Drug Interactions and Over-the-Counter Drugs
Objectives
• Differentiate the four pharmacokinetic processes related to drug interaction.
• Explain the three effects associated with pharmacodynamic interactions.
• Discuss drug interaction, and give examples
• Describe the effects of drug-food interactions.
• Explain the meaning of drug-induced photosensitivity.
• Discuss the nursing implications related to patients’ use of over-the-counter drugs.
Key Terms
additive effect, p. 32
adverse drug reaction, p. 30
antagonistic effects, p. 33
drug incompatibility, p. 30
drug interaction, p. 29
over-the-counter drugs, p. 29
photosensitivity, p. 34
synergistic effects, p. 33
Drug therapy is complex because of the great number of drugs available. Drug-drug, drug-food, and drug-laboratory interactions are an increasing problem. Because of the possibility of numerous interactions, the nurse should be knowledgeable about drug interactions and should closely monitor patient responses. Thorough and timely communication among members of the health team is essential. Patients at high risk for interactions include those who have chronic health conditions, take multiple medications, see more than one health care provider, and use multiple pharmacies. Older adults are at especially high risk for drug interactions; 75% take prescription drugs, and 82% take over-the-counter (OTC) drugs—drugs that are obtainable without a prescription.
Drug Interactions
A drug interaction is defined as an altered or modified action or effect of a drug as a result of interaction with one or multiple drugs. It should not be confused with adverse drug reaction or drug incompatibility. An adverse drug reaction is an undesirable drug effect that ranges from mild untoward effects to severe toxic effects, including hypersensitivity reaction and anaphylaxis. Drug incompatibility is a chemical or physical reaction that occurs among two or more drugs in vitro. In other words, the reaction occurs between two or more drugs within a syringe, IV bag, or other artificial environment outside of the body.
Drug interactions can be divided into two categories: (1) pharmacokinetic interactions and (2) pharmacodynamic interactions.
Pharmacokinetic Interactions
Pharmacokinetic interactions are changes that occur in the absorption, distribution, metabolism or biotransformation, and excretion of one or more drugs.
Absorption
When a person takes two drugs at the same time, the rate of absorption of one or both drugs can change. One drug can block, decrease, or increase the absorption of another drug. It can do this in one of the following ways:
Drugs that increase the speed of gastric emptying (e.g., laxatives, metoclopramide [Reglan]) may cause an increase in gastric and intestinal motility and a decrease in drug absorption. Most drugs are absorbed primarily in the small intestine; exceptions include barbiturates, salicylates, and theophylline (Theo-24), which undergo gastric absorption. Opioids and anticholinergic drugs (e.g., atropine) decrease gastric emptying time and gastrointestinal (GI) motility, causing an increase in absorption rate. For drugs that undergo gastric absorption, the amount or extent of absorption increases the longer the drug remains in the stomach.
When the gastric pH is decreased, a weakly acidic drug like aspirin is less ionized and more rapidly absorbed. Drugs that increase the gastric pH decrease absorption of weak-acid drugs. Antacids such as magnesium hydroxide (Maalox) and aluminum hydroxide (Amphojel) raise the gastric pH and block or slow absorption. Some drugs may react chemically. For example, tetracycline and the heavy-metal ions (calcium, magnesium, aluminum, and iron) found in antacids or iron supplements may lead to the formation of a drug complex and thus prevent the absorption of tetracycline. This phenomenon may also be observed when products that contain divalent cations are ingested with fluoroquinolone antibiotics such as ciprofloxacin (Cipro). Consequently, dairy products, multivitamins, and antacids should be avoided 1 hour before and 2 hours after tetracycline or ciprofloxacin consumption.
Certain cholesterol-lowering drugs, such as cholestyramine and colestipol, also form complexes with drugs like carbamazepine (Tegretol) and furosemide (Lasix). The drugs are then less soluble, resulting in reduced drug absorption. Drug complexation may be desired; for example, activated charcoal may be administered after toxic ingestion of a medication to decrease its absorption and, consequently, diminish or eliminate any untoward effects associated with the overdose.
Alteration of bacteria normally found in the GI tract may affect the pharmacokinetics of a medication. For example, intestinal microflora have the ability to metabolize digoxin (Lanoxin). Digoxin is a digitalis preparation that is used to treat heart failure and arrhythmias. Metabolism of this drug by gut bacteria decreases its bioavailability. Erythromycin, a macrolide antibiotic, and tetracycline are broad-spectrum antibiotics (effective against both gram-positive and gram-negative organisms) that destroy or inhibit the growth of these GI microflora. Consequently, the administration of one of these drugs to a patient on digoxin therapy may lead to an increase in the absorption of digoxin and, ultimately, to toxicity. Another example of this phenomenon occurs with oral contraceptives. Gut bacteria are necessary to hydrolyze estrogen conjugates into free estrogens so they may be optimally absorbed to exert their contraceptive effect. Concurrent antibiotic administration may alter these intestinal bacteria, impairing this process and preventing the optimal absorption and effectiveness of the oral contraceptive. A patient who takes oral birth control to prevent pregnancy should use a barrier form of protection during sex when she takes a course of antibiotics.
Distribution
A drug’s distribution to tissues can be affected by its binding to plasma/serum protein. Only drugs unbound to protein are free active agents and can enter body tissues. Two drugs that are highly protein bound and administered simultaneously can result in drug displacements. Factors that influence displacement of drugs are (1) drug concentration in the blood, (2) protein-binding power of the drugs, and (3) volume of distribution (Vd).
Two drugs that are highly bound to protein or albumin will compete for binding sites in the plasma. The result is a decrease in protein binding of one or both drugs; therefore, more free drug circulates in the plasma and is available for drug action. This effect can lead to drug toxicity. When two highly protein-bound drugs must be taken concurrently, the drug dosage of one or both drugs may need to be decreased to avoid drug toxicity.
Drugs that are highly protein bound include the anticoagulant warfarin, anticonvulsants such as phenytoin and valproic acid, gemfibrozil (antihyperlipidemic), most nonsteroidal antiinflammatory drugs (NSAIDs), sulfisoxazole (sulfonamide), glyburide (antidiabetic), and quinidine (antidysrhythmic). Warfarin (Coumadin) is 99% protein-bound, allowing only 1% to be free drug. If 2% to 3% of warfarin is displaced from albumin binding sites, the amount of free warfarin would be 3% to 4% instead of 1%. This action has the potential to increase the anticoagulant effect of warfarin; excess bleeding may result.
A significant decrease in serum albumin level because of liver disease or poor nutritional status can increase the free amount of highly protein-bound drugs such as phenytoin and warfarin, making more drug available to exert its pharmacologic effect.
Metabolism or Biotransformation
Many drug interactions of metabolism occur with the induction or inhibition of the hepatic microsomal system. A drug can increase the metabolism of another drug by stimulating liver enzymes. These enzymes produce a cascade effect in drug function. Drugs that promote induction of enzymes are called enzyme inducers. Barbiturates like phenobarbital are enzyme inducers. Phenobarbital increases the metabolism of most antipsychotics and methylxanthine (theophylline). Increased metabolism promotes drug elimination and decreases plasma concentration of the drug. The result is a decrease in drug action. Sometimes liver enzymes convert drugs to active or passive metabolites. The drug metabolites may be excreted or may produce an active pharmacologic response.
The anticonvulsant drugs phenytoin and carbamazepine and the antimicrobial medication rifampin are hepatic enzyme inducers that can increase drug metabolism—for example, for the anticoagulant drug warfarin. A larger dose of warfarin is usually needed while the patient takes a hepatic inducer, because metabolism aids in decreasing the amount of drug. If the drug inducer is withdrawn, warfarin dosages must be decreased, because less drug is eliminated by hepatic metabolism. Usually interaction occurs after 1 week of drug therapy and can continue for 1 week after the drug inducer is discontinued. Drugs with narrow therapeutic ranges should be closely monitored.
Some drugs are enzyme inhibitors. The antiulcer drug cimetidine (Tagamet) is an enzyme inhibitor that decreases metabolism of certain drugs such as theophylline, causing an increase in the plasma concentration of theophylline. The theophylline dose must be decreased to avoid toxicity. If cimetidine or any enzyme drug inhibitor is discontinued, the theophylline dosage should be adjusted. Other well-known hepatic enzyme inhibitors are erythromycin (an antibacterial) and itraconazole (an antifungal).
The use of tobacco and alcohol may have variable effects on drug biotransformation. Polycyclic aromatic hydrocarbons found in cigarette smoke induce production of the specific family of enzymes responsible for theophylline metabolism. Chronic cigarette smoking leads to an increase in hepatic enzyme activity and can increase theophylline clearance. Asthmatics who smoke and take theophylline to manage their disease may require an increase in theophylline dosage. The ingestion of alcohol may have variable effects on biotransformation. With chronic alcohol use, hepatic enzyme activities are increased; with acute alcohol use, metabolism is inhibited.
Natural or herbal products may also have an impact on metabolism. St. John’s wort, an OTC herbal product used to manage symptoms of depression, induces the metabolism of certain drugs such as warfarin, digoxin, and theophylline. This action potentially decreases the effectiveness of these medications, possibly necessitating a dose increase to sustain efficacy. Flavonoids, a group of naturally occurring compounds found in the juice and pulp of citrus fruits, are potent inhibitors of the metabolism of certain drugs. Patients who are stabilized on therapeutic doses of carbamazepine, diazepam, statins, calcium channel blockers, or erectile dysfunction drugs may subject themselves to adverse effects from greater-than-expected drug levels if they eat or drink grapefruit products concurrently with the drug.
Certain drugs alter hepatic blood flow, causing a decrease in liver metabolism. Table 4-1 describes the effects of drug enzyme inducers and inhibitors.
TABLE 4-1
DRUGS: ENZYME INDUCERS AND ENZYME INHIBITORS
| DRUG CATEGORY | DRUG EFFECT |
| Drug enzyme inducer | Onset and termination of drug effect is slow, approximately 1 week. |
| Drug dosage may need to be increased with use of drug inducer. | |
| Drug dosage should be adjusted after termination of drug inducer. | |
| Monitor serum drug levels, especially if the drug has a narrow therapeutic drug range. | |
| Drug enzyme inhibitor | Onset of drug effect usually occurs rapidly. |
Half-life (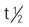 ) of the second drug may be increased, causing a prolonged drug effect. ) of the second drug may be increased, causing a prolonged drug effect. | |
| Interaction may occur related to the dosage prescribed. | |
| Disease entities affect drug dosing. | |
| Monitor serum drug levels, especially if the drug has a narrow therapeutic range. |

Excretion
Most drugs are filtered through the glomeruli and excreted in the urine. With some drugs, excretion occurs in the bile, which passes into the intestinal tract. Drugs can increase or decrease renal excretion and have an effect on the excretion of other drugs. Drugs that decrease cardiac output, decrease blood flow to the kidneys, and decrease glomerular filtration rate can also decrease or delay drug excretion. The antiarrhythmic drug quinidine decreases the excretion of digoxin; therefore, the plasma concentration of digoxin is increased, and digitalis toxicity can occur.
Diuretics promote water and sodium excretion from the renal tubules. Furosemide (Lasix) acts on the loop of Henle, and hydrochlorothiazide (HydroDIURIL) acts on the distal tubules. Both diuretics decrease reabsorption of water, sodium, and potassium. A renal loss of potassium, which may lead to a condition known as hypokalemia, can enhance the action of digoxin, and digitalis toxicity could occur (see “Drug-Laboratory Interactions” later in the chapter).
Two or more drugs that undergo the same route of excretion may compete with one another for elimination from the body. Probenecid, a drug for gout, decreases penicillin excretion by inhibiting the secretion of penicillin in the renal tubules of the kidneys. In some cases, this effect may be desirable to increase or maintain the plasma concentration of penicillin (which has a short half-life) for a prolonged period of time.
Changing urine pH affects drug excretion. The antacid sodium bicarbonate causes the urine to be alkaline. Alkaline urine promotes the excretion of drugs that are weak acids (e.g., aspirin, barbiturates). Alkaline urine also promotes reabsorption of weak base drugs. Acid urine promotes the excretion of weak base drugs such as quinidine.
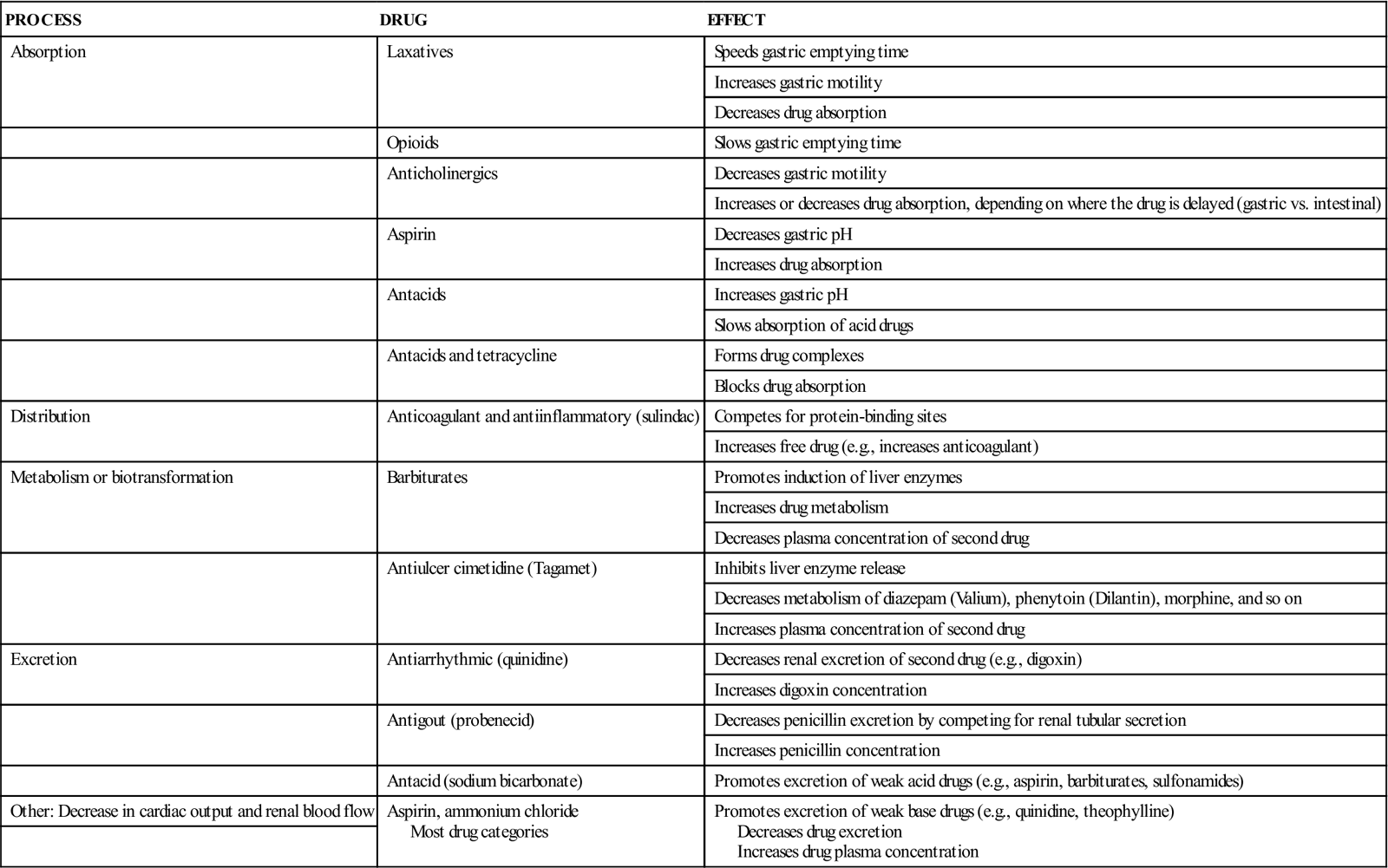
With decreased renal or hepatic function, there is usually an increase in free drug concentration. It is essential to closely monitor these patients for drug toxicity when they take multiple drugs. Checking serum drug levels, a practice also known as therapeutic drug monitoring (TDM), is especially important for drugs that have a narrow therapeutic range and are highly protein bound. Digoxin and phenytoin are two drugs that require TDM. Table 4-2 summarizes the drug interactions that affect pharmacokinetics.
TABLE 4-2
PHARMACOKINETIC INTERACTIONS OF DRUGS
| PROCESS | DRUG | EFFECT |
| Absorption | Laxatives | Speeds gastric emptying time |
| Increases gastric motility | ||
| Decreases drug absorption | ||
| Opioids | Slows gastric emptying time | |
| Anticholinergics | Decreases gastric motility | |
| Increases or decreases drug absorption, depending on where the drug is delayed (gastric vs. intestinal) | ||
| Aspirin | Decreases gastric pH | |
| Increases drug absorption | ||
| Antacids | Increases gastric pH | |
| Slows absorption of acid drugs | ||
| Antacids and tetracycline | Forms drug complexes | |
| Blocks drug absorption | ||
| Distribution | Anticoagulant and antiinflammatory (sulindac) | Competes for protein-binding sites |
| Increases free drug (e.g., increases anticoagulant) | ||
| Metabolism or biotransformation | Barbiturates | Promotes induction of liver enzymes |
| Increases drug metabolism | ||
| Decreases plasma concentration of second drug | ||
| Antiulcer cimetidine (Tagamet) | Inhibits liver enzyme release | |
| Decreases metabolism of diazepam (Valium), phenytoin (Dilantin), morphine, and so on | ||
| Increases plasma concentration of second drug | ||
| Excretion | Antiarrhythmic (quinidine) | Decreases renal excretion of second drug (e.g., digoxin) |
| Increases digoxin concentration | ||
| Antigout (probenecid) | Decreases penicillin excretion by competing for renal tubular secretion | |
| Increases penicillin concentration | ||
| Antacid (sodium bicarbonate) | Promotes excretion of weak acid drugs (e.g., aspirin, barbiturates, sulfonamides) | |
| Other: Decrease in cardiac output and renal blood flow | Aspirin, ammonium chloride Most drug categories | Promotes excretion of weak base drugs (e.g., quinidine, theophylline) Decreases drug excretion Increases drug plasma concentration |
Pharmacodynamic Interactions
Pharmacodynamic interactions are those that result in additive, synergistic, or antagonistic drug effects.
Additive Drug Effect
When two drugs with similar action are administered, the drug interaction is called an additive effect and is the sum of the effects of the two drugs. Additive effects can be desirable or undesirable. For example, a desirable additive drug effect occurs when a diuretic and a beta blocker are administered for the treatment of hypertension. In combination, these two drugs use different mechanisms to have a more pronounced blood pressure–lowering effect. As another example, aspirin and codeine are two analgesics that work by different mechanisms but can be given together for increased pain relief.
An example of an undesirable additive effect is that from two vasodilators: hydralazine (Apresoline) prescribed for hypertension and nitroglycerin prescribed for angina. The result could be a severe hypotensive response. Another example is the interaction of aspirin and alcohol. Aspirin is directly irritating to the stomach, causes platelet dysfunction, and inhibits prostaglandin-mediated mucus production of the gastric mucosa, which protects the underlying tissues of the stomach. Alcohol disrupts the gastric mucosal barrier and suppresses platelet production. Both aspirin and alcohol can prolong bleeding time and, when taken together, may result in gastric bleeding.
Synergistic Drug Effect or Potentiation
When two or more drugs are given together, one drug can potentiate or have a synergistic effect on another. In other words, the clinical effect is substantially greater than the combined effect of the two. An example of this is the combination of meperidine (Demerol, synthetic opioid analgesic) and promethazine (Phenergan, antihistamine). One of the major side effects of promethazine is sedation. When used with meperidine in postsurgery patients, promethazine enhances or potentiates the drowsiness effect of meperidine. Less meperidine is required when it is combined with promethazine, which can be desirable. An example of an undesirable effect occurs when alcohol and a sedative-hypnotic drug, such as chlordiazepoxide (Librium) or diazepam (Valium), are combined. The resultant effect of this example is increased central nervous system (CNS) depression.
Some antibacterials (antibiotics) have an enzyme inhibitor added to the drug to potentiate the therapeutic effect. Examples are ampicillin with sulbactam (Unasyn) and amoxicillin with clavulanate (Augmentin), in which sulbactam and clavulanate potassium are bacterial enzyme inhibitors. Ampicillin and amoxicillin can be given without these inhibitors; however, the desired therapeutic effect may not occur because of the bacterial beta-lactamase, which inactivates the drugs and causes bacterial resistance. The combination of the antibiotic with either sulbactam or clavulanate inhibits bacterial enzyme activity and enhances the effect or broadens the spectrum of activity of the antibacterial agent. Not all bacteria secrete beta-lactamase enzyme; only those that do will inhibit these antibiotics and result in bacterial resistance. (Refer to Unit IX for information on antibacterial agents.)
Antagonistic Drug Effect
When two drugs that have opposite effects, or antagonistic effects, are administered together, each drug cancels the effect of the other. In other words, the actions of both drugs are nullified. An example of an antagonistic effect occurs when the adrenergic beta stimulant isoproterenol (Isuprel) and the adrenergic beta blocker propranolol (Inderal) are given together. Isoproterenol is a drug that is used in emergency situations to treat bradycardia, which is defined as a heart rate of less than 60 beats per minute. If given to a patient receiving a drug that decreases blood pressure and heart rate, the action of each drug is canceled. Neither drug delivers the expected therapeutic effect.
There are some situations in which the antagonistic effect is desirable. In morphine overdose, naloxone is given as an antagonist (antidote) to block the narcotic response. This is a beneficial drug interaction of an antagonist.
The use of two prescription drugs can have additive, synergistic, antagonistic, or no effect. This is especially true of warfarin; when taken with another drug, the effects of the second drug can increase, decrease, or have no effect on the anticoagulant. Table 4-3 lists the drugs that may be taken with an anticoagulant and the effects that the second drug has on the anticoagulant.
TABLE 4-3
DRUG INTERACTIONS WITH ANTICOAGULANTS AND PRESCRIPTION DRUGS
| PRESCRIPTION DRUGS | ANTICOAGULANT EFFECTS |
| Selected Antilipidemics | |
| Fibrate Group | Increased effect; may cause increased bleeding |
| Statin Group | |
| Lovastatin | Increased effect |
| Pravastatin | No known effects |
| Angiotensin-Converting Enzyme Inhibitors | No known effects |
| Aminoglycosides | No known effects |
| Aspirin | Strong effects; can cause bleeding |
| Antineoplastic Drugs | |
| Cytoxan, 5-fluorouracil, methotrexate, doxorubicin, vincristine | Increased effects; can cause bleeding |
| Cytoxan, mercaptopurine, mitotane | Decreased effects; Cytoxan may cause increased or decreased effects |
| Barbiturates | Reduced effect of anticoagulants |
| Benzodiazepines | No known effects |
| Beta Blockers | No known effects |
| Selected Cephalosporins | |
| Cefamandole | Increased effects; may cause bleeding |
| Nonsteroidal Antiinflammatory Drugs (NSAIDs) | |
| Ibuprofen | Normal doses; no effects |
| Diclofenac, ketoprofen, tolmetin | May increase effects |
| Quinolone Antibiotics | Usually no effects |
| Ciprofloxacin, norfloxacin, ofloxacin | Increased effects in isolated cases |
| Sulfonamides | |
| Bactrim-cotrimoxazole | Increased effects in 35% of persons |
| Tricyclic Antidepressants | No known effects |
| Vitamin K | Decreased effects of anticoagulants |
| Food | |
| Aspartame (artificial sweetener) | Increased effects |
| Green vegetables (spinach, broccoli, brussels sprouts) | Decreased effects; vegetables are rich in vitamin K |
| Alcohol | |
| Mild to moderate drinking | No effects unless heavy drinking |
| Heavy drinking | Increased effects if liver function is impaired |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


