Practice Point
The microbes that inhabit one body site may not be the same as found in another site on the same person.
The skin is a mechanical barrier to organisms, and skin oils and sweat are chemicals that help prevent their entrance into tissue.1 When a wound occurs, organisms from the skin, gastrointestinal track, and mucous membranes as well as the external environment have free access to tissue. Bioburden is a measure of the number of viable microbes on a tissue surface or device.
 Practice Point
Practice Point
Bioburden is a measure of the viable organisms in a wound or on a tissue surface.
Until recently, scientists and clinicians understood that infection was due to planktonic organisms, those who float free when placed in water.3 These organisms are individual cells that have a predictable life cycle and work independently. There is a good understanding of planktonic organisms, as seen by the fact, that antibiotics have been developed that target-specific sites to kill or immobilize them. Our growing understanding of biofilm has radically changed our perspective on microorganisms, how they function, and their effect on wound infection.4
 Practice Point
Practice Point
Antibiotic treatment is based on knowledge of planktonic organisms.
Planktonic organisms of all types float freely in the air and exist on the skin and surfaces in the environment. With injury, organisms contaminate the wound surface. The organisms attach to the surface, change their genetic code (become biofilm genotype) and exude the extracellular polymeric substance (EPS) that provides the structure for the biofilm. Biofilm is defined as communities of microorganisms attached to a surface (living or not) embedded within a hydrated matrix of extracellular polymeric substance (EPS).5,6 It is composed of polysaccharide-based substance, although it includes proteins, lipids, and DNA. Biofilm provides protection against antimicrobials and host defenses. The communities of organisms that form are genetically different from each other as well as their planktonic predecessors.7 The organisms, in the biofilm community, work synergistically, communicate with each other and other communities (through a process called quorum sensing), are able to change their metabolism, and are resistant to antibiotics.3,8
Biofilm communities are usually composed of multiple organisms. They form fluid-filled channels that allow oxygen and nutrients to get to the cells and carry away waste. Their ability to reduce their metabolism allows these organisms to use little oxygen and nutrients and become dormant, only later to be reactivated. Biofilms impair healing as the EPS helps the organisms evade the immune system and contributes to perpetuating the inflammatory phase so the wound turns into a chronic nonhealing wound.5,8
 Practice Point
Practice Point
Biofilm protects organisms from antibiotics.
At some point, the biofilm communities come to exceed their nutrient/oxygen capacity and send off organisms into the wound environment (seed the environment).3 Essentially, the cells released are planktonic organisms that float freely in the moisture of the wound fluid and go on to repeat the process of setting up a new biofilm. The newly released planktonic organisms also stimulate a continuation of the inflammatory response. Meanwhile, the EPS prevents antibiotics from reaching the organisms; in addition, the organisms constantly change their genetic code, making it difficult for antibiotics to kill them.
 Practice Point
Practice Point
Scientific discoveries have radically changed the way we think of infection. Evidence-based practice requires current scientific knowledge about biofilm as the basis for safe care.
Biofilm can form throughout the body, for example, plaque on teeth, thick secretions with cystic fibrosis.
The biofilm generally is not visible because of its location, except perhaps in wounds. Some authors suggest that biofilm in wounds may be observed by expert clinicians as translucent films or opaque yellow-green material.6 Others do not believe it is visible.3,5 For the most part, clinicians can only infer the presence of biofilm genotype organisms based on signs and symptoms. Sophisticated diagnostic tools needed to identify biofilms are not currently available in clinical practice.3,5
Seminal work showed that biofilms occur more frequently in chronic wounds than acute wounds (60% of chronic wounds and 6% of acute wounds).9 Thus, in this chapter where appropriate, the differentiation between acute and chronic wounds is presented.
 Practice Point
Practice Point
Biofilm occurs much more frequently in chronic wounds (every 3 out of 5 wounds) than in acute wounds (1 in 12 wounds). The physical characteristics of biofilm have not been identified; the presence of biofilm is inferred from signs and symptoms.
Wound Bioburden Cycle
Predictable processes occur that help us understand how exposure to bioburden can lead to infection or eradication of invading organisms in wounds. Risk of wound infection involves the interaction of the individual’s immune system, the environment, and the organisms.3,10 Increased risk of infection occurs in patients who are immunocompromised as occurs with therapeutic chemotherapy for cancer and persons receiving prednisone. Infection risk also is increased when the environment is moist, warm, lacks appropriate pH, and sufficient oxygen and when there is a large load of bioburden present. The nature of the organisms also affects the risk of infection and depends on their constitutional needs such as oxygen requirement (aerobic/anaerobic) and inherent virulence, for example, high virulence as seen in beta hemolytic Streptococcus.
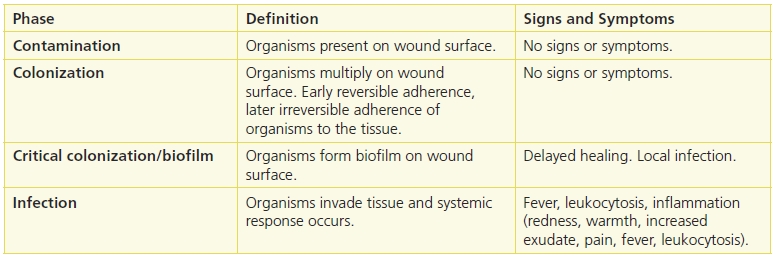
The wound bioburden cycle describes the processes associated with the increasing invasion of a wound by organisms (Table 7-1). The first phase of this cycle is contamination, and it begins with injury that disrupts the skin. Examples of tissue injury are a surgical incision, trauma, or a bite. Microorganisms present in the environment contaminate the wound. The full range of microorganisms may be included (bacteria, fungi, viruses), and they may be endogenous, that is, from the patient’s skin or gastrointestinal track, mucous membranes or exogenous, that is from external contamination, for example, healthcare workers, trauma. Contamination cannot be seen nor does it generate clinical signs and symptoms. The organisms are planktonic genotype (free floating) and are normally managed by host defenses and the bioburden cycle terminated at this phase.3
Table 7-1 Phases of the Wound Bioburden Cycle
 Practice Point
Practice Point
Contamination and colonization cannot be seen and produce no signs or symptoms. This phase of the wound bioburden cycle offers the best opportunity for removal of organisms from the wound, before the organisms become attached to the wound surface.
If the microorganisms are not removed, they assess the environment with receptors on their cell wall and attach to the surface. At this point, the organisms’ attachment is reversible, that is, the organism can be washed away with irrigation.3 Once lightly attached, the organisms use the wound bed as a source of nutrition, oxygen, etc. The organisms soon become irreversibly attached, and early stages of biofilm are formed. Over time, the biofilm genotype develops, and the organisms begin to modify their genetic profile and multiply. Colonization cannot be seen and does not produce signs and symptoms.
Within 24 hours of contamination, the organisms begin to develop a thin layer of EPS, and the layer becomes thicker as the biofilm organisms continue to lay down layers of ESP.6 Biofilm forms during the critical colonization/biofilm phase, and this phase is characterized by local infection and delayed healing.5,10 In fact, it has been proposed that the concept of critical colonization represents the effects of the biofilm,11 so in this chapter, critical colonization is termed critical colonization/biofilm.
 Practice Point
Practice Point
The critical colonization/biofilm phase occurs when organisms create local communities in the biofilm on the wound. Local infection and delayed healing are visible to the clinician and characterize this phase.
Infection occurs when the organisms invade the tissue and produce systemic signs. Infection is characterized by inflammation (redness, heat, edema, pain, and increased exudate.12,13 It is treated with antibiotics, but they are effective only when the organisms are metabolically active.
 Practice Point
Practice Point
Infection occurs when organisms invade the tissue and produce systemic signs. Antibiotics used to treat wound infection function only when organisms are metabolically active.
Table 7-1 summarizes the phases of the wound bioburden cycle and their associated signs and symptoms. It should be noted that with the increasing availability of DNA-based diagnostic protocols, a change in this paradigm may be needed that alters the phases of the cycle. One such proposed paradigm is contamination, attachment, proliferation, critical concentration of signaling molecules, adaptation of the biofilm phenotype, and polymicrobial biofilm.14
Defenses Against Infection
Injury mobilizes a predictable, albeit complex, sequence of events that are designed to lead to healing (see chapter 5, Acute and chronic wound healing). These processes also provide a network of protection against wound invasion by microorganisms. With initiation of the inflammatory response to tissue injury, there is an influx of polymorphonucleocytes (PMNs) and monocytes. Of the PMNs, neutrophils are the most common and arrive immediately after injury and predominate for the first 48 hours.15 The monocytes enter the wound space immediate after the PMNs and are transformed into macrophages, reaching their peak within 24 hours of injury. Both the leukocytes and macrophages are important in quickly infiltrating the area of tissue damage and removing microorganisms by phagocytosis. These WBCs work best in a well-perfused oxygen-rich environment as phagocytes require oxygen (oxygen burst).16
Proinflammatory mediators, reactive oxygen species, proteases, and antimicrobial peptides are released into the wound and result in microbial killing as well as tissue damage. Normally, the processes stimulated by the proinflammatory mediators that lead to inflammation and immune cell activation are reversed when the neutrophils die (apoptosis) and are engulfed by macrophages.15 In acute wounds, this occurs by about day 5, and wound infection is circumvented.
 Practice Point
Practice Point
Inflammation is important to fighting infection early after injury. Continued inflammation over time characterizes chronic nonhealing wounds.
What was a positive response to injury becomes a negative one when the inflammatory cycle continues beyond its normal duration. Specifically, chronic wounds inflammatory cells continue to be recruited; generate reactive oxygen species, proteases, and antimicrobial peptides that kill microorganisms; and result in tissue damage. The organisms themselves may participate in protective processes, for example, Pseudomonas aeruginosa may contribute to inflammation by producing rhamnolipids that protect them from phagocytosis.17
Proteases are especially problematic as they cause degradation of the extracellular matrix and tissue damage. A negative spiral is set up where damage caused by neutrophil generated proteases and chronic inflammation delays wound healing (delayed reepithelialization, impaired collagen generation, cells at the edge of the wound).15,18 The biofilm protective mechanisms are in place, and wound infection occurs when the microorganisms overtake the individual’s immune system.
From a clinical perspective, it is important to recognize that the body’s defense against micro-organisms works effectively in most acute wounds as seen by the low rate (<2%) of surgical site infection (SSI), recognizing that even at this rate, SSI is the most common hospital acquired complication.19 Comparatively, chronic wounds present a grim picture with the prevalence of wound infection estimated to be 53%.20 With more than 40 million inpatient surgeries each year and the increasing incidence of chronic diseases that often involve chronic wounds, for example, diabetes, venous disease, it would behoove us to be vigilant in applying evidence-based care to mitigate wound infection and its sequelae. While the etiology of various types of chronic wounds differ (e.g., diabetic ulcers are due to arteriosclerotic peripheral vascular disease often complicated with peripheral neuropathy, pressure ulcers are caused by unrelieved pressure), there is agreement in the field that the pathway for chronic wound infection is applicable across the chronic wound types.3
Defining Infection
Wound infection is the invasion and multiplication of microorganisms in wound tissue resulting in pathophysiologic effects or tissue injury21 or the presence on any beta-hemolytic Streptococcus.22 The definition sets out the criteria for a wound infection: tissue must be viable, organisms invade tissue, organisms multiple, patient defenses are overwhelmed, and signs and symptoms of infection are visible. Also, beta-hemolytic Streptococcus needs to be identified early because of their well-recognized virulence. Necrotic tissue and slough by definition are dead so infection cannot be present in them; however, dead tissue does support proliferation of organisms and so needs to be removed.18 Also, pus is the product of phagocytosis and digestion of dead microorganisms by WBCs; pus is not indicative of infection.
 Practice Point
Practice Point
Infection occurs in living tissue so cannot occur in debris; however, debris (slough, necrotic tissue, old sutures) does support proliferation of organisms and so needs to be removed.
The definition of infection was established based on acute wounds and the existing understanding of planktonic organisms. Since then, our understanding of biofilm in chronic wounds and biofilm genotype organism behavior has grown. In addition, the National Institutes of Health (NIH) has declared that 80% of infections are due to biofilm.23 As invasion during critical colonization/biofilm results in delayed healing, the question must be posed whether wounds in the critical colonization/biofilm phase of the wound bioburden cycle are infected and should be treated as infected. The expectation is that in the near future, the availability of bedside molecular diagnostic techniques will allow better definition of bioburden and biofilm and development of more effective treatment of offending organisms.14
Wound infection currently is diagnosed based on its signs and symptoms.5,13 Classic characteristics of wound infection are erythema, heat, swelling, and pain.3,12 One of the challenges in clinical practice is differentiating infection from inflammation. Inflammation normally is present for 3 to 5 days after injury and is characterized by the same signs.5,13 The question becomes how to differentiate these two processes and especially in chronic wounds where the inflammatory response often is more muted.
The most common acute wounds are surgical and the criteria for SSI are used for acute wounds. The definition for SSI was established more than 20 years ago24 and continue to be the criteria used by the Centers for Disease Control (CDC).
Chronic wounds, on the other hand, are characterized by a chronic inflammation and biofilm. However, the identification of infection in chronic wounds is tricky as the signs and symptoms may be more subtle than in acute wounds and indicators of moving from critical colonization to infection may not be recognized.
To date, there are not universal criteria for infection in chronic wounds. Lack of criteria contributes to inconsistent diagnosis of chronic wound infection and potentially to inappropriate treatment (under or overtreatment). The problem of lack of widely accepted criteria is compounded by the lack of clinical diagnostic tools to evaluate the contribution of the biofilm to infection in chronic wounds.4,5
Signs and Symptoms of Wound Infection
SSI is divided into incisional and organ/space. Incisional SSI is further divided into superficial and deep SSI.24 Superficial SSI sites include the skin and subcutaneous tissue while deep SSI designates infection in soft tissues, fascia, and muscle layers of the incision. Organ/space SSIs involve any part of the anatomy other than the incision or that manipulated during the operation (Table 7-2).
Table 7-2 Diagnostic Criteria for Incisional and Organ/Space Surgical Site Infection24
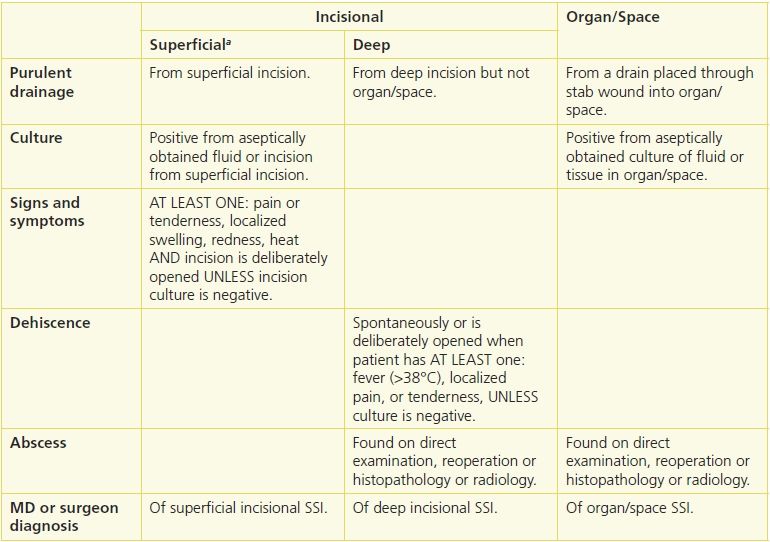
aNot superficial SSI: stitch abscess, episiotomy or neonate circumcision, infected burn, incisional SSI that extends into fascia and muscle.
SSI refers to infection at the site of an incision. All SSI occurs within 30 days of surgery, except deep SSI that involves an implant. The timeframe for these may extend up to 1 year if an implant is in place, the SSI appears related to the implant surgery, and the infection meets the anatomic criteria for deep SSI.
 Practice Point
Practice Point
Diagnosis of wound infection in acute wounds is based on the depth of the infection. In addition, the criteria for infection in acute and chronic wounds are not the same.
A number of researchers have examined the signs and symptoms of infection in chronic wounds. Cutting and Harding12 identified traditional infection criteria (abscess, cellulitis, discharge [serous exudate with inflammation, seropurulent, hemopurulent, pus]) as well as the additional criteria for granulating tissues: delayed healing, discoloration, friable granulation tissue that bleeds easily, unexpected pain/tenderness, pocketing at the base of the wound, bridging of epithelium or soft tissue, abnormal smell, and wound breakdown.12 The validity of these criteria was tested on ward nurses (n = 20) working in pairs, who were asked to identify if each of four wounds was infected. Findings were compared with wound infection diagnosis by the principal investigator (an MD) and swab culture results. Descriptive statistics were reported that showed the nurses had less than chance agreement with the MD (47.5%), and the MD had nearly perfect agreement (97.5%) with culture findings.25 The relationship of the nurses’ infection classification with the culture data was not reported. These data indicate that the assessment of the presence of wound infection is not consistent among healthcare providers. The author concluded that the nurses needed more education to improve their performance.
 Practice Point
Practice Point
Practitioners responsible for identifying wound infection need knowledge about the criteria and practice in applying them.
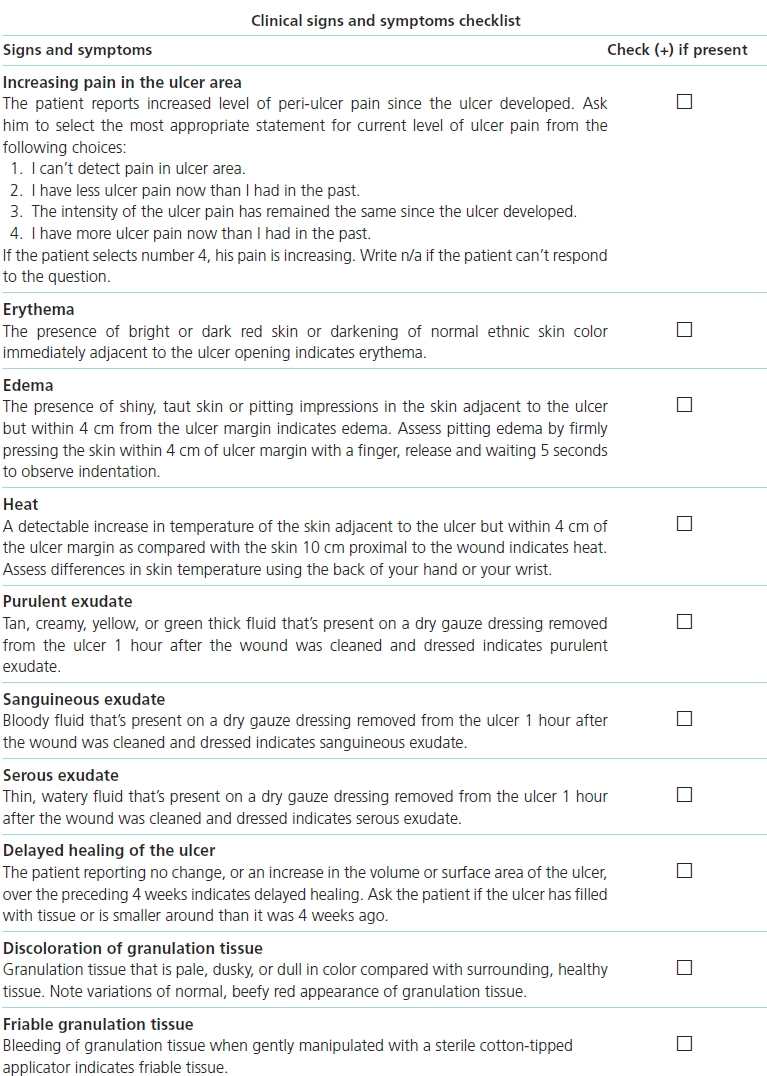
Subsequently, a landmark study by Gardner et al.13 tested the Cutting and Harding.12 They studied signs and symptoms of infection in patients with chronic wounds (n = 36), 11 of whom were infected based on biopsy with both aerobic and anaerobic analyses.13 Classic signs of infection (pain, erythema, edema, heat, purulence) were compared with signs of infection in secondary wounds (serous drainage with inflammation, delayed healing, discolored granulation tissue, friable granulation tissue, pocketing at the base of the wound, foul odor, and wound breakdown). The Clinical Signs and Symptoms Checklist,13 comprised the signs and symptoms with operational definitions and mutually exclusive evaluative categories, was used to measure the signs and symptoms (Box 7-1). Findings showed that the signs and symptoms of chronic wounds were better indicators of infection in chronic wounds than were the classic signs (sensitivity 0.62, specificity 0.38). Valid indicators of infection in chronic wounds were based on a combination of the sensitivity, specificity, discrimination power, and positive predictive power and were increasing pain, friable granulation tissue, foul odor, and wound breakdown. Specificity of increasing pain (1.0) and wound breakdown (1.0) were robust enough to be considered signs of infection.
 Practice Point
Practice Point
Signs of wound infection in chronic wounds are increasing pain, friable granulation tissue, foul odor, and wound breakdown; the strongest of these indicators are increasing pain and wound breakdown.
The Gardner26 research team then went on to examine individually the same signs and symptoms in chronic diabetic foot ulcers (DFUs), those of the Guidelines of the Infection Disease Society of America (IDSA),27 and a composite predictor based on all the signs and symptoms of infection.26 Of the 64 participants, 25 had a DFU with a high microbial load (>106). Using the same methods as in the 2001 study, researchers evaluated whether the IDSA predicted ulcers. Findings showed no individual sign was a significant predictor of high microbial load nor was the IDSA combination. The composite predictor performed moderately well (concordance = 0.645 with 95% confidence interval 0.559 to 0.732).
At the same time, Sibbald and colleagues10 explored ways to translate existing research findings, so they could be used in clinical practice. They proposed diagnostic criteria using the acronym NERDS for critical colonization (Fig. 7-1) and STONEES for infection (Fig. 7-2). With NERDS acronym, critical colonization/biofilm is present in chronic wounds when three or more of the signs/symptoms are present. With the STONEES criteria, infection was present when any three of the STONEES criteria are observed.
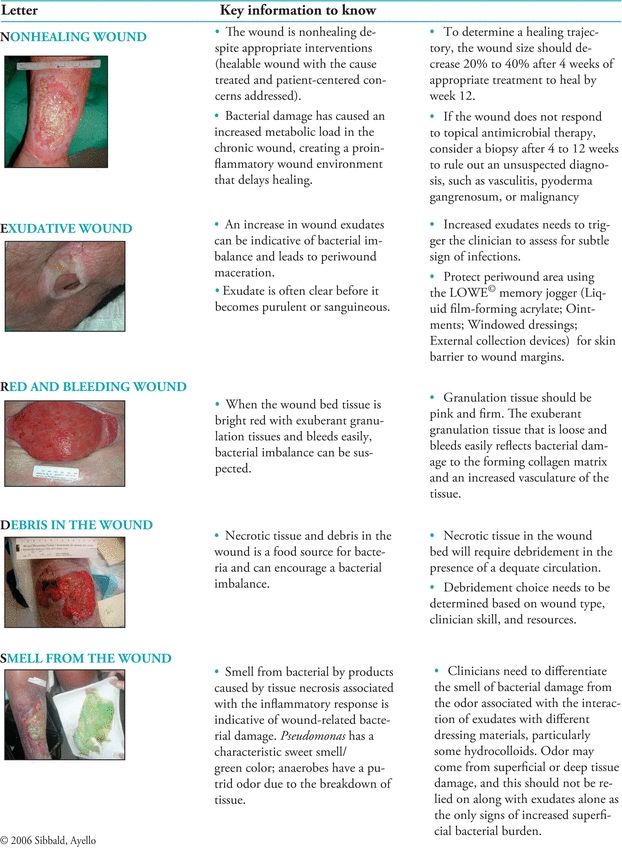
Figure 7-1. NERDS©: Superficial increased bacterial burden.
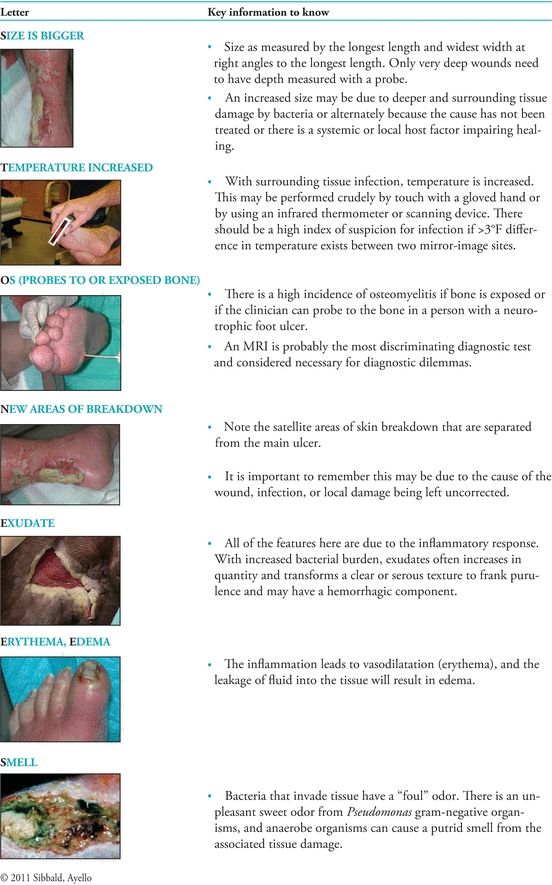

Figure 7-2. STONEES©: Deep compartment infection.
A cross-sectional validation study of patients with leg and foot ulcers conducted by Woo and colleagues28 found that the presence of any three NERDS signs had a sensitivity of 73% and a specificity of 80.5% when compared with semiquantitative swab cultures with scan or light growth. They also found that any three STONEES signs had a sensitivity of 90% and a specificity of 69.4% when compared with semiquantitative swab cultures with moderate-to-heavy growth.
 Practice Point
Practice Point
Any three NERDS signs are indicative of critical colonization/biofilm, and any three STONES signs are indicative of wound infection.
Cutting and colleagues29 sought expert opinion from international interdisciplinary wound experts to determine the clinical signs and symptoms of infection present in chronic wounds as well as those specific to a particular type of wound. Data showed that signs and symptoms common to all chronic (not acute wounds or burns) were cellulitis, malodor, pain, delayed healing, wound deterioration or breakdown, and an increased amount of exudate. Signs or symptom-specific criterion for infection in various chronic wounds are crepitus for pressure ulcers; phlegmon for neuropathic/diabetic ulcers; increase in local skin temperature for venous ulcers; and dry necrosis (that may become moist and boggy at the edges of the necrotic tissue) for arterial disease–associated tissue breakdown.
 Practice Point
Practice Point
Expert opinion of international wound experts using the Delphi technique showed that signs and symptoms of infection in chronic wounds were cellulitis, malodor, pain, delayed healing, wound deterioration or breakdown, and an increased exudate.
A review by Reddy et al.20 summarizes the literature that examines the relationship between deep tissue biopsy (gold standard) and signs and symptoms of wound infection in patients with chronic wounds. Likelihood ratios (LRs) were used to identify the presence of infection. A positive LR predicts the presence of infection while negative values indicate that wound infection is not present. The likelihood of infection increases when an ulcer causes increasing pain (LR range 11 to 20); however, absence of decreasing pain is not as helpful in identifying the absence of pain (LR 0.64 to 0.88).13,26 Classic signs of wound infection were not helpful in diagnosing chronic wound infection (LR small and including one).13,26
 Practice Point
Practice Point
Classic signs and symptoms of wound infection are not helpful in identifying infection in chronic wounds.
When Reddy et al.20 examined the literature, they found few studies that systematically examined signs and symptoms and compared them with tissue biopsy culture. Lack of using the gold standard was a major issue. For example, the often cited work of Cutting and Harding12 proposed signs and symptoms of wound infection based on their clinical experience. In their subsequent work that examined the ability of nurses to identify wound infection based on their list of signs and symptoms, the standard of comparison was MD evaluation; swab cultures were used to test MD diagnosis based on the signs and symptoms from the list he had generated.25 In the Delphi study that assessed the opinion of experts on signs and symptoms of chronic wound infection,29 the signs and symptoms elicited were not tested against a standard criterion.
Reddy’s team noted that the majority of studies had examined a single sign or symptom and its relationship with criteria whose validity had not been established. They also acknowledged that many of the signs are quite subjective (e.g., friable granulation tissue; odor) and interrater reliability had not been reported. Reddy et al.20 concluded that data from the Gardner research team provides the strongest evidence for the relationship between signs and symptoms and tissue biopsy cultures. Data from the Sibbald10 paradigm and the validation study of Woo and colleagues28 were not considered in the review because they did not compare the signs and symptoms with tissue biopsy.
Diagnostic Tests
Wound Cultures
Tissue biopsy is the gold standard for wound infection. Those with greater than 105 CFU have been recognized as diagnostic of wound infection.30 Krizek et al.31 showed that skin grafts did not “take” when there were more than 105 organisms per gram of tissue. Similarly, Robson et al.32 found that bacteria that exceeded 105 per gram of tissue inhibited successful secondary closure of wounds. Also at this same time, Mack33 explored the effect of topical antibiotic on skin flora. Classic work of Robson and Heggers30 using a sample of wounds (n = 41) of mixed etiology showed that the quantitative tissue biopsy method had a sensitivity of 100%, a specificity of 93.5%, and an accuracy of 95.1% in predicting the success of delayed closure laid the foundation for biopsy as the gold standard for infection.
Subsequently, studies used 106 as the criterion for wound infection. Krizek and Davis34 found that fatal sepsis was associated with visceral or blood cultures greater than 106 or 107 organisms per gram of tissue or milliliter of blood. These researchers also demonstrated that fatal wound sepsis was related to the number of bacteria in the wound.35 In addition, Noyes and colleagues36 found that wound exudates with greater than 106 bacteria per milliliter were associated with invasive infection. The U.S. Army Surgical Research Unit provided a series of studies that found burn wound sepsis was associated with bacterial levels exceeding 105 organisms per gram of tissue.37,38
Part of the issue is how to interpret greater than 105 CFU. Does it mean 100,000 or does it mean 1,000,000? References to greater than 105 have been interpreted as 100,000 or 1,000,00039 organisms per gram of tissue. Some research30,40 has used greater than 105, while others41 have used 106. In addition, gram stain criteria for infection is the presence of 106 or more organisms.42 While there is not universal agreement on the number, use of 106 to define infection is recommended.
Wound cultures measure planktonic organisms and underestimate the number of organisms present. In addition, recent understanding of bioburden raises the question of whether culture findings can accurately capture the bioburden in a wound, especially in chronic wounds.4 However, for the moment, until strategies to measure bioburden in biofilm and ways to interpret those data are more universally available, culture will remain the gold standard for wound infection.
 Practice Point
Practice Point
Tissue biopsy with 106 or greater organisms or the presence of beta-hemolytic Staphylococcus is recommended as the criterion for wound infection.
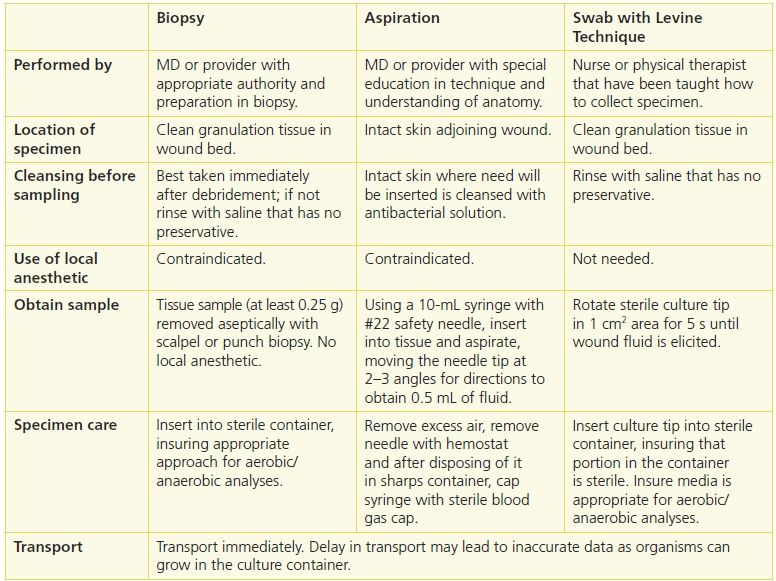
Three approaches to culturing are available: biopsy, aspiration, and swab. Table 7-3 describes important dimensions of the procedure.
Table 7-3 Comparison of Three Techniques for Wound Culture
Regardless of the culture technique employed, preparation of the lab slip that accompanies the specimen is critical to accurate interpretation of the culture findings. The lab needs to know the site on the body where the specimen was obtained, the type of wound, the time the specimen was obtained, the age and gender of the patient, whether receiving antibiotics (name, dose, started when) as well as any special information, for example, occupation (farmer vs. ocean diver), anticoagulated. Prompt delivery of the specimen also is essential so organisms do not have time to grow. Failure to complete these seemingly mundane aspects of wound culture can result in false-positive or false-negative results.43
 Practice Point
Practice Point
Accurate wound culture data depend on aseptic collection of the specimen, essential data about the wound and patient that are incorporated into the lab slip, and timely transport of the specimen to the lab.
Specimens can be processed qualitatively, quantitatively, or semiquantitatively. Qualitative processing, results in isolation and identification of the organisms present. The quantitative processing allows the specific number of organisms grown in media to be identified and counted. The number of organisms is expressed as number per gram of tissue, milliliter of fluid, or swab. The number of organisms present provides information regarding the rate of microorganism multiplication. Few facilities are equipped to perform quantitative analyses, and most are restricted to research studies. The majority of cultures are processed using semiquantitative technique where the cultures are inoculated onto media using a sterile loop that dilutes the specimen from the first to the fourth quadrant. Results are reported as 1+, 2+, 3+, and 4+ (trace, few, moderate, abundant).41,43 With semiqualitative analyses, authors10,44 suggest that 4+ should be used as the cutoff for diagnosing infection. The sensitivity of the organisms isolated to antibiotics can be obtained, regardless of which processing technique is used. Normally, results from a culture are available in 24 to 48 hours. Also, analyses for anaerobic organisms and fungi are normally not performed unless requested.
Each of these wound culturing techniques has strengths and limitations. Tissue biopsy is the gold standard and so is the standard against which the other approaches are compared. However, it is a labor-intensive process where the tissue must be weighed, homogenized, and serially diluted prior to inoculating media, and then plating it and growing it in aerobic and anaerobic conditions.43 Biopsy also is limited because it causes patients pain when performed, many facilities do not have the resources to perform the laboratory analysis, and it requires that a MD or specially educated provider with appropriate education perform it.41,43
Few studies have been performed that address aspiration wound culturing. A study by Lee et al.45 examined aspiration but did not compare it with biopsy. Another found that aspiration underestimated infection identified by biopsy but that the organisms obtained were not consistent with the organisms identified with deep tissue biopsy.46 Currently, there are insufficient data about the use of aspiration wound cultures to recommend it for culturing wounds, although it is appropriate for draining abscesses.
Swab cultures have been extensively studied in an effort to identify a less invasive but accurate method of culturing wounds. Early work by Levine et al.42 showed a high concordance (R2 = 0.76) between their specific method of obtaining a swab culture (1 cm2 area, 5-second culture, obtain bloody fluid) and biopsies of the same sites (Fig. 7-3). Other approaches to surface cultures have been used, most recently the Z-stroke where the tissue is swabbed across the wound from edge to edge in a Z pattern. The Levine technique sensitivity was 90% and specificity 57%, while that from the Z technique at a sensitivity of 90%, specificity was 27%. The concordance of the Levine technique with tissue biopsy was identical (78%), and concordance with respect to recovering the specific organisms Staphylococcus aureus, P. aeruginosa, and beta-hemolytic Streptococcus was 96%, 96%, and 99%, respectively.41
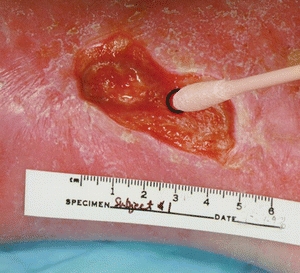
Figure 7-3. The Levine technique. (Photo courtesy of Dr. Rita Frantz.)
Swab culture has the advantage of being easy to use and require minimal training to ensure appropriate sampling. However, it has been criticized because it only accesses wound surface organisms as well as contaminants. It also does not sample organisms deep in the tissue, including anaerobic organisms.47 An addition threat to accuracy is the possibility of dry swabs or swabs with insufficient specimen.43 One approach proposed to overcome the concern of contaminants is wound cleansing, recommended to remove exudate, topical therapies, and loose nonviable tissue prior to obtaining the culture.47,48 Moistening the swab with normal saline or transport medium is recommended prior to specimen collection to address the potential dryness issue.46,48,49
Although the accuracy of swab cultures as compared with biopsy cultures has been studied, methodological issues interfere with our ability to obtain little information as the basis for clinical practice.40,42,46,50 The most serious methodological problem presented by these studies is that the specific swabbing techniques employed were not described. Swabbing techniques vary greatly according to wound preparation, area of the wound sampled, duration of sampling, and even the type of swab employed (e.g., alginate).51
To address this problem, Gardner et al.41 compared culture findings from swab specimens obtained using wound exudate, the Z-stroke technique, and Levine’s technique with culture findings from viable wound specimens. Culture findings based on swab specimens obtained using Levine’s technique were more accurate and concordant with culture findings based on tissue specimens than swabs taken with either wound exudate or the Z-stroke technique.
At a critical threshold of 37,000 organisms per swab, the Levine technique had a sensitivity of 90% and specificity of 57% when the true infection status of the wound was defined as 1,000,000 or more organisms per gram of tissue. The Z technique using the critical threshold of 62,500 organisms had a sensitivity of 90% but only a 27% specificity. In terms of recovering any and all organisms, swab specimens obtained using Levine’s technique were 78% concordant with tissue specimens. Gardner et al.41 as well as others52 recommend the Levine technique. The researchers, however, note that further study is needed to identify the optimal critical threshold for practice.
 Evidence-Based Practice
Evidence-Based Practice
Swab cultures should be obtained using the Levine technique as it obtains fluid from the wound tissue, not just surface organisms.
A review by Reddy et al.20 was conducted to determine the preferred noninvasive method of culturing chronic wounds. Few (four) studies met the inclusion criteria that included comparison of the techniques with tissue biopsy, and they calculated likelihood ratios (LR) to identify predictors of infection (positive LR) and predictors of no infection (negative LR). The Gardner study41 that examined nonarterial chronic wound ulcers using the Levine technique (when compared with biopsy) had a likelihood ratio (LR) of 6.3 (95% CI 2.5 to 15), showing its strength in identifying infection. Similarly, a negative wound culture (LR 0.47 with 95% CI 0.31 to 0.73) indicated that infection was less likely. In contrast, the Z-technique (superficial surface culture of entire wound surface moving from side to side while rotating the swab between the thumb and forefinger) neither predicted nor excluded wound infection. In a later study by Gardner et al.26 on patients with nonarterial diabetic ulcers, Levine technique did not predict infection, but negative cultures predicted the absence of infection. The third study40 in patients with mixed chronic wounds, compared quantitative swab culture with the Levine technique with tissue biopsy, did not predict infection while the negative culture predicted the absence of wound infection (LR 0.35; 95% CI 0.15 to 0.85). A serious limitation of the study is the lack of information about the exact number of organisms that were defined infection, that is, does greater than 105 actually mean 106? Other concerns were lack of information about who the participants were; age and gender of participants were not reported.
In many ways, this review summarizes the existing literature about noninvasive wound culture in patients with chronic wounds. First, the prevalence of chronic wounds is high, especially in spinal cord–injured patients, long-term care residents, and diabetics. Wound infection in the chronic wound populations is high, the range depending to some extent on the quality of the research.20 Yet, even the high prevalence of infection in patients with chronic wounds may be an underestimation as patients with infected wounds often are excluded from many research studies. This review shows that the identification of wound infection remains challenging. Clinical signs and symptoms of infection are important in wound assessment. Inflammation as markers are especially important in acute wounds.
However, the signs and symptoms associated with inflammation may not be present in some patients with acute wounds or in patients with chronic wounds. The signs specific to secondary wounds continue to require ongoing attention. Wound pain and wound breakdown need to be routinely incorporated into assessment. Consideration also needs to be given to routine evaluation of a set of signs and symptoms of infection in chronic wounds such as is provided by the Clinical Symptom Assessment List13 and the Sibbald et al.10 NERDS and STONEES approach. The use of criteria that have established operational definitions will aid providers in evaluating chronic wounds consistently.
Wounds suspected of infection, especially those with delayed healing, are often cultured to confirm the diagnosis. While qualitative cultures provide useful information in wounds that are demonstrating obvious clinical signs of infection, they may not be as useful in diagnosing infection in the absence of signs and symptoms unless certain pathogens are isolated. In the absence of clinical signs and symptoms, quantitative cultures remain the gold standard method for diagnosing localized wound infection. Newer technique on the horizon hold hope for a revolution in point of care diagnostics for wound infection.
Newer Technique to Identify Bacteria in Wounds
Advanced techniques have been used in research to evaluate the presence of biofilm and the nature of organisms in it. Early on, microscopic approaches were used to identify the biofilm EPS. Initially light microscopy was used and later confocal scanning microscopy. At times, the microscopy was complemented with various staining techniques.
More recently, various molecular methods have been developed and utilized. Polymer chain reaction (PCR) identification of bacteria and fungi has been used to sequence the DNA in biofilm organisms. Sequencing specific regions of bacteria and fungi present in chronic wounds has been used but is expensive and not widely available to clinicians. The goal is to develop large databases to identify patterns of organisms that correlate with nonhealing.14,53
The recognition that most chronic wounds have high levels of proteases has led to the development of point of care detectors for use with wound fluid. While approved for use in some European countries, these sensors are not currently available in the United States.53
The goal of this diagnostic work is to identify specific organisms so focused care can be provided. At the moment, personalized care seems to be needed that addresses the specific organisms identified in a specific wound in a given patient.14,53 Such an approach will require improved diagnostics and pharmacies able to prepare combination of drugs that can be applied in the immediate postdebridement period to kill biofilm organisms. Much research is focused on this area, and clinicians will need to continue to update their knowledge to provide evidence-based care.
 Practice Point
Practice Point
A bedside test for identifying microorganisms in biofilm does not currently exist. Ongoing research continues to develop and test this needed diagnostic tool.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree