M. Linda Workman
Care of Patients with Noninfectious Lower Respiratory Problems
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
5 Use assessment information to identify people at increased genetic risk for a respiratory disease.
6 Encourage everyone to not smoke or to quit smoking.
9 Teach patients using any type of inhaler for drug delivery the correct way to use these devices.
11 Teach patients with asthma to have their reliever (rescue) inhalers with them at all times.
Psychosocial Integrity
Physiological Integrity
14 Compare the pathophysiology and clinical manifestations of asthma, bronchitis, and emphysema.
15 Identify risk factors for COPD and lung cancer.
17 Interpret peak expiratory flow (PEF) readings for the need for intervention.
18 Coordinate care for the patient immediately following lung volume reduction surgery.
20 Coordinate nursing interventions for the patient with chest tubes.

http://evolve.elsevier.com/Iggy/
Animation: Asthma
Answer Key for Decision-Making Challenges and NCLEX Examination Challenges
Audio Clip: High- and Low-Pitched Wheezes
Audio Clip: Pleural Friction Rub
Audio Glossary
Concept Map Creator
Concept Map: Respiratory Acidosis (COPD-Related)
Key Points
Review Questions for the NCLEX® Examination
Direct gas exchange occurs in the alveoli and the smallest airways. Any problem of these tissues reduces gas exchange and interferes with oxygenation. Many lower airway problems are chronic and progressive, requiring changes in a person’s lifestyle. The older patient with a lower airway problem may need special help before the disorder becomes severe because of age-related changes in breathing effectiveness. Chart 32-1 lists nursing issues for the older patient with a respiratory problem.
Chronic Airflow Limitation
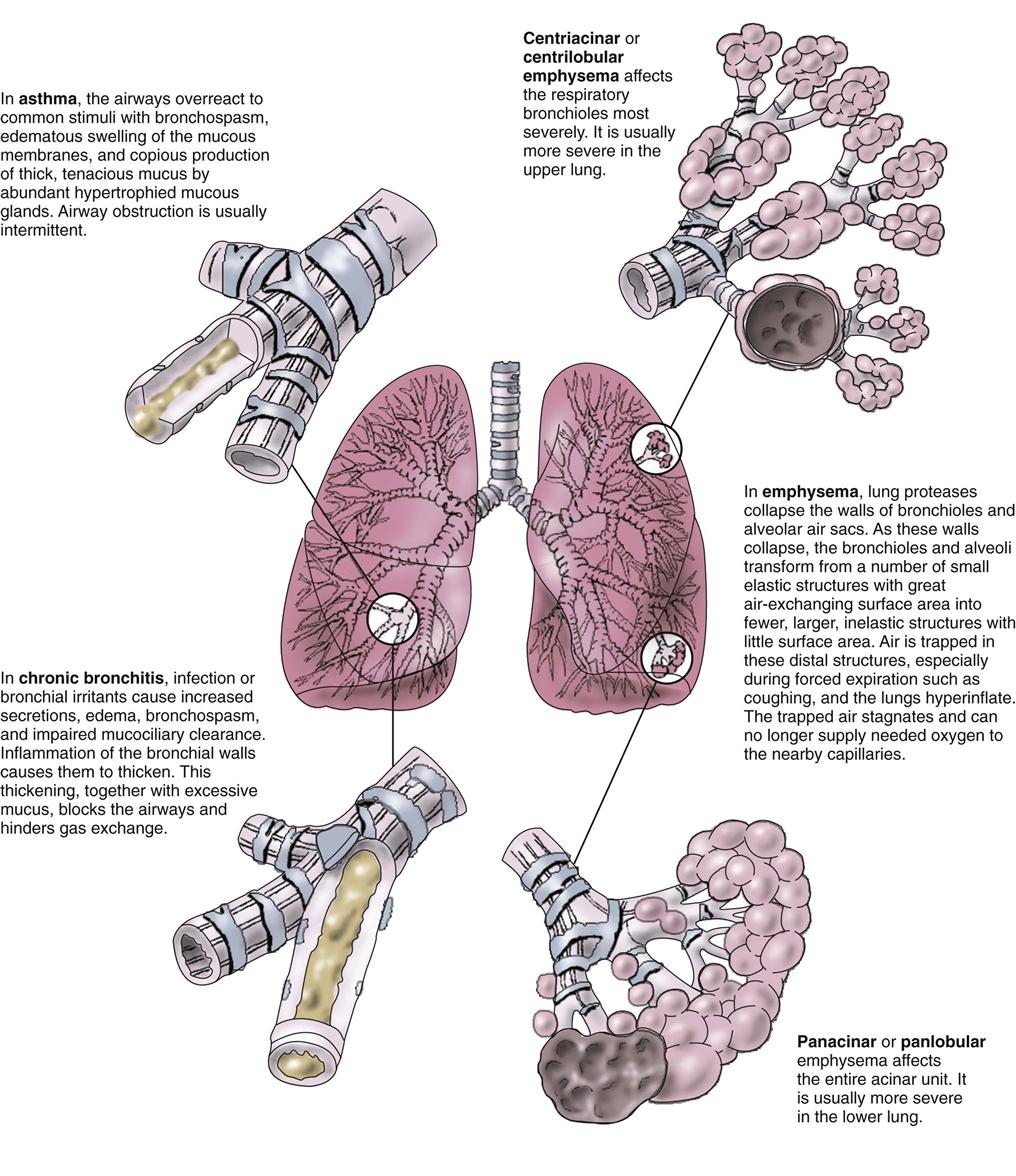
Chronic airflow limitation (CAL) is a group of chronic lung diseases that includes asthma, chronic bronchitis, and pulmonary emphysema. Emphysema and chronic bronchitis, termed chronic obstructive pulmonary disease (COPD), are characterized by bronchospasm and dyspnea (Fig. 32-1). The tissue damage is not reversible and increases in severity over time, eventually leading to respiratory failure. Asthma, unlike COPD, is a chronic disease with intermittent reversible airflow obstruction and wheezing.
More than 40 million Americans suffer from some form of CAL, and 1 million people between the ages of 40 and 65 years have moderate to severe disability from CAL (Centers for Disease Control and Prevention [CDC], 2010). Although some of the problems are not reversible, good management strategies can help maintain adequate oxygenation and improve overall health.
Asthma
Pathophysiology
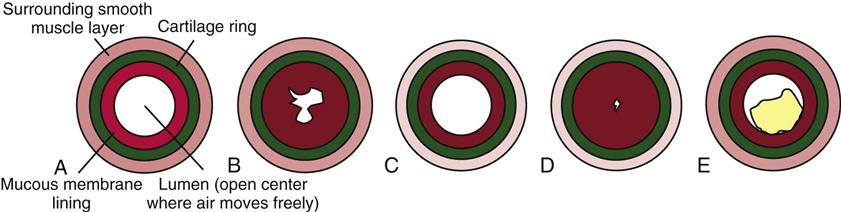
Asthma is usually a chronic condition in which reversible airflow obstruction in the airways occurs intermittently (see Fig. 32-1). Airway obstruction can occur in two ways: (1) inflammation and (2) airway hyperresponsiveness that leads to bronchoconstriction. Inflammation obstructs the lumen (i.e., the inside) of airways (Fig. 32-2). Airway hyperresponsiveness and constriction of bronchial smooth muscle cause a narrowing of the airway from the outside. Airway inflammation can trigger bronchiolar hyperresponsiveness, and many people with asthma have both problems at the same time. Severe airway obstruction can be fatal. More than 3400 deaths from acute asthma occur in the United States each year (CDC, 2010).
Etiology and Genetic Risk
Asthma is classified into different types based on how the attacks are triggered; however, the effect on oxygenation is similar for all types of asthma. Inflammation of the mucous membranes lining the airways is a key event in triggering an asthma attack. Inflammation occurs in response to the presence of specific allergens; general irritants such as cold air, dry air, or fine airborne particles; microorganisms; and aspirin and other NSAIDs. Hyperresponsiveness can occur with exercise, with an upper respiratory illness, and for unknown reasons.
When asthma is well controlled, the airway changes are temporary and reversible. With poor control, chronic inflammation can lead to damage and hyperplasia of the bronchial epithelial cells and of the bronchial smooth muscle (Global Initiative for Asthma [GINA], 2010). When asthma attacks are frequent, even exposure to low levels of the triggering agent or event may stimulate an attack.
Inflammation triggers asthma for some people when allergens bind to specific antibody molecules (especially immunoglobulin E [IgE]). These molecules are attached to tissue cells called mast cells and white blood cells (WBCs) called basophils. These cells are filled with granules containing chemical mediators that can start local inflammatory responses (see Chapters 19 and 22). Some mediators, such as histamine, start an immediate inflammatory response, which can be blocked by drugs like diphenhydramine (Benadryl). Others, such as leukotriene and eotaxin, are slower and cause later, prolonged inflammatory responses, which can be blocked by drugs like montelukast (Singulair), zafirlukast (Accolate), and zileuton (Zyflo). Mediators also attract more WBCs (eosinophils, macrophages, basophils) to the area, which then release even more inflammation-inducing mediators. Inflammation of airway mucous membranes causes blood vessel dilation and capillary leak, leading to tissue swelling with increased secretions and mucus production (McCance et al., 2010). Inflammation can also occur through general irritation rather than allergic responses.
Bronchospasm is a narrowing of the bronchial tubes through constriction of the smooth muscle around and within the bronchial walls. It can occur as a result of hyperresponsive airways when small amounts of pollutants or respiratory viruses stimulate nerve fibers, causing constriction of bronchial smooth muscle. If an inflammatory response is stimulated at the same time, the mediators released during inflammation also trigger constriction. Severe bronchospasm alone, especially in smaller bronchioles, can profoundly limit airflow to the alveoli.
Aspirin and other NSAIDs can trigger asthma in some people, although this response is not a true allergy. It results from increased production of leukotriene when aspirin or NSAIDs suppress other inflammatory pathways.
Gastroesophogeal reflux disease (GERD) is now thought to be a major trigger for asthma in some people, especially in those who have more asthma manifestations at night (GINA, 2010). It does not cause the airway hyperresponsiveness, but the highly acidic stomach contents appear to make the hyperresponsiveness worse when these materials enter the airway.
Incidence/Prevalence
Asthma can occur at any age. About half of adults with asthma also had the disease in childhood. Asthma affects nearly 19 million adults in the United States and Canada (CDC, 2010). It is more common in urban settings than in rural settings.
Patient-Centered Collaborative Care
Assessment
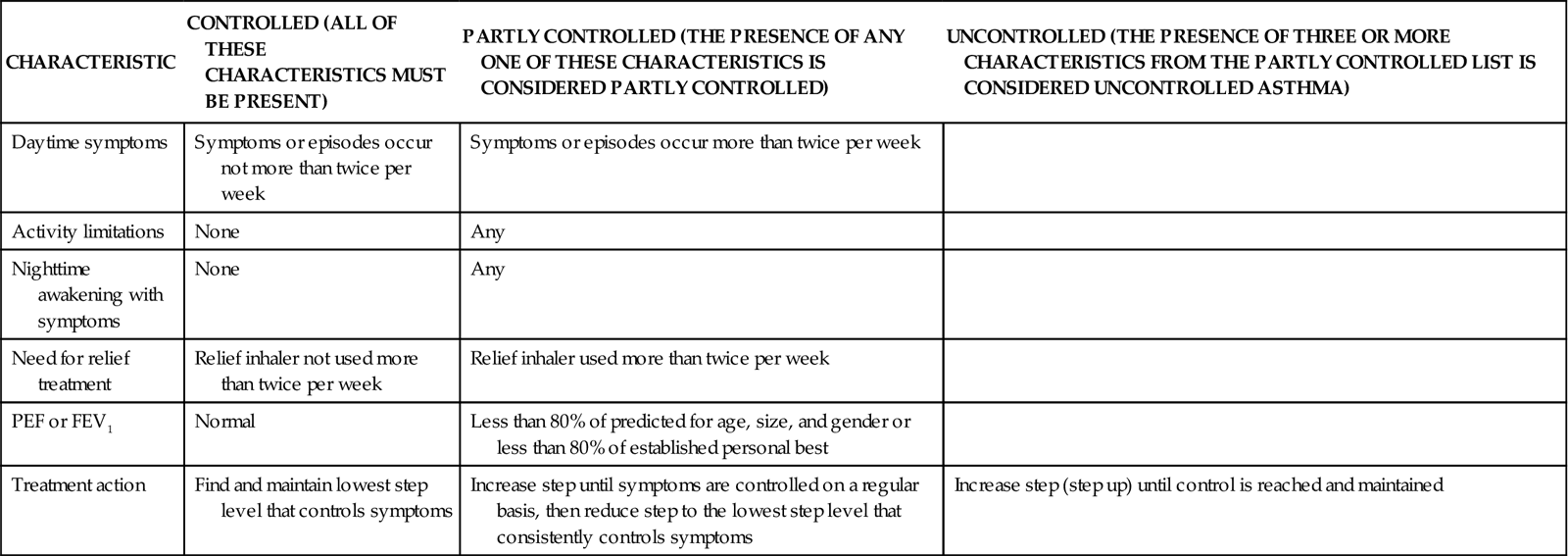
Asthma is classified on the basis of how well controlled the manifestations are, as well as on the patient’s response to asthma drugs. These classes are the basis for current asthma therapy (Charts 32-2 and 32-3).
History
The patient with asthma usually has a pattern of intermittent episodes of dyspnea (shortness of breath), chest tightness, coughing, wheezing, and increased mucus production. Ask whether the manifestations occur continuously, seasonally, in association with specific activities or exposures, or more frequently at night. Some patients have these manifestations for 4 to 8 weeks after a chest cold or other upper respiratory tract infection. The patient with atopic (allergic) asthma often has other allergic problems such as rhinitis, skin rash, or pruritus. Ask whether any family members have asthma or respiratory problems. Ask about current or previous smoking habits. If the patient smokes, use this opportunity to teach him or her about smoking cessation (Chart 32-4). Wheezing in nonsmokers is an important symptom in the diagnosis of asthma.
Physical Assessment/Clinical Manifestations
The patient with mild to moderate asthma may have no manifestations between asthma attacks. During an acute episode, the most common manifestations are an audible wheeze and increased respiratory rate. At first, the wheeze is louder on exhalation. When inflammation occurs with asthma, coughing may increase.
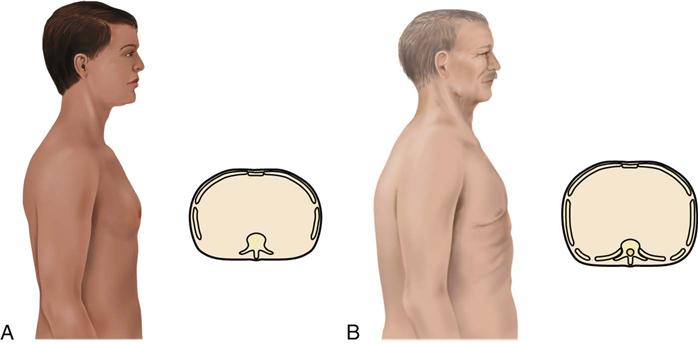
The patient may use accessory muscles to help breathe during an attack. Observe for muscle retraction at the sternum and the suprasternal notch and between the ribs. The patient with long-standing, severe asthma may have a “barrel chest,” caused by air trapping (Fig. 32-3). The anteroposterior (AP) diameter (diameter between the front and the back of the chest) increases with air trapping, giving the chest a rounded rather than an oval shape. The normal chest is about 1.5 times as wide as it is thick. In the patient with severe, chronic asthma, the AP diameter may equal or exceed the lateral diameter. Compare the AP diameter of the chest with the lateral diameter. Chronic air trapping also flattens the diaphragm and increases the space between the ribs.
Along with an audible wheeze, the breathing cycle is longer with prolonged exhalation and requires more effort. The patient may be unable to speak more than a few words between breaths. Hypoxia occurs with severe attacks. Pulse oximetry shows hypoxemia (poor blood oxygen levels). Examine the oral mucosa and nail beds for cyanosis. Other indicators of hypoxemia include changes in the level of cognition or consciousness and tachycardia.
Laboratory Assessment
Laboratory tests can help determine the type of asthma and the degree of breathing impairment. Arterial blood gas (ABG) levels show how well the patient is obtaining oxygen (see Chapter 14 for discussion of ABGs). The arterial oxygen level (PaO2) may decrease during an asthma attack. Early in the attack, the arterial carbon dioxide level (PaCO2) may be decreased as the patient increases respiratory effort. Later in an asthma episode, PaCO2 rises, indicating carbon dioxide retention. Allergic asthma often occurs with an elevated serum eosinophil count and immunoglobulin E levels. The sputum may contain eosinophils and mucus plugs with shed epithelial cells (Curschmann’s spirals).
Pulmonary Function Tests
The most accurate tests for asthma are the pulmonary function tests (PFTs) measured using spirometry. Baseline PFTs are obtained for all patients diagnosed with asthma. The most important PFTs for a patient with asthma are:
• Forced vital capacity (FVC) (volume of air exhaled from full inhalation to full exhalation)
• Peak expiratory flow (PEF) (fastest airflow rate reached at any time during exhalation)
A decrease in either the FEV1 or the PEF of 15% to 20% below the expected value for age, gender, and size is common for the patient with asthma. An increase of 12% in these values after treatment with bronchodilators is diagnostic for asthma. Airway responsiveness is tested by measuring the PEF and FEV1 before and after the patient inhales the drug methacholine, which induces bronchospasm in susceptible people.
Interventions
The purposes of asthma therapy are to control and prevent episodes, improve airflow, and relieve symptoms. Adult asthma is best managed when the patient is an active partner in the management plan. Priority nursing actions focus on patient education about proper implementation of a personal asthma action plan, which includes drug therapy and lifestyle management strategies to assist the patient in understanding his or her disease and its treatment (GINA, 2010).
Teaching for Self-Management
Asthma often has intermittent overt symptoms. With guided self-care, patients can co-manage this disease, increasing symptom-free periods and decreasing the number and severity of attacks (Ellis, 2008; Pruitt, 2011). Good management decreases the number of hospital admissions and increases participation in patient-chosen work and leisure activities. Self-care requires extensive education for the patient to be able to self-assess respiratory status, self-treat (including adjusting the frequency and dosage of prescribed drugs), and know when to consult the health care provider.
Ideally, a personal asthma action plan is developed by the health care provider and the patient. This plan is tailored to meet the patient’s personal triggers, asthma manifestation pattern, and drug responses. This plan includes the prescribed daily controller drug(s) schedule and prescribed reliever drug directions, patient-specific daily asthma control assessment questions, directions for adjusting the daily controller drug schedule, when to contact the health care provider (in addition to regularly scheduled visits), and what emergency actions to take when asthma manifestations are not responding to controller and reliever drugs.
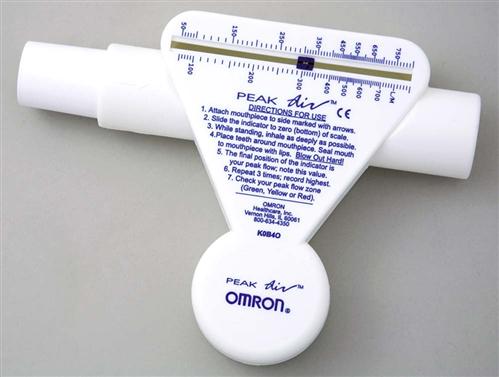
Teach the patient to assess symptom severity at least twice daily with a peak flowmeter (Fig. 32-4) and to adjust drugs according to his or her personal asthma action plan to manage inflammation and bronchospasms to prevent or relieve symptoms. Chart 32-5 describes the correct method to use the meter. Teach the patient to first establish a baseline or “personal best” peak expiratory flow (PEF) by measuring his or her PEF twice daily for 2 to 3 weeks when asthma is well controlled and recording the results. This way, the patient will know when his or her peak flow is reduced to the point that more drugs are needed or that emergency assistance is needed. When the patient has established a “personal best,” all other readings are compared with this value in terms of percent of personal best. Some meters are color-coded to help the patient interpret the results. Green zone readings are at least 80% or above the “personal best.” This is the ideal range for asthma control and indicates that no increases in drug therapy are needed. Yellow is a range between 50% and 80% of personal best. When a patient has a reading in this range, he or she needs to use the prescribed “reliever drug” (formerly called a rescue drug). Within a few minutes after using the reliever drug, another PEF reading should be made to determine whether the reliever drug is working. Frequent readings in the yellow zone or increasing use of reliever drugs is a warning that indicates the need to reassess the asthma plan for a temporary or permanent change in controller (preventive) drugs. Red is a range below 50% of the patient’s personal best and indicates serious respiratory obstruction.
Teach the patient to keep a symptom and intervention diary to learn specific triggers of asthma symptoms, early cues for impending attacks, and personal response to drugs. Stress the importance of proper use of his or her personal asthma action plan for any severity of asthma (Corbridge & Corbridge, 2010). Chart 32-6 lists areas to emphasize when teaching the patient with asthma.
Drug Therapy
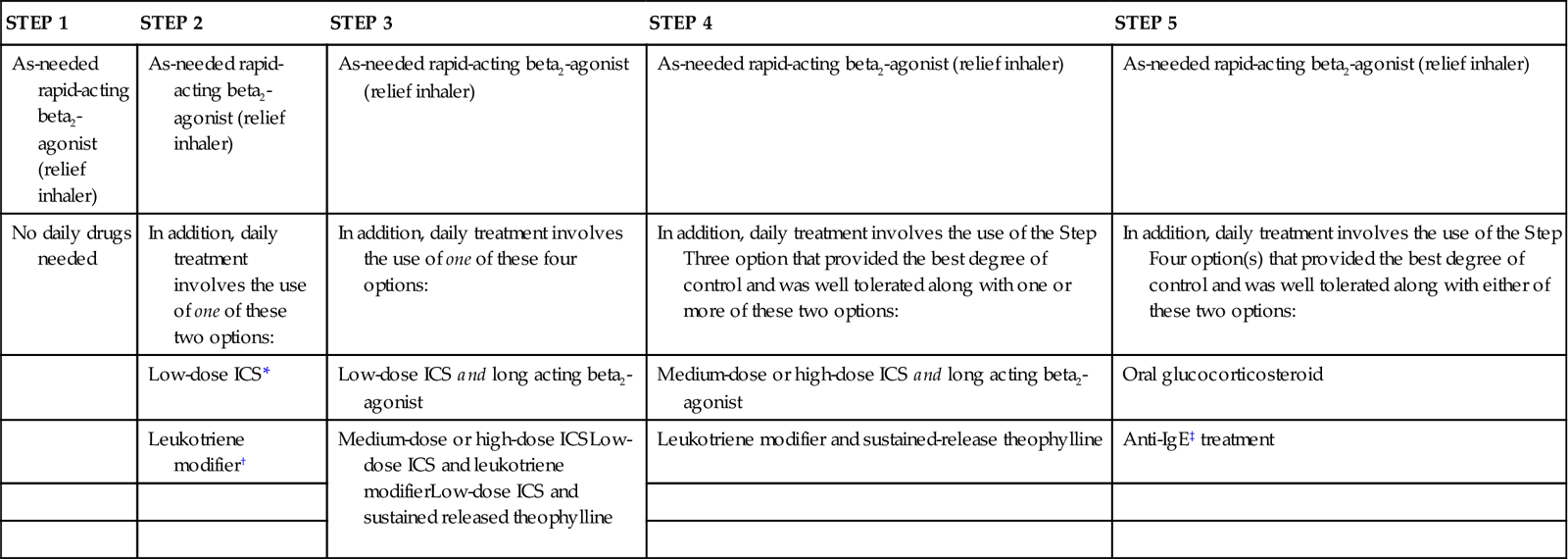
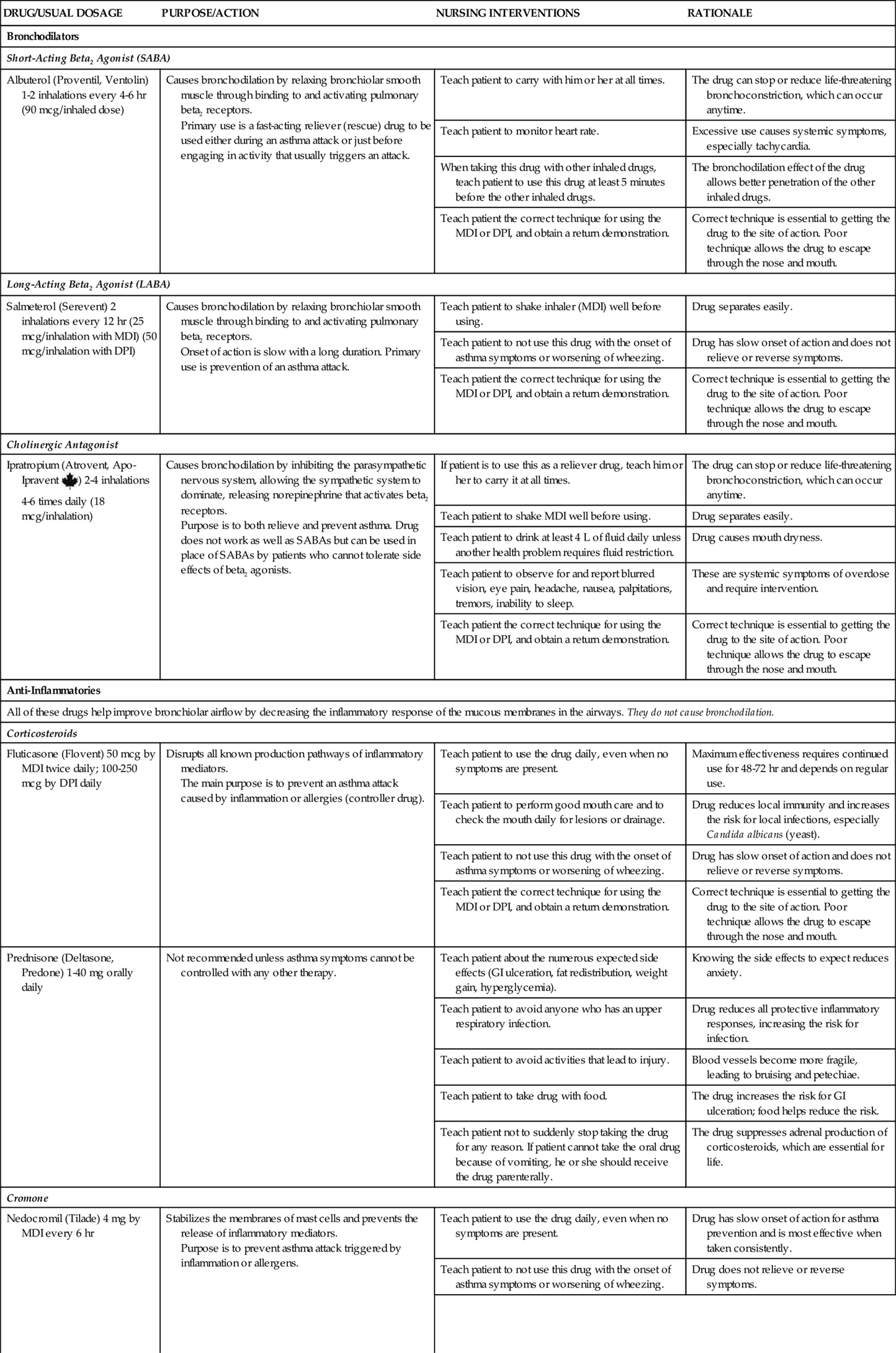
Pharmacologic management of adult patients with asthma is based on the step category for severity and treatment (see Charts 32-2 and 32-3) (GINA, 2010). Control therapy drugs (formerly called preventive drugs) are drugs used to reduce airway responsiveness to prevent asthma attacks from occurring. They are used every day, regardless of symptoms. Reliever drugs are those used to actually stop an attack once it has started. Some patients may need drug therapy only during an asthma episode. For others, daily drugs are needed to keep asthma episodic rather than a more frequent problem. This therapy involves the use of bronchodilators and various drug types to reduce inflammation. Some drugs reduce the asthma response, and other drugs actually prevent the response. Combination drugs are two agents from different classes combined together for better response. Chart 32-7 lists the most common preferred drugs in each class for control (prevention) and relief (rescue) therapy of asthma. The actions, interventions, and rationales for most drugs within a single class are similar although drug dosages may differ. Be sure to consult a pharmacology text or drug handbook for more information on a specific drug.
Bronchodilators.
Bronchodilators increase bronchiolar smooth muscle relaxation. They have no effect on inflammatory processes. Thus when a patient with asthma has airflow obstruction by both bronchospasm and inflammation, at least two types of drug therapy are needed. Some bronchodilators work by stimulating the beta2-adrenergic receptors on bronchial smooth muscle in much the same way that the sympathetic nervous system transmitters epinephrine and norepinephrine do; others work by blocking the parasympathetic nervous system. Bronchodilators include beta2 agonists and cholinergic antagonists.
Beta2 agonists bind to the beta2-adrenergic receptors and cause an increase in the intracellular level of a substance called cyclic adenosine monophosphate (cAMP). This substance sets pathways into motion that trigger smooth muscle relaxation.
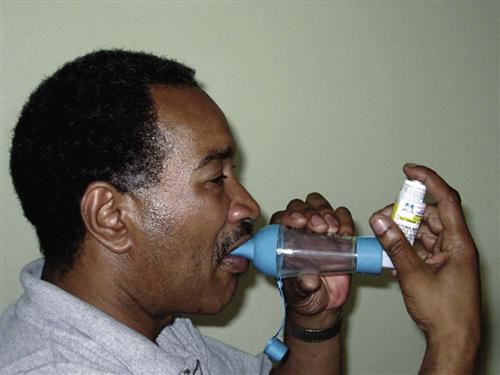
Short-acting beta2 agonists (SABAs) provide rapid but short-term relief. These inhaled drugs are most useful when an attack begins (as relief) or as premedication when the patient is about to begin an activity that is likely to induce an attack (GINA, 2010). Such agents include albuterol (Proventil, Ventolin), bitolterol (Tornalate), levalbuterol (Xopenex), pirbuterol (Maxair), and terbutaline (Brethaire). When inhaled from either a metered dose inhaler (MDI) or a dry powder inhaler (DPI), the drug is delivered directly to the site of action and systemic effects are minimal (unless the agent is overused). Teach the patient the correct technique for using an inhaled drug to achieve the greatest benefit. Chart 32-8 describes the proper way to use an MDI. Fig. 32-5 shows a patient using a “spacer” with an MDI. Spacer use increases the amount of drug delivered to the lungs. Chart 32-9 describes the proper care and use of a DPI. Fig. 32-6 shows a patient using a DPI.
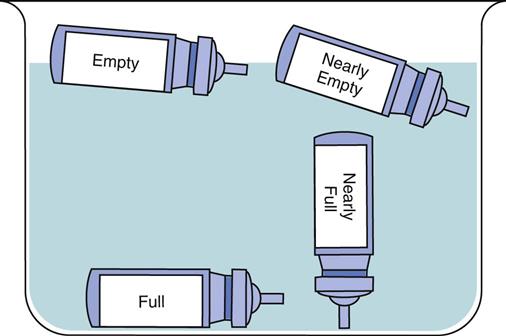
Dry powder inhalers indicate the amount of remaining drug; however, aerosol inhalers (MDIs) do not. Demonstrate how to check approximate aerosol inhaler drug levels by placing the inhaler in water (Fig. 32-7). Full inhalers sink to the bottom. An empty inhaler floats on its side. This technique is controversial because it is only a general approximation. The more recommended technique is for the patient to count the number of doses as they are used; however, many patients have difficulty keeping the dose count accurate.
Long-acting beta2 agonists (LABAs) are also delivered by inhaler directly to the site of action—the bronchioles. Proper use of the long-acting agonists can decrease the need to use reliever (rescue) drugs as often. Unlike short-acting agonists, long-acting drugs need time to build up an effect but the effects are longer lasting. Thus these drugs are useful in preventing an asthma attack but have no value during an acute attack. Therefore teach patients not to use LABAs alone to relieve (rescue) them during an attack or when wheezing is getting worse but, instead, to use a SABA. Relying on LABAs during an attack can lead to worsening of symptoms and death (Aschenbrenner, 2010). Examples of LABAs include formoterol (Foradil) and salmeterol (Serevent). These drugs are never prescribed as the only drug therapy for asthma. Teach the patient to use these control drugs daily as prescribed, even when no symptoms are present.
Cholinergic antagonists, also called anticholinergic drugs, are similar to atropine and block the parasympathetic nervous system. This blockade allows the sympathetic nervous system to dominate, resulting in increased bronchodilation and decreased pulmonary secretions. The most common drug in this class is ipratropium (Atrovent), which is used as an inhalant. Most cholinergic antagonists are short acting and must be used several times a day, although long-acting agents such as tiotropium (Spiriva) are available for use once a day. These drugs are not as effective as beta2 agonists and are recommended as first-line asthma control therapy only for those patients who cannot tolerate the side effects of beta2 agonists.
Xanthines, such as theophylline and aminophylline, are used only when other types of management are ineffective. These drugs are given systemically, and the dosage that is effective is close to the dosage that produces many dangerous side effects. Blood levels must be monitored closely to ensure the drug level is within the therapeutic range.
Anti-Inflammatory Agents.
Anti-inflammatory agents decrease the inflammatory responses in the airways. Those given systemically have more side effects. Others are used as inhalants and have fewer systemic side effects.
Corticosteroids decrease inflammatory and immune responses in many ways, including by preventing the synthesis of mediators. Inhaled corticosteroids (ICSs) can be helpful in controlling (preventing) the manifestations of asthma. Newer high-potency steroid inhalers, such as fluticasone (Flovent), budesonide (Pulmicort), and mometasone (Asmanex), may be used once per day for maintenance. Systemic corticosteroids, because of severe side effects, are avoided for mild to moderate intermittent asthma and are used on a short-term basis for moderate asthma. For some patients with severe asthma, daily oral corticosteroids may be needed. Both inhaled corticosteroids and those taken orally are control drugs (preventive). They are not effective in reversing symptoms during an asthma attack and should not be used as reliever (rescue) drugs. Teach patients the difference between ICSs and relief drug inhalers. Control drugs must be used on a scheduled basis, even when asthma symptoms are not present.
Cromones, both those that are inhaled and those that are taken orally, are useful as preventive asthma therapy when taken on a scheduled basis. These agents have different mechanisms of action to reduce airway inflammation. Nedocromil (Tilade) inhibits the release of inflammatory mediators from respiratory cells and WBCs. Mast cell stabilizers, such as cromolyn sodium (Intal), prevent mast cell membranes from opening when an allergen binds to IgE. Thus these drugs help prevent atopic asthma attacks but are not useful during an acute episode. The inhaled cromones are not effective in reversing symptoms during an asthma attack and should not be used as reliever (rescue) drugs.
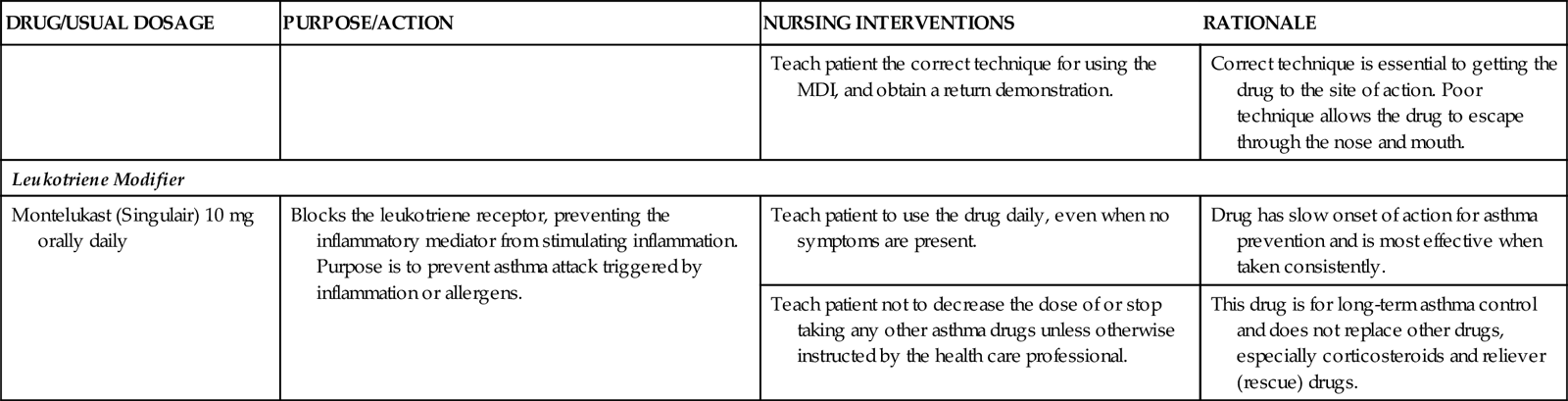
Leukotriene modifiers are oral drugs that work in several ways to prevent an asthma episode when taken on a scheduled basis. Montelukast (Singulair) and zafirlukast (Accolate) block the leukotriene receptor. Zileuton (Zyflo) prevents leukotriene synthesis. These drugs do not reverse symptoms during an asthma attack and should not be used as reliever (rescue) drugs.
Anti-IgE agents are monoclonal antibodies directed against part of the immunoglobulin E (IgE) structure. When these agents bind to IgE molecules that have allergens attached to them, the complex cannot bind to mast cells and basophils. This action prevents the IgE-allergen complex from triggering the release of mediators from mast cells and basophils. Thus these drugs help prevent atopic asthma attacks but are not useful during an acute episode. Omalizumab (Xolair) is currently the only drug in this class and is used only for people with asthma who have high blood levels of IgE. It is injected subcutaneously every 2 to 3 weeks.
Exercise/Activity
Regular exercise is a recommended part of asthma therapy (Holcomb, 2008). Aerobic exercise assists in maintaining cardiac health, enhancing skeletal muscle strength, and promoting ventilation and perfusion. Teach patients with asthma to examine the conditions that trigger an attack and adjust the exercise routine as needed. Some may need to premedicate with inhaled SABAs before beginning activity. For others, adjusting the environment may be needed (e.g., changing from outdoor ice-skating in cold, dry air to indoor ice-skating).
Oxygen Therapy
Supplemental oxygen is often used during an acute asthma attack. Oxygen is delivered by mask, nasal cannula, or endotracheal tube. High flow rates or concentrations may be needed when bronchospasms are severe and limit flow of oxygen through the bronchiole tubes. Heliox, a mixture of helium and oxygen (often 50% helium and 50% oxygen), can help improve oxygen delivery to the alveoli. This gas mixture is lower in density than oxygen alone or room air and flows even when airway resistance is high.
Status Asthmaticus
Status asthmaticus is a severe, life-threatening acute episode of airway obstruction that intensifies once it begins and often does not respond to usual therapy. The patient arrives in the emergency department with extremely labored breathing and wheezing. Use of accessory muscles for breathing and distention of neck veins are observed. If the condition is not reversed, the patient may develop pneumothorax and cardiac or respiratory arrest. IV fluids, potent systemic bronchodilators, steroids (to decrease inflammation), epinephrine, and oxygen are administered immediately to reverse the acute condition. Prepare for emergency intubation. Sudden absence of wheezing may indicate complete airway obstruction and require a tracheotomy. When breathing improves and wheezing decreases, management is similar to that for any patient with asthma.
Chronic Obstructive Pulmonary Disease
Pathophysiology
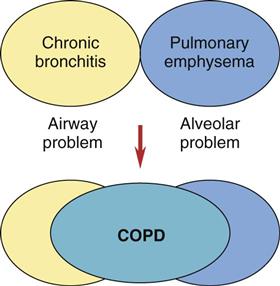
Chronic obstructive pulmonary diseases (COPD) include emphysema and chronic bronchitis. Although these are separate disorders with different pathologic processes, many patients with emphysema also have chronic bronchitis at the same time (Fig. 32-8).
Emphysema
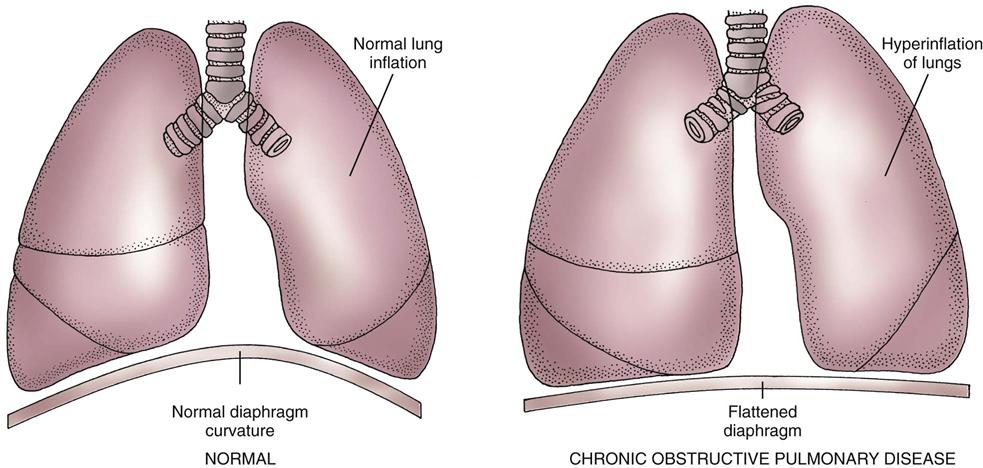
The two major changes that occur with emphysema are loss of lung elasticity and hyperinflation of the lung (see Fig. 32-1). These changes result in dyspnea and the need for an increased respiratory rate.
In the healthy lung, protein degrading enzymes called proteases are present to destroy and eliminate particulates and organisms inhaled during breathing. If these proteases are present in higher-than-normal levels, they damage the alveoli and the small airways by breaking down elastin. High protease levels cause the alveolar sacs to lose their elasticity and the small airways to collapse or narrow. Some alveoli are destroyed, and others become large and flabby, with decreased area for effective gas exchange.
An increased amount of air is trapped in the lungs. Causes of air trapping are loss of elastic recoil in the alveolar walls, overstretching and enlargement of the alveoli into air-filled spaces called bullae, and collapse of small airways (bronchioles). These changes greatly increase the work of breathing. The hyperinflated lung flattens the diaphragm (Fig. 32-9), weakening the effect of this muscle. As a result, the patient with emphysema needs to use additional muscles (accessory muscles) in the neck, chest wall, and abdomen to inhale and exhale. This increased effort increases the need for oxygen, making the patient work harder and have an “air hunger” sensation. Often, inhalation starts before exhalation is completed, resulting in an uncoordinated pattern of breathing.
Oxygenation is affected by the increased work of breathing and the loss of alveolar tissue. Although some alveoli enlarge, the curves of alveolar walls decrease and less surface area is available for gas exchange. Often the patient adjusts by increasing the respiratory rate, so arterial blood gas (ABG) values may not show gas exchange problems until the patient has advanced disease. Then carbon dioxide is produced faster than it can be eliminated, resulting in carbon dioxide retention and chronic respiratory acidosis (see Chapter 14). The patient with late-stage emphysema also has a low arterial oxygen (PaO2) level, because it is difficult for oxygen to move from diseased alveoli into the blood.
Emphysema is classified as panlobular, centrilobular, or paraseptal depending on the pattern of destruction and dilation of the gas-exchanging units (acini) (see Fig. 32-1). Each type can occur alone or in combination in the same lung. Most are associated with smoking or chronic exposure to other inhalation irritants.
Chronic Bronchitis
Bronchitis is an inflammation of the bronchi and bronchioles caused by exposure to irritants, especially cigarette smoke. The irritant triggers inflammation, vasodilation, mucosal edema, congestion, and bronchospasm. Bronchitis affects only the airways, not the alveoli.
Chronic inflammation increases the number and size of mucous glands, which produce large amounts of thick mucus. The bronchial walls thicken and impair airflow. This thickening, along with excessive mucus, blocks some of the smaller airways and narrows larger ones. Smaller airways are involved first. In addition, the increased mucus provides a breeding ground for microorganisms and leads to chronic low-grade infection.
Chronic bronchitis hinders airflow and gas exchange because of mucus plugs and infection narrowing the airways. As a result, the PaO2 decreases (hypoxemia) and the arterial blood carbon dioxide (PaCO2) level increases (respiratory acidosis).
Etiology and Genetic Risk
Cigarette smoking is the greatest risk factor for COPD. The patient with an 8–pack-year history usually has obstructive lung changes but no manifestations of disease. The patient with a 20–pack-year history or longer often has early-stage COPD with changes in pulmonary function tests (PFTs).
The harmful effects of tobacco occur because inhaled smoke triggers the release of excessive amounts of the proteases from cells in the lungs. These enzymes break down elastin, the major component of alveoli. By impairing the action of cilia, smoking also inhibits the cilia from clearing the bronchi of mucus, cellular debris, and fluid.
In addition to the increased risk for COPD from active smoking, passive smoking (or secondhand smoke), as well as smoke that clings to hair and clothing (sometimes called “thirdhand” smoke), contributes to upper and lower respiratory problems. The risk is greater when exposure occurs in small, confined spaces.
Alpha1-antitrypsin deficiency is a less common but important risk factor for COPD. A special enzyme, alpha1-antitrypsin (AAT), is normally present in the lungs. One purpose of AAT is to regulate the proteases that are present to break down inhaled pollutants and organisms. AAT, a protease inhibitor, prevents the proteases from working on lung structures.
The production of normal amounts of AAT depends on the inheritance of a pair of normal gene alleles for this protein. The AAT gene is recessive. Thus if one of the pair of alleles is faulty and the other allele is normal, the person makes enough AAT to prevent COPD unless there is significant exposure to cigarette smoke and other precipitating factors. This person, however, is a carrier for AAT deficiency. If both alleles are faulty, COPD develops at a fairly young age even when the person is not exposed to cigarette smoke or other irritants.
About 100,000 Americans have severe AAT deficiency, and many more have mild to moderate deficiencies (Nussbaum et al., 2007). Although an AAT deficiency also can cause problems in the skin and liver, lung diseases are more common.
Incidence/Prevalence
The prevalence of chronic bronchitis and emphysema in the United States has been estimated at about 13.5 million (for chronic bronchitis) and 2 million (for emphysema). Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of morbidity and mortality in the United States (Global Initiative for Chronic Obstructive Lung Disease [GOLD], 2009).
Complications
COPD affects oxygenation of all tissues. Complications can result in tissue anoxia and death. Major problems occur, such as hypoxemia, acidosis, respiratory infection, cardiac failure, and dysrhythmias.
Hypoxemia and acidosis occur because the patient with COPD has reduced gas exchange, leading to decreased oxygenation and increased carbon dioxide levels. These problems reduce cellular function.
Respiratory infection risk increases because of the increased mucus and poor oxygenation. Bacterial infections with Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are common. Acute lung infections make COPD manifestations worse by increasing inflammation and mucus production and inducing more bronchospasm. Airflow becomes even more limited, the work of breathing increases, and dyspnea results.
Cardiac failure, especially cor pulmonale (right-sided heart failure caused by pulmonary disease), occurs with bronchitis or emphysema. Air trapping, airway collapse, and stiff alveolar walls increase the lung tissue pressure, making blood flow through lung vessels more difficult. The increased pressure creates a heavy workload on the right side of the heart, which pumps blood into the lungs. As the disease progresses, the amount of oxygen in the blood decreases, causing major blood vessels in the lung to constrict. To pump blood through these narrowed vessels, the right side of the heart generates high pressures. In response to this heavy workload, the right chambers of the heart enlarge and thicken, causing right-sided heart failure with backup of blood into the general venous system. Chart 32-10 lists key features of cor pulmonale.
Cardiac dysrhythmias are common in patients with COPD. They result from hypoxemia (from decreased oxygen to the heart muscle), other cardiac disease, drug effects, or acidosis.
Health Promotion and Maintenance
The incidence and severity of COPD would be greatly reduced by smoking cessation. COPD is rare among people who have never smoked cigarettes. Disease progression can be slowed by smoking cessation. Urge all people who smoke to quit smoking. Chart 32-4 provides tips to teach people about smoking cessation.
Other measures to reduce the incidence of COPD are to avoid inhalation irritants in all environments. Teaching all people to use masks when working in areas with high levels of particulate matter can reduce individual exposure. Proper venting of workplaces and recreation areas that have airborne or particulate matter also reduces exposure.
Patient-Centered Collaborative Care
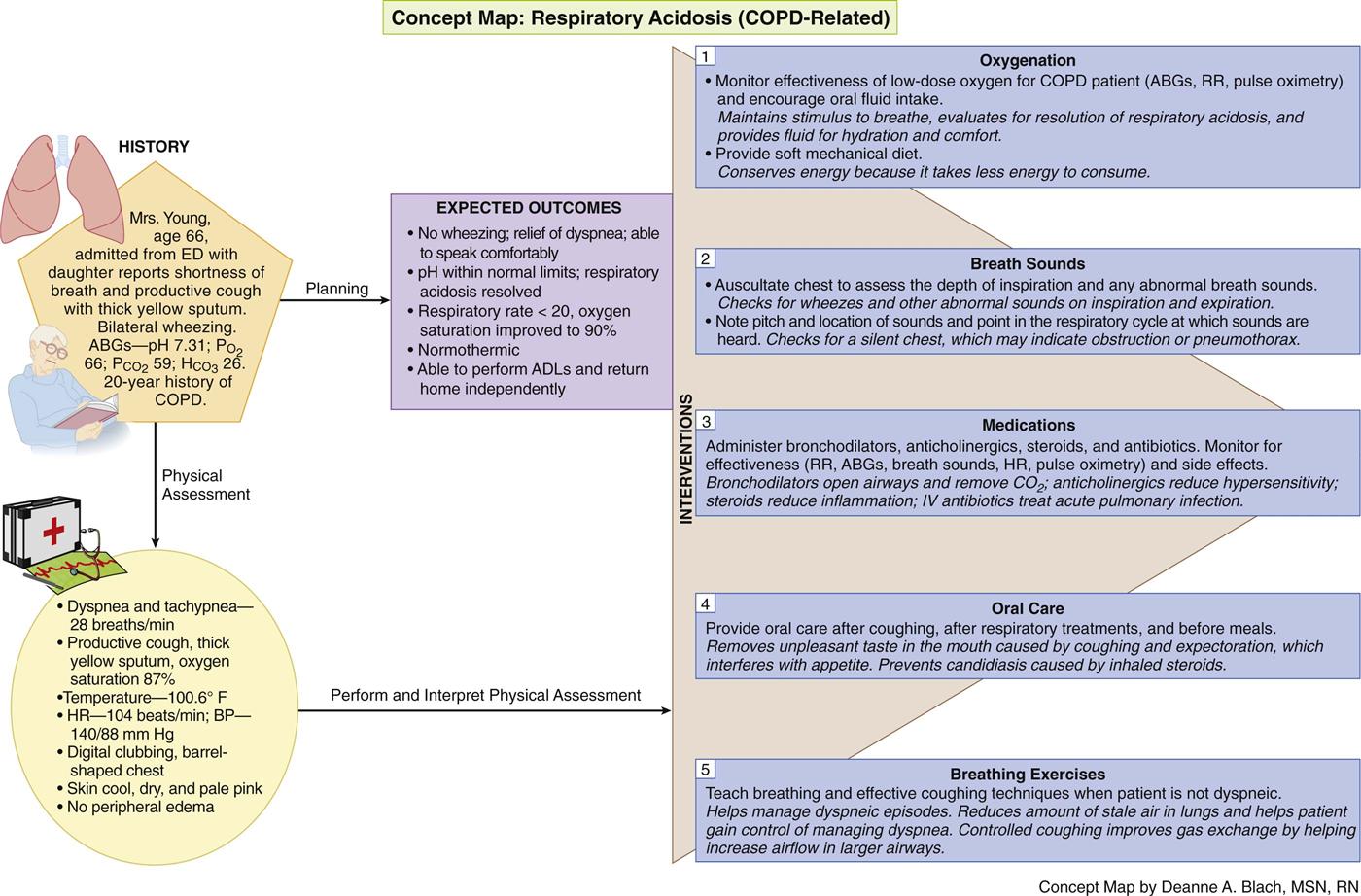
The Concept Map on p. 615 addresses assessment and nursing care issues related to COPD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree