic)
Long-acting maintenance medications
 Formoterol
Formoterol
 Salmeterol
Salmeterol
 Salmeterol/fluticasone (combines a beta2-agonist bronchodilator and an inhaled steroid)
Salmeterol/fluticasone (combines a beta2-agonist bronchodilator and an inhaled steroid)
 Formoterol/budesonide (combines a beta2-agonist bronchodilator and an inhaled steroid)
Formoterol/budesonide (combines a beta2-agonist bronchodilator and an inhaled steroid)
Pharmacokinetics
Albuterol is rapidly absorbed following oral administration. With the oral drug, the onset of action is 15 to 30 minutes (extended release, 30 minutes), the peak is 2 to 3 hours, and the duration of action is 8 to 12 hours. With the inhaled drug (MDI), the onset of action is 5 to 15 minutes, the peak activity is 1 to 1 1/2; hours, and the duration of action is 3 to 6 hours. The drug quickly undergoes extensive metabolism in the liver. It enters breast milk.
Action
Albuterol and other drugs in the class stimulate beta2-adrenergic receptors in the smooth muscle of bronchi and bronchioles. The receptors, in turn, stimulate the enzyme adenyl cyclase to increase production of cyclic AMP. The increased cyclic AMP produces bronchodilation.
Use
Health care providers use albuterol to treat or prevent bronchospasm in people with asthma and other reversible obstructive airway disease. They also use the drug to prevent exercise-induced bronchospasm. Table 31.2 gives route and dosage information for albuterol and other adrenergic bronchodilators. In general, children and adolescents may take antiasthmatic medications for the same indications as for adults.
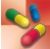 TABLE 31.2
TABLE 31.2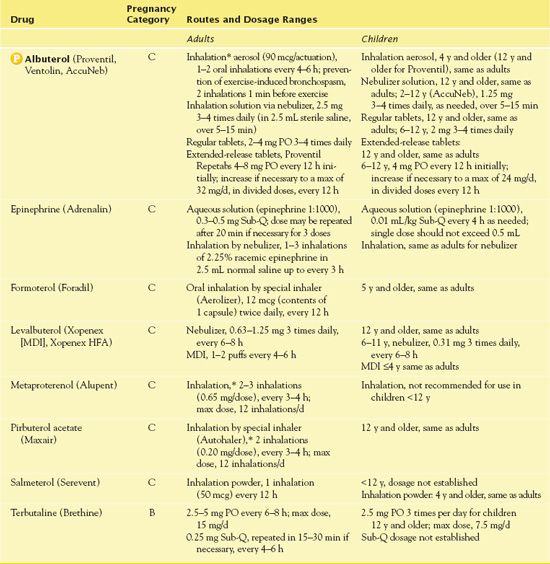
*Short-acting adrenergic bronchodilators are used mainly by inhalation, as needed, rather than on a regular schedule; MDI, metered-dose inhaler.
Use in Older Adults
Older adults often have chronic pulmonary disorders for which albuterol and other bronchodilators are used.
QSEN Safety Alert 
The main risks with adrenergic bronchodilators, particularly in older adults, are excessive cardiac and central nervous system (CNS) stimulation.
As with other populations, administering the medications by inhalation and giving the lowest effective dose decrease adverse effects.
Use in Patients With Critical Illness
People who are critically ill often use albuterol. Larger doses of bronchodilators (inhaled, systemic, or both) are usually required to relieve the symptoms of acute, severe bronchoconstriction or status asthmaticus. Then, it is necessary to reduce doses to the smallest effective amounts to attain the most therapeutic effects and produce the fewest adverse effects.
Use in Patients Receiving Home Care
Home administration of albuterol is common. Visits by a home care nurse should include assessment that the patient follows directions for administration; is not taking the drug more often than prescribed; is using the inhaler correctly; and is not manifesting signs of adverse effects, including excessive cardiac and CNS stimulation.
Adverse Effects
Administration of albuterol by MDI is associated with fewer systemic effects than administration of higher dosages orally or by nebulizer. Muscle tremor is the most frequent adverse effect. Major adverse effects are excessive cardiac and CNS stimulation. Symptoms of cardiac stimulation include angina, tachycardia, and palpitations. Symptoms of CNS stimulation consist of agitation, anxiety, insomnia, seizures, and tremors. Other reported effects may include serious dysrhythmias and cardiac arrest.
Contraindications
Contraindications to albuterol include known hypersensitivity to the drug, as well as cardiac tachydysrhythmias and severe coronary artery disease. Caution is warranted in hypertension, hyperthyroidism, diabetes mellitus, and seizure disorders.
Nursing Implications
Preventing Interactions
Some drugs interact with albuterol. Beta-blockers inhibit bronchodilation and can induce bronchospasm in patients with asthma who are taking albuterol. Thyroid hormones, theophylline, and some cold products can enhance the stimulatory adverse effects of the drug. Monoamine oxidase inhibitors should be avoided within 14 days of initiating treatment with albuterol to prevent hypertensive crisis. Caffeine-containing products, such as coffee, tea, or cola drinks, can also increase the adverse effects of cardiac and CNS stimulation.
Administering the Medication
Self-administration of albuterol and the other beta2-agonists is usually by MDI. Patients should use an albuterol inhaler before they use any other inhaler. This opens the airways and allows for better absorption of the other drug. The patient should wait 5 minutes or more between using different inhalers, such as one for a corticosteroid. Although most drug references still give a regular dosing schedule (e.g., every 4-6 hours) for beta2-agonists, asthma experts recommend that the drugs be used when needed (e.g., to treat acute dyspnea or prevent dyspnea during exercise). With overuse, they lose their bronchodilating effects because the beta2-adrenergic receptors become unresponsive to stimulation. (However, this tolerance does not occur with the long-acting beta2-agonists.)
Assessing for Therapeutic Effects
The nurse observes for decreased dyspnea, wheezing, and respiratory secretions; relief of bronchospasm and wheezing; reduced rate and improved quality of respirations and pulmonary function; and reduced anxiety and restlessness.
Assessing for Adverse Effects
The nurse observes for tachycardia, dysrhythmias, palpitations, restlessness, agitation, and insomnia. These signs and symptoms result from cardiac and CNS stimulation.
Patient Teaching
QSEN Safety Alert 



