Paul Kim, DPM, MS
Javier La Fontaine, DPM, MS
Lawrence A. Lavery, DPM, MPH
“We gratefully acknowledge the contributions of James B. McGuire, DPM, PT, and Steven R. Kravitz, DPM, for their work on the third edition of this chapter.”
Objectives
After completing this chapter, you’ll be able to:
- state the significance of foot ulcers in patients who have diabetes mellitus
- list strategies for preventing foot ulcers in patients with diabetes
- describe wound characteristics and assessment parameters for a patient with diabetes
- list options for reducing pressure for a patient with diabetes who has a foot ulcer
- discuss the therapy options for patients with a diabetic foot ulcer.
Diabetes: A Growing Problem
Diabetes mellitus is a chronically metabolic disease with increasing prevalence worldwide.1 The American Diabetes Association (ADA) defines diabetes as “a disease in which the body doesn’t produce or properly use insulin.” Diabetes mellitus is not only a major health crisis in the United States, with an increase in the incidence by 48% over the past 10 years, but also now a pandemic. However, of the 20.8 million Americans (7% of the population) who have diabetes, only 14.6 million are diagnosed, leaving more than one-third unaware they have the disease. Blacks, Hispanics, Native Americans, and Asian-Americans have the highest prevalence of diabetes mellitus.2
Foot problems are one of the most common complications of diabetes that lead to hospitalization.3,4 Admissions for foot complications account for 20% to 25% of all hospital days for patients with diabetes.4 Between 2% and 6% of patients with diabetes will develop a foot ulcer every year.5
Diabetes has become the most common underlying cause of lower-extremity amputation in the United States. Recent data from the Centers for Disease Control (CDC) show an annual number of 111,000 hospitalizations for the diabetic foot in 2003, thereby surpassing the number attributed to peripheral arterial disease (PAD).6 The annual rate of amputations in the United States has almost halved in the past decade, and most of this decrease has been in the above-ankle amputations.7 The risk of lower-extremity amputation in people with diabetes is 15 to 46 times higher than in nondiabetic patients.4 After the initial amputation, the risk of reamputation or amputation of the contralateral extremity is also high: 9% to 17% of patients will experience a second amputation within the same year.4,8 The incidence of lower-extremity amputation is 1.5 times higher in Hispanics and 2.1 times higher in African Americans compared with non-Hispanic Whites9 (Box 16-1).
Box 16-1 ADA Contact Information
The American Diabetes Association (ADA)2 offers much information for diabetic patients and their families as well as for healthcare professionals. General information about diabetes is available, along with advice on exercise, nutrition, and daily meal planning. To contact the ADA:
1701N. Beauregard Street
Alexandria, VA 22311
1-800-DIABETES
Etiology of and Risk Factors for Foot Ulcers
A number of local and systemic risk factors for foot ulceration and amputations should be considered in the prevention and treatment of the diabetic foot. Perhaps the strongest and easiest risk factor to identify is the presence of a previous ulceration or amputation, which indicates the potential for recurrence due to scar formation or biomechanical abnormalities. The underlying pathology usually is not reversible, and most disease processes affecting the diabetic foot will continue to worsen over time. Three primary pathways or mechanisms of injury have been identified in the development of foot ulcers. These include wounds that result from ill-fitting shoes (low-pressure injuries that are associated with prolonged or constant pressure), ulcers on weight-bearing areas (repetitive moderate pressure and shear forces on the sole), and penetrating injuries from puncture wounds or other traumatic events (high-pressure injuries with a single exposure of direct pressure).10
Peripheral Sensory Neuropathy
Diabetes affects sensory, motor, and autonomic nerve function. In patients with sensory neuropathy, pain—the primary natural warning system that alerts the body to take action and seek medical care—is defective. Sensory neuropathy contributes to an inability to perceive injury to the foot due to what is commonly referred to as loss of protective sensation (LOPS).11 LOPS represents a level of sensory loss where patients can injure themselves without recognizing the injury. Motor neuropathy contributes to wasting of the intrinsic muscles of the foot; muscle imbalance; structural foot deformity, such as claw toes and subluxated metatarsophalangeal joints; and limited joint mobility. Autonomic neuropathy causes shunting of blood12 and loss of sweat and oil gland function, which leads to dry, scaly skin that can easily develop cracks and fissures. The combined effect of these neuropathies results in a foot with structural deformity and biomechanical faults; dry, poorly hydrated integument; and an inability to respond to pain and repetitive injury.
Neuropathy is one of the most common risk factors for lower-extremity complications. It is unusual to see a patient with a foot ulcer who does not have sensory neuropathy.13 Several screening methods can be used to identify sensory neuropathy, including a systematic clinical examination, vibration perception threshold (VPT) testing with a VPT Meter, and pressure assessment with Semmes-Weinstein monofilaments14 (Fig. 16-1). Although these methods are noninvasive and have good sensitivity and specificity to identify patients with LOPS,15 Semmes-Weinstein monofilaments in particular may present several problems, which should be considered before using the device. Semmes-Weinstein monofilaments should be purchased from a vendor that sells calibrated instruments because considerable variability exists among different brands of monofilament.16 Booth and Young found that some brands of monofilaments buckled at 8 g of force rather than at the 10 g for which they were designated. In addition, the material properties of the monofilament wear out after repetitive testing. Young et al.17 found that after 500 cycles of testing (or the equivalent of testing 10 sites on each foot for 25 patients), there was an average reduction of 1.2 g of testing force. A worn-out monofilament may result in patients being diagnosed as having sensory neuropathy with LOPS when they are not at risk.
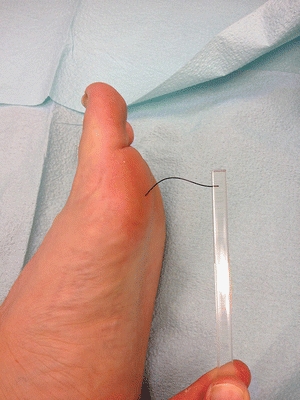
Figure 16-1. Semmes-Weinstein monofilament exam. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
 Practice Point
Practice Point
A Semmes-Weinstein 10-g (5.07 log) monofilament is commonly used to assess protective sensation in the feet of patients with diabetes. You can order the Semmes-Weinstein monofilament from the following companies:
- Center for Specialized Diabetic Foot Care: 1-800-543-9055
- North Coast Medical, Inc.: 408-283-1900
- Sensory Testing Systems: 1-888-289-9293
- Smith & Nephew Rehabilitation Division: 1-800-558-8633.
Use the 10-g (5.07 log) monofilament wire on each foot at the following 10 sites:
- plantar aspect of the first, third, and fifth digits
- plantar aspect of the first, third, and fifth metatarsal heads
- plantar midfoot medially and laterally
- plantar heel
- dorsal aspect of the midfoot.
Performing the Test
Place the patient in a supine or sitting position. Remove his socks and shoes and provide support for his legs. Touch the monofilament to the patient’s arm or hand to demonstrate what it feels like. Then ask him to respond “yes” each time he feels the monofilament on his foot (Fig. 16-2).
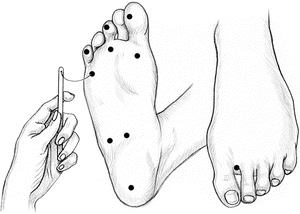
Figure 16-2 Assessing Protective Sensation with a Monofilament.
Place the patient’s foot in a neutral position with his toes pointing straight up, and tell him to close his eyes. Remind him to say “yes” when he feels the monofilament on his foot. Hold the monofilament perpendicular to the patient’s foot and press it against the first site, increasing the pressure until the monofilament wire bends into a C shape. Make sure it doesn’t slide over the skin. Hold the monofilament in place for about 1 second. Record the patient’s response on a foot-screening form. Use a “φφ” for a positive response and a “–” for a negative response. Then move to the next site.
Test all 10 sites at random and vary the time between applications so that the patient won’t be able to guess the correct response. If he has a scar, callus, or necrotic tissue at a test site, apply the monofilament along the perimeter of the abnormality, not directly on it.
Loss of protective sensation is indicated if the patient can’t feel the monofilament at any site on his foot. It is essential to teach a patient who has lost protective sensation to inspect and protect his feet.
Adapted from Sloan, H.L., Abel, R.J. “Getting in Touch with Impaired Foot Sensitivity,” Nursing 28(11):50–51, November 1998; and Armstrong, D.G., et al. “Choosing a Practical Screening Instrument to Identify Patients at Risk for Diabetic Foot Ulceration,” Archives of Internal Medicine 158(3):289–92, February 9, 1998, with permission.
 Practice Point
Practice Point
Not all monofilaments are of the same quality or last forever; be sure to use a calibrated instrument in your patient assessments.
A systematic clinical examination can be an effective way to diagnose neuropathy and identify high-risk patients. Abbott et al. used a modification of the neuropathy disability score to evaluate a large cohort of patients (n = 9,710) with diabetes.18 The neuropathy disability score evaluates vibration with a 128-Hz tuning fork, pinprick, hot–cold perception, and Achilles deep tendon reflex (Fig. 16-3). Abbott showed that high neuropathy disability scores (>6) were associated with a higher ulcer incidence.
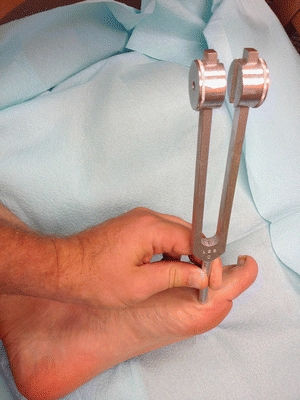
Figure 16-3. Tuning fork exam over bony prominence. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
VPT testing is a quantitative evaluation that measures large myelinated nerve function. It is less prone to interoperator variation than are monofilaments, and it does not need to be replaced to continue providing accurate results. The VPT Meter is a handheld device with a rubber head that is applied to a bony prominence, such as the medial aspect of the first metatarsal head or the tip of the great toe. The unit contains a linear scale that displays the applied voltage, ranging from 0 to 100 V. The amplitude is then slowly increased until the patient can feel the vibration. The inability to feel greater than 25 V is indicative of LOPS and puts the patient at risk for ulceration and amputation.
Peripheral Arterial Disease
Tissue perfusion is arguably the most important aspect of the diabetic limb. PAD in patients with diabetes is characterized by multiple occlusive plaques of small- and medium-sized arteries of the infrapopliteal vessels.19,20 Hence, diabetes induced PAD effects both small and large vessels. This becomes an important consideration for interpreting the results of arterial diagnostic tests as well as developing a treatment plan. PAD puts the patient with diabetes at a significantly greater risk for foot ulcers, infections, and amputations.10 Several theories attempt to explain the microvascular changes that occur in diabetes. One theory proposes that increased microvascular pressure and flow results in direct injury to the vascular endothelium, which in turn causes the release of extravascular matrix proteins. This leads to microvascular sclerosis and thickening of the capillary basement membrane. Capillary fragility also leads to microhemorrhage, which could be the reason that infection spreads through the tissue planes in patients with diabetes.20 In addition to the direct effect on the vessels, an additional indirect effect on the microvasculature is mediated by the autonomic nervous system. LoGerfo et al.21 believe that there is no microcirculatory occlusive process; rather, they suggest that some other indirect physiologic abnormality occurs. Altered microvascular blood flow is a complication of diabetic autonomic neuropathy that causes a shunting of blood away from the skin, making it prone to ulceration and impairing the healing process20 (Fig. 16-4).
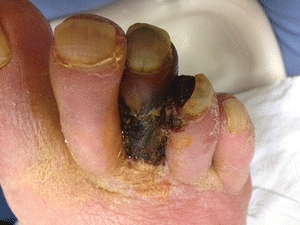
Figure 16-4. Focal necrosis of the third toe. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
It is likely that any theory of microvascular involvement in the process of diabetic ulceration and healing must include both the direct effects of glycosylation and local inflammation and the indirect effect of alteration of microvascular hemodynamics associated with autonomic dysfunction. Regardless of the underlying mechanism, the result is a decrease in perfusion to the tissue thereby decreasing healing potential and placing the diabetic limb at risk.
Evaluating vascular status should include a thorough history of symptoms of intermittent claudication, ischemic rest pain, and peripheral vascular surgery; clinical signs of ischemia, such as skin temperature, dependent rubor, pallor, hair loss, and shiny skin; and a clinical assessment of lower-extremity pulses.22 According to the American College of Cardiology (ACC)/American Hospital Association (AHA) guidelines23 for the management of patients with PAD, physicians should screen their patients for a diagnosis of PAD by determining bilateral resting ankle–brachial indices (ABIs) (Fig. 16-5). In addition, a toe–brachial index should be used when the ABI is not reliable because of excessively high systolic pressures in the ankle. When the ABI is greater than 1.3, the arteries are considered to be noncompressible due to calcifications within the endothelial walls. Segmental pressure measurements are useful to localize the site of lower-extremity PAD when planning a vascular intervention. However, in the advanced disease state of diabetes and particularly end-stage renal disease, ABIs may have limited utility due to the lack of compressibility and may require vascular surgery consultation.
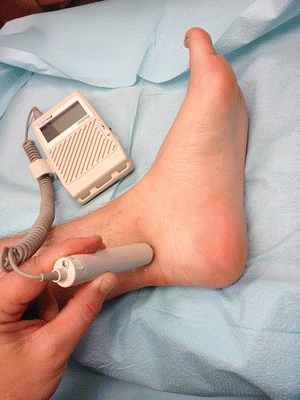
Figure 16-5. Handheld Doppler arterial exam of posterior tibial artery. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
Skin and Nail Examination
Evaluation of the skin and nails is critical to identify the subtle signs of impending injury, including high-pressure areas, cracks, maceration, blistering, or fissures in the skin. Patient education is an important aspect of care. All patients should be instructed on how to perform skin self-examinations as a preventive measure. Discoloration of a callus or bleeding under a callus is a sign of a preulcerative lesion. Likewise, deformed and thickened nails are commonly the source of abnormal pressure on the nail bed that can cause subungual ulcerations. Common nail disorders seen in patients with diabetes mellitus include onychomycosis (tinea unguium) and onychocryptosis (ingrown toenail). While these are usually minor problems in persons without diabetes, they can result in cellulitis, osteomyelitis, neuropathy, and vascular impairment in patients with diabetes.
Musculoskeletal Examination
In patients with neuropathy, ulcerations typically develop as a result of repetitive pressure and shear on the sole of the foot or from shoe pressure on the top or sides of the foot; however, no specific level of pressure has been determined to be abnormal or pathologic.24 Diabetes alters biomechanics in patients with preexisting structural and functional foot deformities (Fig. 16-6). Motor neuropathy is thought to contribute to atrophy and weakness of the intrinsic muscles of the foot. This leads to what has been called the “intrinsic minus foot,” which describes wasting of the small (intrinsic) muscles that originate in the foot (flexor digitorum brevis, flexor hallucis brevis, extensor digitorum brevis, extensor hallucis brevis, lumbricales, interossei, and abductor hallucis).
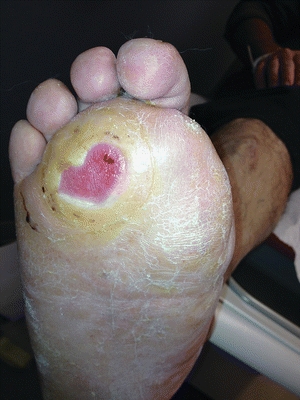
Figure 16-6. Ulcer under two to three metatarsophalangeal joints after great toe amputation. The two to three metatarsophalangeal joints are dislocated causing increased pressure on the sole of the foot. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
Metatarsal ulcers can develop when digital deformities are irritated by the toe box of a shoe or because of loss of fat pad and increased pressure under the metatarsal head (Figs. 16-7 and 16-8). The lesser digits contract and sublux dorsally, resulting in a claw toe deformity and a strong plantar flexor force at the metatarsophalangeal joints.25 As the toes deform and the metatarsophalangeal joints dislocate, the metatarsal heads are literally driven through the bottom of the foot. The tips and dorsal aspects of the toes and the area beneath the metatarsophalangeal heads are subjected to increased pressure and friction, which, in the presence of LOPS, can lead to ulceration.26 Limited mobility of the ankle and metatarsophalangeal joints has been associated with soft tissue glycosylation involving the gastroc-soleus-Achilles complex and periarticular tissues.27 Limited motion of the ankle, subtalar, and metatarsophalangeal joints has been associated with high pressures in the forefoot. Often patients with an intrinsic minus foot will appear to have a high arch; however, this is not a congenital deformity but rather is due to atrophy of the abductor hallucis muscle belly on the medial side of the foot. A profound example of musculoskeletal abnormality of the diabetic foot is represented by Charcot neuroarthropathy, which is characterized by fracture, subluxation, and/or dislocation of joints in the foot or ankle. These structural deformities typically cause plantar bony deformities resulting in areas of high pressure leading to ulceration.
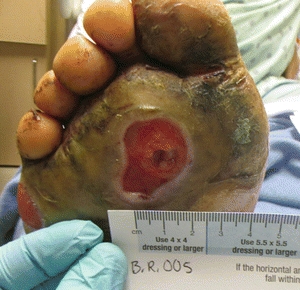
Figure 16-7. Metatarsal ulcer. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
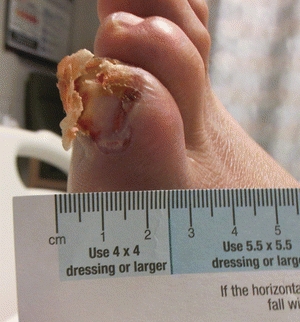
Figure 16-8. Ulcer on digital deformity. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
Infection
Soft tissue and bone infections are very common in persons with diabetic foot ulcerations. The majority of patients with diabetic foot ulcers (56%) will be treated for soft tissue infection during the course of their ulceration. Approximately 20% of these patients will develop infection of the underlying bone.28 Classically defined signs and symptoms of infection as outlined in the Infectious Disease Society of America (IDSA) guidelines are certainly limb threatening.29
Identification of foot infections in patients with diabetes requires vigilance because the normal signs of infection may be blunted or absent. Hyperglycemia impairs the humoral innate immune response by increasing the proinflammatory cytokine levels, dysregulating vasoactive cytokines, such as bradykinin and nitric oxide (NO), and decreasing complement activation. This, in turn, can lead to increased insulin resistance through several pathways, causing more hyperglycemia.30,31 The polymorphonuclear cells (PMNs) and monocytes of the cellular innate immune system show impaired chemotaxis, adherence, phagocytosis, and intracellular killing in patients with diabetes.32,33 The influence of hyperglycemia on the adaptive immune system (i.e., T cells and immunoglobulins) is less well understood.
The lower-extremity wounds are often colonized with microorganisms regardless of the presence of a “true infection.” Routine cultures of the wounds and superficial swabs should not be done when the wound is clinically not infected.34 When cultures are obtained, deep tissue should be obtained rather than superficial swabs. In cases of mild or moderate infection, curettages from the ulcer base after debridement should be obtained prior to the initiation of antibiotics.35 In severe infections, empiric antibiotic therapy should be started as soon as possible. Bone biopsy, preferably under radiographic guidance, is the most reliable method to obtain bone cultures in patients suspected of having osteomyelitis.36 (See Chapter 7, Bioburden and Infection.) Wound depth is the strongest predictor of both soft tissue and bone infections. Compared with superficial wounds, the risk of infection for wounds that extend to the bone is 23 times higher for soft tissue and 6.7 times higher for bone (Table 16-1).
Table 16-1 Risk Factors for Soft Tissue and Bone Infections
Adapted from Lavery, L.A., Armstrong, D.G., Wunderlich, R.P., et al. “Risk Factors for Foot Infections in Individuals with Diabetes,” Diabetes Care 29(6):1288, June 2006; and Lavery, L.A., Peters, E.J., Armstrong, D.G., et al. “Risk Factors for Developing Osteomyelitis in Patients with Diabetic Foot Wounds,” Diabetes Research in Clinical Practice 83(3):347–52, March 2009.
Biofilm is a significant growing concern that is less understood, its impact underappreciated, and not effectively treated. Biofilm is a colony of bacteria, fungus, or yeast that can populate a wound within 10 to 24 hours.37,38 Once established, a complex and biodiverse community evolves protected by a glycocalyx shell.39,40 Thus, conservative debridement may not be able to reach these deeper layers.
Unlike planktonic (unattached/free floating) bacteria that are metabolically active and can be generally treated with antibiotic therapy, microbes within a biofilm are relatively senescent. Thus, the mechanism of action of antibiotics, which is to interfere with protein synthesis (disruption of cell wall, cell membrane synthesis) is largely ineffectual against biofilm. Although biofilm under ideal circumstances provides a steady-state ecology of checks and balances between the microbial species, the chronic wound may be dominated by one species that develops into wound chronicity and periods of limb-threatening acute infection. Further troubling is the fact that qualitative and standard quantitative culturing techniques selectively identify specific and limited number of bacteria, fungus, and yeast. Thus, the true pathogenic microbe may not be detected and subsequently not treated.41 Biofilm’s definitive influence on wound healing and infection has yet to be determined. However, it is important to acknowledge that infection and biofilm are different entities that require treatment strategies.
Diagnostic Imaging
Whenever there’s an open wound on the foot, it’s always wise to order an x-ray initially to check for the presence of osteomyelitis. If the wound has been open for several weeks or if bone can be palpated with a sterile probe, further investigation is warranted even if standard x-rays are negative.
Several studies showed that the magnetic resonance imaging (MRI) is the diagnostic modality of choice to detect the presence of osteomyelitis.42,43 However, MRI is not always available and requires a well-trained and experienced reader. If MRI is unavailable and plain x-ray reveals changes suggestive of osteomyelitis (cortical erosion, active periosteal reaction, mixed lucency, and sclerosis), a bone biopsy should be obtained prior to treatment with an antibiotic regimen or surgical resection. If the x-ray shows no evidence of osteomyelitis, the patient should be treated with an empiric antibiotic regimen, and radiographs of the foot should be repeated.
Multidisciplinary Framework for Prevention
Evaluation of risk factors and risk stratification is important to prioritize the patient’s treatment according to his or her individual needs.44 Many healthcare providers either never evaluate the feet or generally consider everyone with diabetes to be “at-risk” for foot problems. This usually leads to no preventive care, but it can also contribute to unnecessary services for low-risk patients. To help evaluate individual risk factors, a risk classification system endorsed by the International Working Group on the Diabetic Foot13 provides a validated scheme to stratify subjects based on their risk of ulceration and amputation. Key elements of the lower-extremity examination should help to risk-stratify subjects in order to identify the frequency and level of preventive care required (Table 16-2).
Table 16-2 Lavery-Peters Diabetic Foot Risk Classification
Adapted from Lavery, L.A., Peters, E.J., Williams, J.R., et al. “Reevaluating the Way We Classify the Diabetic Foot: Restructuring the Diabetic Foot Risk Classification System of the International Working Group on the Diabetic Foot,” Diabetes Care 31(1):154–6, January 2008.
Many strategies can be used to help prevent foot complications in diabetic patients. Several clinical studies have reported a 48% to 78%45 reduction in amputations and a 47% to 49% reduction in lower-extremity–related hospitalizations46 when high-risk diabetic patients are treated in specialty clinics. These clinics often include multiple specialties that focus on both prevention and care of acute complications in patients with diabetes. Further, consensus documents for prevention measures related to the “diabetic foot” have been developed by the American Orthopaedic Foot and Ankle Society,47 the American College of Foot and Ankle Surgery,48 the Registered Nurses’ Association of Ontario, and the International Working Group on the Diabetic Foot.
While multidisciplinary clinics is an important principle in the comprehensive care of these patients, the practice of multidisciplinary care falls short. In most cases, these multidisciplinary care clinics employ specialty providers that spend a few half days each week in clinic and work in their own specialty offices for the remainder of the week. Thus, continuity of care and continuous communication between specialists are limited. Ideally, all specialists are colocated within the same clinic/institution. However, this may not be practical in many situations. Thus, a new “spoke and hub” model of wound care may make more sense. This model utilizes community-based wound care centers to screen, assess, diagnose, and treat the majority of wounds while referring the most difficult cases to tertiary referral centers within a regional area where a more resource intensive approach can be utilized. Thus, a distinction between a “wound care center” (community based) and a “limb salvage center” (tertiary referral center) should be implemented. 49
Another evolution of wound care thought may include a period of intensive inpatient care. Currently, weekly or biweekly clinic-based treatment is the norm that may result in little demonstrable progress. An inpatient course that includes rigid glucose control, nutrition optimization, perfusion maximization, intravenous antibiotic therapy, and serial operating room wound debridement with a plan for definitive wound closure or coverage may be of benefit.
Further complicating the treatment of chronic wounds is the diversity of wound care providers. Wound care is a discipline that spans multiple disciplines. Thus, wound treatment is driven by the scope of training, licensing, and differing philosophical approaches. This is another reason for colocated wound care where the strengths of each discipline can be combined to a single, comprehensive treatment approach.
Foot Care
Regular foot evaluation is essential to identify new risk factors and prevent impending complications. Podiatric physicians provide for debridement of callus and nails as well as regular evaluation of shoes and insoles. These routine encounters offer an additional opportunity to reinforce key educational elements, such as the need to avoid going barefoot, hydrate the skin, and inspect the feet daily. Protective footwear and insoles can be prescribed for the patient and then evaluated and monitored for their effectiveness.
Protective Footwear and Pressure Redistribution
The primary role of therapeutic footwear is to protect the foot from repetitive injuries and eliminate the shoe as a source of pathology. Extra-depth shoes have a high toe box with enough depth throughout the shoe to accommodate a total contact molded insole or orthotic. These are often recommended for patients with structural foot deformities, such as claw toes or dislocated metatarsophalangeal joints. These types of shoes usually allow for up to a ⅜”-thick accommodative insole to fit without irritating the top or sides of the foot (Fig. 16-9).

Figure 16-9. Hemisection of diabetic extra-depth shoe with insole.
The combination of a correctly sized shoe and a protective insole can reduce pressure on the sole, top, and sides of the foot by as much as 20%.50,51
Several studies52,53 have shown a significant reduction in foot ulcers in patients who receive therapeutic shoes, compared to shoes, patients would normal select themselves. There is little clinical evidence to help us understand the effectiveness of the types of therapeutic shoes and insole that are commonly used to prevent foot complications. There are a variety of insole materials and material combinations as well as different accommodations that can be built into the insole. Likewise, the type of shoe and outer sole accommodations are numerous. Most of the decisions for protective shoes and insoles are left to technicians that have little working knowledge of the medical literature. When patients reulcerate, they do not return to the pedorthist or shoemaker for care, so these providers have no follow-up to determine if their approach is effective.
There are four randomized clinical trials and two prospective cohort studies that describe the benefit of various types of shoes and insoles for high-risk patients with diabetes.54 Most studies include patients with a previous foot ulcer and use a control group of patients with self-selected footwear. In some studies, this is because they cannot afford therapeutic shoes,55 they refuse recommended shoes,56 or their insurance does not pay for shoes and insoles.57 Reulceration is much higher among patients who do not use therapeutic shoes and insoles. About 60% of patients reulcerate with self-selected shoes. Among patients who receive therapeutic shoes and insoles, there is a two- to fourfold reduction in reulceration compared to patients that use shoes they have selected. Even with standard preventative care such as therapeutic shoes and insoles, education, and regular foot care, about 24% to 50% of patients will develop another foot ulcer within the next year.58
The four randomized clinical studies that evaluate therapeutic shoes and insoles provide a glimpse into the complexity of evaluating this intervention to prevent ulcers. Reiber et al. compared two insole constructs with off-the-shelf footwear compared to patient-selected shoes.59 Uccioli et al.60 compared custom-made shoes and insoles to patient-selected shoes. Lavery et al.53 randomized patients to receive a shear-reducing insole compared to a standard insole and off-the-shelf shoe, as well as education and regular foot care. Rizzo et al. 61assessed the impact of a structured follow-up program on the incidence of diabetic foot ulceration in high-risk diabetic patients.
Custom-molded shoes (Figs. 16-10 and 16-11) are individually made from a mold of the patient’s foot. Custom-molded shoes can be expensive and require several weeks or months to make. However, they are only necessary in a small percentage of high-risk patients with severe foot deformities that cannot be accommodated by off-the-shelf shoes. For most patients with less severe deformities, there are a number of more affordable athletic, comfort, and therapeutic shoes with multiple sizes and extra depth to accommodate a wide variety of foot deformities. Even with the appropriate shoes, recurrence of ulcers is a problem. In a study by Waaijman et al., 171 neuropathic diabetic patients with a recently healed plantar foot ulcer and custom-made footwear were followed for 18 months or until ulceration. A total of 71 patients had a recurrent ulcer62. Significant independent predictors were presence of minor lesions, day-to-day variation in stride count, and cumulative duration of past foot ulcers. They concluded that the presence of a minor lesion was clearly the strongest predictor, and the use of adequately off-loading footwear was a strong protector against ulcer recurrence from unrecognized repetitive trauma.62
Figure 16-10. Charcot deformity. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)

Figure 16-11. Custom-molded high top boot designed to fit a patient with Charcot deformity.
The Diabetic Shoebill
Patients with diabetes who are “at risk” for foot disease and who have Medicare Part B are eligible for Medicare’s Therapeutic Shoe Bill.63 In order to qualify, a patient must have diabetes and one or more of the following: previous amputation of part or all of either foot; a history of previous foot ulceration or preulcerative calluses; peripheral neuropathy with evidence of callus formation; foot deformity; or poor circulation.
The bill covers one of the following annually: one pair of off-the-shelf extra-depth shoes and three additional pairs of multidensity inserts or custom-molded orthoses; one pair of off-the-shelf extra-depth shoes, including a modification and two additional pairs of multidensity inserts; or one pair of custom-molded shoes and two additional pairs of multidensity inserts (Fig. 16-12).



Figure 16-12. Wide-toe shoe with insert. Shoes with a deep toe box and that are “extra depth” throughout the shoe are the mainstay of diabetic wound preventive care. The shoes typically come with laces, as shown here, or with Velcro closures. The combination of a correctly sized shoe and an accommodative insole can reduce pressure on the sole, top, and sides of the foot. The insert is customized to relieve pressure, according to the patient’s needs, and then placed inside the shoe. (Photographs courtesy of Royce Medical Co.)
Preventive Education
Education has long been assumed to be an essential component of any program designed to reduce the incidence of diabetic foot ulcers. Preventive education usually takes the form of an intensive introduction to the disease and includes practical steps to cope with the manifestations of diabetes over time. In a 2004 Cochrane Review64 of nine randomized, controlled trials to determine the effectiveness of educational programs in preventing diabetic foot ulceration, the authors concluded that there was only weak evidence to suggest that education reduces foot ulceration and amputations in high-risk patients. On the other hand, the studies reinforced the idea that increased knowledge of foot care had a positive effect on patient behavior in the short term. Continual education and reinforcement may be helpful, especially among high-risk patients, to improve patient outcomes over the long term.
To complicate matters, many patients with diabetes have severe limitations to classical education methods. A large proportion of patients both with and without foot ulcers lacked the visual acuity, manual dexterity, or joint flexibility to perform the self-examination necessary to care for their feet.4,11 Forty-nine percent of patients with ulcers could not position or see their feet, and 15% were legally blind in at least one eye. When patients are obese or have limited joint mobility or impaired vision, education and self-assessment skills should be directed to both the patient and his or her spouse or caregiver.4,11 Repetition and regular reinforcement should be practiced by every member of the healthcare team to help the patient and family maintain an understanding of the disease process and continue to practice protective behaviors to avoid some of the serious complications of diabetes.
Temperature Monitoring
At-home temperature monitoring (Fig. 16-13) is a new concept for high-risk patients to identify early warning signs of tissue injury before a foot ulcer actually develops. Because neuropathy inhibits the natural warning system, local inflammation and pain as a result of tissue trauma go unnoticed. Several studies have used temperature assessment as a surrogate to identify tissue injury in patients at risk for diabetic foot ulcers and pressure ulcers. Indeed, two randomized clinical trials demonstrated a 3- to 10-fold reduction in foot complications among high-risk patients using at-home temperature assessment as compared with standard prevention therapy.65,66
Figure 16-13. Plantar foot temperature exam. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
 Practice Point
Practice Point
At-home temperature monitoring can provide objective feedback to warn patients with neuropathy that their feet are injured before an ulcer develops.
Wound Characteristics and Assessment
Several classification systems can be used to classify diabetic ulcers. The University of Texas ulcer classification system (Table 16-3) is a validated system that includes a mechanism to document wound depth as well as the presence of infection and vascular impairment—two pivotal factors in predicting clinical outcomes. Using this system, the risk of amputation has been shown to be predictive of amputation as wounds increase in depth (grade 0 to III) and progress from no infection (class A), to infection (class B), to PAD (class C), and to infection and PAD (class D) (Fig. 16-14).
Table 16-3 University of Texas Diabetic Wound Classification System
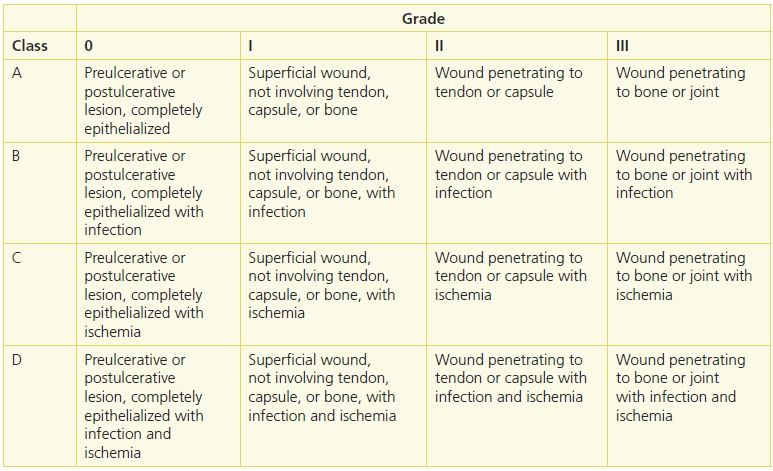
Reprinted from Armstrong, D.G., et al. “Validation of a Diabetic Wound Classification System: The Contribution of Depth, Infection, and Ischemia to Risk of Amputation,” Diabetes Care 21(5):855–69, May 1998, with permission.
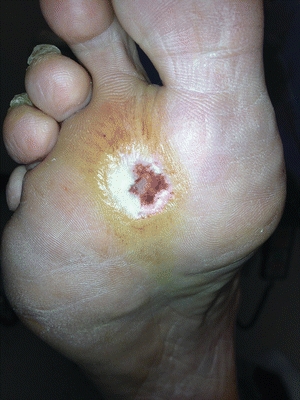
Figure 16-14. UT1A full-thickness ulcer without involvement of deep structures with no PAD or infection. (Photo courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
A classification scheme first described by Meggitt and popularized by Wagner has also been used extensively but has the disadvantage of not consistently including wound depth or the presence of infection (Table 16-4). Osteomyelitis is the only type of infection included, and end-stage disease events of gangrene are the only vascular parameters included. Furthermore, the system is difficult to use for more subtle disease processes that are critical for clinical decision making.
Table 16-4 Meggitt-Wagner Ulcer Classification
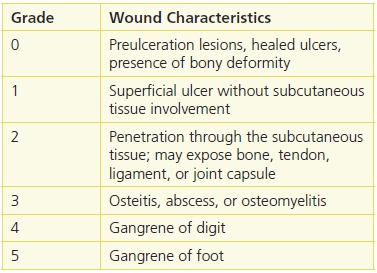
Reprinted from Wagner, F.W. “The Dysvascular Foot: A System for Diagnosis and Treatment,” Foot & Ankle 2(2):64–122, September 1981; and Meggitt, B. “Surgical Management of the Diabetic Foot,” British Journal of Hospital Medicine 16:227–32, 1976, with permission.
The ADA Consensus report2 recommends that a systematic wound assessment include the following questions in the evaluation:
- Has the patient experienced trauma? Is the ulcer a result of penetrating trauma, blunt trauma, or burn?
- What is the duration of the wound? Is the ulcer acute or chronic?
- What is the progression of local or systemic signs and symptoms? Is the wound getting better, is it stable, or is it deteriorating?
- Has the patient had any prior treatment of the wound or previous wounds? What treatments worked? What failed?
In addition, blood glucose control and comorbidities should be evaluated. Clinical assessment should identify:
- signs of ischemia—adequate blood flow to heal the wound
- signs of soft tissue or bone infection—cellulitis, abscess, or osteomyelitis
- wound depth—undermining or exposed tendon, joint capsule, or bone
- appearance—surrounding callus, devitalized tissue, granulation tissue, drainage, eschar, or necrosis.
 Practice Point
Practice Point
Six essentials of the ADA treatment algorithm
- debridement, early and often
- reducing pressure
- moist wound healing
- treating infection
- correcting ischemia (below the knee disease)
- preventing amputation.
Treatment Plan
Debridement (Surgical, Enzymatic, Maggot, Ultrasound)
Sharp debridement of the ulcer removes devitalized tissue, reduces the bacterial load of the wound, eliminates proteases from the wound bed, and provides a bleeding wound bed. A diabetic ulcer typically has a thick rim of keratinized tissue surrounding it. Debridement must remove all of the callus and devitalized tissue, so that a clean wound edge is created and all edge pressure from the callus is removed. Enzymatic or autolytic debridement may be an option if sharp debridement is not possible or if the patient has PAD.22 Ongoing debridement may be needed throughout the healing process.67 Indeed, higher healing rates have been observed in patients who have had more frequent debridement. In addition, in a post hoc evaluation from the becaplermin gel pivotal trial, Steed reported a higher proportion of healed wounds in both the treatment and placebo study groups when wound debridement was performed more frequently.68
It is important to acknowledge that not all forms of sharp debridement are the same. Sharp debridement performed in the clinic has significant limitations that may result in insufficient removal of devitalized tissue and pathogens (Fig. 16-15A and B). The inability to control significant bleeding, pain control, and the nonsterility of the environment in which it is performed limit aggressive debridement. Operating room debridement eliminates these barriers. An evolution in thought for the treatment of chronic wounds should include “excisional debridement.”69 This technique describes the excision of the entirety of the wound and its perimeter much like the approach to a soft tissue tumor. Further, in the operating room setting, after this type of debridement is performed, a definitive closure or graft can be placed.
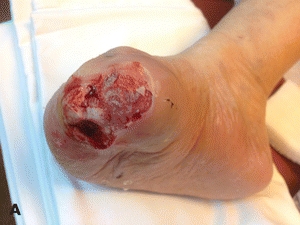
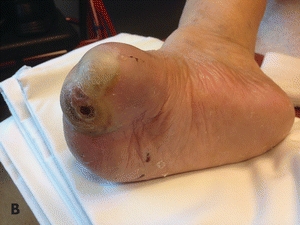
Figure 16-15. (A) Full-thickness ulcer with undermining predebridement. (B)Ulcer postdebridement (Note: increased in actual size). (Photos courtesy of S. van Asten and J. Lafontaine, MD. Used with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


