Robert S. Kirsner, MD, PhD
Objectives
After completing this chapter, you’ll be able to:
- recognize the importance of identifying atypical wounds
- explain the need for wound biopsies in determining the etiology of an atypical wound
- describe the various clinical manifestations of atypical wounds.
Types of Atypical Wounds
Prolonged pressure (pressure ulcers), venous insufficiency (venous leg ulcers), complications of long-standing diabetes mellitus (diabetic foot ulcers), and poor vascular supply (arterial ulcers) are the most common causes of chronic wounds. Wounds resulting from uncommon etiologies, called atypical wounds, are less frequently encountered and less well understood. Their prevalence has not been studied extensively, but it is estimated that at least 10% of more than 500,000 leg ulcers in the United States may be due to unusual causes.1,2 A variety of etiologies may cause atypical wounds,3 such as infections, external or traumatic causes, metabolic disorders, genetic diseases, neoplasms, and inflammatory processes.
It is critical to recognize when a wound is caused by an etiology other than prolonged pressure, neuropathy, or abnormal vascular supply so that a correct diagnosis can be made and the appropriate therapy provided. A wound should be evaluated for an atypical etiology if:
- it is present in a location different from that of a common chronic wound
- its appearance varies from that of a common chronic wound
- it does not respond to conventional therapy.
For example, the thigh is an atypical location for a pressure, venous, arterial, or diabetic ulcer and should raise the suspicion of an atypical cause. A wound on the medial aspect of the leg but extending deep to the tendon would be considered atypical despite being in a common location because the depth of this wound is atypical for venous ulcers. Finally, any wound that is not healing after 3 to 6 months of appropriate treatment should raise the consideration of an atypical cause, even if the distribution and clinical appearance are classic for a common chronic wound.
Once a wound is deemed atypical, a tissue sample is critical for histologic evaluation with special stains, tissue culture (for infectious causes), and immunofluorescence testing (for some inflammatory or immune-based causes).
 Evidence-Based PracticeEvidence-Based Practice
Evidence-Based PracticeEvidence-Based Practice
Tissue samples are mandatory for atypical wounds because many of the unusual causes of wounds can resemble each other, making visual diagnosis alone difficult and risky.
Etiologies of Atypical Wounds
Some of the most commonly encountered etiologies for an atypical wound include inflammatory causes, infections, vasculopathies, metabolic and genetic factors, malignancies, and external causes (Table 20-1). However, a thorough medical history, including epidemiological exposure, family history, personal habits, medications, and concomitant systemic diseases, along with a thorough physical examination, histologic evaluation, and laboratory testing, will provide critical information necessary for a correct diagnosis of an atypical wound.
Table 20-1 Potential Etiologies of Atypical Wounds
Inflammatory Causes
Among the most interesting—and probably more common—causes of atypical wounds are the inflammatory ulcers. Although a variety of inflammatory and immunologic diseases affect the skin, two relatively common causes of inflammatory ulcers are vasculitis and pyoderma gangrenosum.
Vasculitis
Vasculitis is defined as inflammation and necrosis of the blood vessels, which can ultimately result in end-organ damage—in this case, the skin.4 Although about half of the cases are idiopathic,5 vasculitis is a reaction pattern that may be triggered by certain reactants, among which are underlying infections, malignancy, medications, and connective tissue diseases (Table 20-2). Clinically, vasculitis varies depending on the size of the underlying vessel affected. For example, lesions may include a reticulated erythema due to disease of the superficial cutaneous plexus, or they may present as widespread palpable purpura, necrosis, and ulceration due to disease in larger, deeper vessels (Fig. 20-1). Often, vasculitic lesions on the skin are just one component of the patient’s pathology—they may also have involvement of different end organs such as the kidney, lung, central nervous system, and gastrointestinal tract.6
Table 20-2 Potential Etiologies of Vasculitis

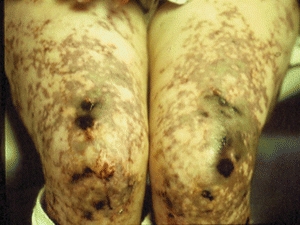
Figure 20-1 Vasculitis. This photograph shows reticulated erythema and necrotic ulcers on the thighs of a patient with vasculitis.
Circulating immune (antibody–antigen) complexes, which deposit in blood vessel walls, are the cause of many types of vasculitis.7 Tissue biopsies will confirm the presence of vasculitis if performed early, and biopsies of perilesional skin may detect the type of immunoglobulin involved in the process. Biopsies performed later in the course of lesion development may fail to reveal immunoreactants or inflammatory cells, and their by-products will degrade immunoglobulins. Tissue culture may be helpful if the vasculitis is due to an infectious process. A histologically confirmed diagnosis mandates evaluation of other organ systems and an attempt to determine the etiologic factor.
If identified—and if possible—the causative agent should be addressed. Additionally, treatment of the vasculitis is based on the extent of the disease (Table 20-3). Mild disease that is limited to the skin can be treated with supportive care, such as leg elevation and dressings. Treatments with limited adverse effects, such as colchicine, dapsone, antihistamines, or nonsteroidal anti-inflammatory agents, also may be used. If skin disease is extensive or systemic involvement is present, more aggressive treatment such as systemic steroids, anti-inflammatory agents, or immunosuppressants may be needed8 (Table 20-4).
Table 20-3 Diagnostic Tests for Vasculitis
Table 20-4 Vasculitis Treatment Options
Pyoderma Gangrenosum
The term pyoderma gangrenosum actually refers to a disorder that is neither infectious nor gangrenous. Rather, it is an inflammatory process causing painful skin ulcers of which the etiology is still not fully understood. It is believed to be due to dysregulation of the innate immune system and alterations in neutrophil chemotaxis. Pyoderma gangrenosum is characterized by the appearance of one or more chronic ulcerations with violaceous undermined borders9 (Fig. 20-2). It mainly affects adults, and its usual course is that of recurring, destructive ulcers, which begin as pustules and resolve with cribriform scars. Several clinical variants of pyoderma gangrenosum have been described, including ulcerative, pustular, bullous, vegetative, and peristomal types.
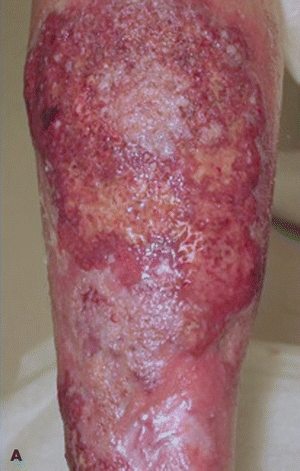
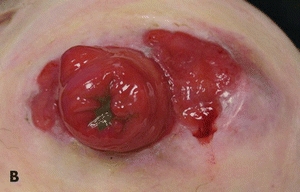
Figure 20-2. (A) Pyoderma gangrenosum in a patient with inflammatory bowel disease and pyoderma gangrenosum, this ulcer on the lateral leg shows areas of cribriform scarring. (B) Peristomal pyoderma gangrenosum.
 Practice PointPractice Point
Practice PointPractice Point
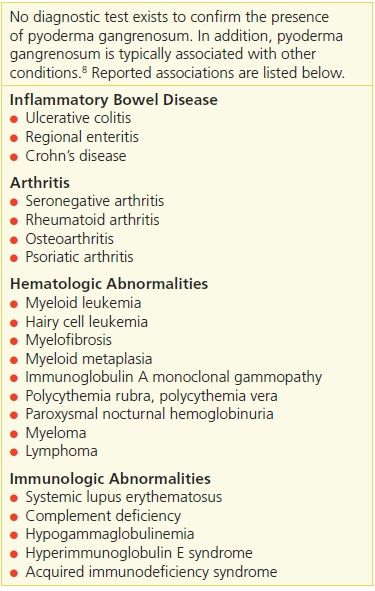
Because a diagnostic test to confirm pyoderma gangrenosum does not exist and a number of other conditions may resemble it clinically, a correct diagnosis relies on the clinical presentation and exclusion of other causes.
It is important for the clinician to search for underlying diseases when a diagnosis of pyoderma gangrenosum is rendered because it is associated with other conditions in more than 50% of patients10 (Table 20-5). Among these are inflammatory bowel disease, arthritis (seropositive and seronegative), monoclonal gammopathies, and other hematologic disorders and malignancies. Pyoderma gangrenosum lesions can occur around the stoma in persons with inflammatory bowel disease11 (Fig. 20-2).
Table 20-5 Systemic Diseases Associated with Pyoderma Gangrenosum
Pathergy (the development of lesions in areas of trauma) plays a role in the development of pyoderma gangrenosum wounds. In susceptible people, even minimal trauma to the skin can result in the production of pyoderma gangrenosum lesions, such as pustules or ulcers.
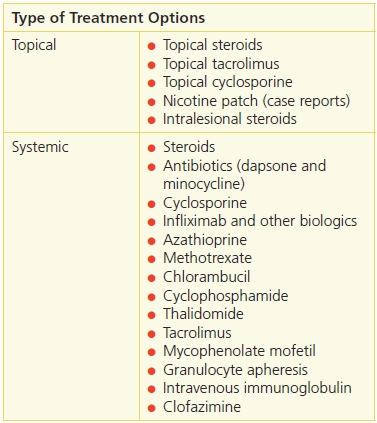
Curative treatment does not exist. The course of pyoderma gangrenosum waxes and wanes; however, corticosteroids usually are helpful.12 For limited or mild disease, topical or intralesional steroids may be used. For more severe or widespread disease, systemic steroids can be used, although their adverse effects limit long-term use. In addition, immunosuppressant or anti-inflammatory agents may also be of value; for example, cyclosporine also appears quite effective in treating this disorder (Table 20-6). Infliximab, a monoclonal antibody to tumor necrosis factor-α, has been shown to be useful for patients with pyoderma gangrenosum in a controlled, randomized, double blinded study.13 Infliximab is FDA approved to treat Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and ulcerative colitis.
Table 20-6 Pyoderma Gangrenosum Treatment Options
Infectious Causes
Infectious causes of atypical wounds may be due to a variety of different organisms, some of which are not commonly encountered in the United States. For example, atypical mycobacterial infections (other than leprosy and tuberculosis) and fungal infections (other than dermatophytes and Candida) occasionally are detected upon diagnostic testing. Infection caused by Vibrio vulnificus may be responsible for lower leg ulcers in geographic areas where there is warm salt water.
Atypical Mycobacterial Infections
Atypical mycobacteria are ubiquitous in the environment and were not generally viewed as human pathogens until the 1950s, when several cases of disease caused by these organisms were reported.14 Cutaneous infection usually results from exogenous inoculation, and predisposing factors include a history of preceding trauma, immunosuppression, or chronic disease. While Mycobacteria marinum is the most common agent of skin infection by atypical mycobacteria,15 many others have been reported in recent decades (Table 20-7). The cutaneous lesions vary depending on the causative agent and may present as granulomas, small superficial ulcers, sinus tracts, or large ulcerated lesions localized in exposed areas (Fig. 20-3).
Table 20-7 Mycobacterium Species That Cause Skin Ulcers
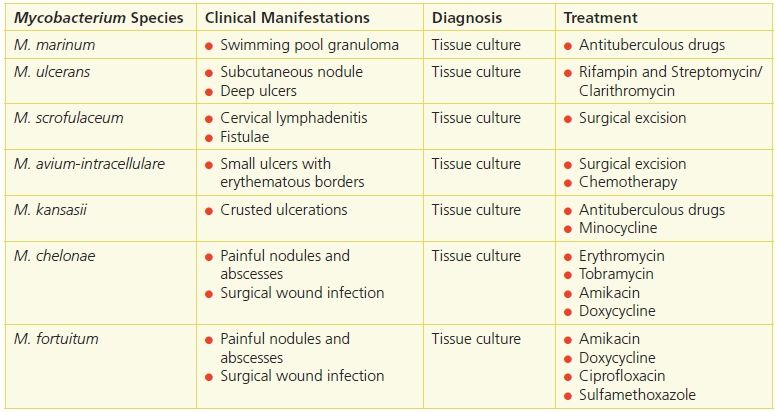
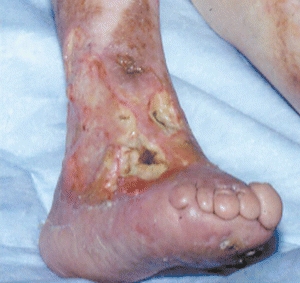
Figure 20-3 Hansen’s disease. Shown here are the leg and foot of a patient with Hansen’s disease caused by Mycobacterium leprae. In addition to neuropathic changes of the toes and plantar aspect of the foot, this patient has a large lateral leg ulcer.
Histologically, mycobacterial infections present as granulomas and abscesses that are difficult to distinguish from those of leprosy and cutaneous tuberculosis. Diagnosis invariably will depend on tissue culture or more recent techniques, such as polymerase chain reaction and gene rearrangement studies.
The appropriate therapy will depend on the causative agent because susceptibility to antibiotics varies. In some cases, simple excision of the cutaneous lesions or a combination of excision and systemic treatment often is most beneficial to the patient.
Buruli Ulcer
Buruli ulcer is a health problem in the tropical areas of many developing countries. Since 1980, Buruli ulcer has emerged as an important cause of human suffering. It was first encountered in 1897 when Sir Albert Cook described large ulcers in patients in Uganda. In the late 1940s, MacCallum identified an organism similar to Mycobacterium ulcerans in an ulcer in a 15-year-old in Bairnsdale, Australia. Large ulcers were subsequently observed in patients living along the Nile River in Buruli County, Uganda, in the 1960s, hence the name of the disease. Buruli ulcer is caused by M. ulcerans, from the family of bacteria that causes tuberculosis and leprosy.
The World Health Organization (WHO) has defined Buruli ulcer as an infectious disease of the skin and subcutaneous tissue characterized by painless nodules, papules, plaque, or edema evolving into a painless ulcer with undermined edges and edema. Progression of the disease is associated with extensive sloughing and massive ulceration, particularly over joints, that may lead to contractures. Significant areas of the torso, face, or an entire limb may be involved. Amputation is often required when a limb is involved.16
Extensive cutaneous ulcers occur as the disease progresses from the early, more treatable, nodular form to the ulcerative form, which can result in the loss of skin and soft tissue. Although Buruli ulcer is rarely fatal, the associated disabilities and disfigurement are extensive and have a profound impact on quality of life in affected patients16,17 (Fig. 20-4).
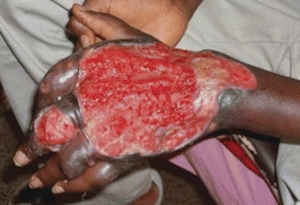
Figure 20-4 Buruli ulcer. This photo shows extensive sloughing and massive ulceration, typically leading to contractures and extensive disability and disfigurement.
The majority of those suffering from Buruli ulcer are between 5 and 15 years of age, but it can affect patients of any age. A prospective study showed that 8-week regimen of rifampicin and streptomycin (injectable) for patients with all stages of Buruli ulcer led to healing in 93% of patients without the need for surgery.18 A more recent prospective study determined that clarithromycin (oral) could replace the injectable streptomycin after 2 weeks of standard rifampin/streptomycin treatment without loss of efficacy.19
Healing often is slow and can be associated with significant functional incapacity due to contractures and amputation. The course of disease can be complicated by the ability of the organism to suppress the immune system of the host. Disease progression is related directly to a decrease in the level of interferon-γ, a Th1 cytokine, as well as a concomitant increase in the Th2 cytokine interleukin-10.16 Reversing the deficiency of interferon-γ and/or decreasing the interleukin-10 level has been identified as a novel therapeutic target for interventional development.
The disease, its treatment, and the resulting disabilities are a significant burden to patients, their families, and their society. In some countries such as Ghana, patients and families are reluctant to seek treatment due to lack of money or misunderstanding that treatment can lead to amputation.17 Educational sessions for clinicians to recognize Buruli ulcer early and to change patient, family, and societal resistance to seeking early treatment have met with success, and the number of late cases of this disease in Ghana has declined over the past decade.16,17
Deep Fungal Infections
Deep fungal infections of the skin can be divided into subcutaneous and systemic mycoses. The subcutaneous mycoses result from traumatic implantation of the etiologic agent into the subcutaneous tissue, development of localized disease, and eventual lymphatic spread. In rare instances, hematogenous dissemination can occur, especially in immunocompromised hosts. As may occur with sporotrichosis or chromomycosis, ulcers from deep fungal infections are found worldwide and can present in a wide variety of clinical settings.20
Systemic mycoses are the result of systemic penetration of pathogenic fungi, with the lungs being the most common port of entry. These infections are restricted to the geographic areas where the fungi occur, especially tropical regions such as Central and South America. After an initial pulmonary infection, the fungi can spread hematogenously or via lymphatic vessels to other organs, including the skin. A decrease in immunity will lead to expression of the fungal infection, as is commonly observed in patients infected with human immunodeficiency virus (HIV).
 Practice PointPractice Point
Practice PointPractice Point
A thorough patient history assists the clinician in considering a diagnosis of systemic mycosis because of the limited area in which the causative fungi occur.
Sporotrichosis
Sporotrichosis is a subacute or chronic fungal infection caused by the fungus Sporothrix schenckii. Occurring as a consequence of traumatic implantation of the fungus into the skin, it is often associated with lymphangitis. Less commonly, inhalation of the conidia can lead to pulmonary infection and subsequent spread to the bones, eyes, central nervous system, and viscera. Systemic disease is seen in individuals with impaired immunity, such as alcoholics and AIDS patients.21
Sporothrix schenckii, a saprophyte in the environment, has been isolated in a variety of plants and other fauna as well as in animals (bites or scratches from animals, such as armadillos and cats). Individuals whose professional or leisure activities (such as rose gardening or landscaping) expose them to the environment are at greater risk of acquiring the infection. Lymphocutaneous sporotrichosis begins as a papule, which then ulcerates with time. The lesions spread and occur via lymphatic channels traveling from the inoculation site. Sporotrichosis is treated with systemic medications, including saturated solution of potassium iodide, itraconazole, terbinafine, and amphotericin B. Topically applied heat also may be used because the organism grows at low temperatures.
Chromoblastomycosis
Chromoblastomycosis is a subcutaneous mycosis caused by several pigmented fungi, including Fonsecaea pedrosoi, Fonsecaea compacta, Phialophora verrucosa, Cladosporium carrionii, and Rhinocladiella aquaspersa. These fungi are acquired through inoculation of the causative agents in the skin, after which a mycotic infection develops at the site of entry. These microorganisms can be found in soil throughout the world; however, the disease is most common in tropical and subtropical climates, with the majority of cases seen in South America.22
Primarily affecting men ages 30 to 50, the principal lesion is a slow-growing papule that eventuates into a verrucous nodule. Exposed areas are involved, with extremities—especially the lower limbs—being affected in 95% of the cases.20 The surface of the lesion may be covered by scales or may be ulcerated with serosanguineous crusts. Black dots can be often observed; these dots are rich in fungi and represent the site of transepidermal elimination of necrotic tissue.
Diagnostic examinations should include scrapings from the lesion with potassium hydroxide 20%; tissue samples should be obtained from biopsies for tissue culture and histology.
The disease tends to be chronic and difficult to treat and may lead to lymphedema and elephantiasis. Ulcerated and cicatricial lesions have been reported to develop into carcinoma. Small lesions can be cured by surgical excision; however, chronic lesions are often resistant to treatment.
Systemic antifungal agents, such as ketoconazole, itraconazole, terbinafine, and amphotericin B, have been used, both alone and in combination, with variable results. Oral flucytosine, alone or in combination with an antifungal, has also been reported to be therapeutic.22 The new-generation triazole antifungals show promise, as demonstrated by the positive results with posaconazole, although they may be prohibitively expensive.23,24 Cryosurgery has also been used alone and in combination with antifungal chemotherapy. In addition, local heat therapy of 42°C to 45°C can also be an effective therapeutic modality.
Paracoccidioidomycosis
Paracoccidioidomycosis (South American blastomycosis) is a chronic, infectious disease caused by the fungus Paracoccidioides brasiliensis, a saprophyte of soil and decaying vegetation found in tropical and subtropical climates. Infection occurs primarily via a respiratory route with occasional dissemination to other organs, including the skin. Rarely, direct inoculation into the skin can occur. Patients present with painful ulcerative lesions of the mouth, the face or, less frequently, the extremities. Involvement of regional lymphatics is characteristic.25
Diagnosis can be established by isolation and identification of the etiologic agent with direct mycologic examination, histopathologic or cytopathologic examination, or culture. Treatment includes trimethoprim—sulfamethoxazole, azole derivatives such as itraconazole and ketoconazole, and amphotericin B for severe cases.
Mycetoma
Mycetoma is a chronic infection of the skin and subcutaneous tissue characterized by local edema, sinus tract formation, and the presence of grains—hard concretions representing colonies of the etiologic agent. It occurs worldwide, but most commonly in tropical and subtropical regions. Mycetomas can be divided into eumycetomas, caused by fungi, and actinomycetomas, caused by actinomycetes. The most common agent in Central and South America is the bacteria Nocardia brasiliensis, which is found in soil.26 This agent is rarely found in the United States but when it does occur, the fungus Pseudallescheria boydii is the most commonly isolated agent.
Male rural workers ages 20 to 40 are most frequently affected. After trauma, a slow-growing, painless nodule develops, which may discharge purulent material and grains. Neighboring lesions may interconnect with each other, giving rise to the sinus tracts that are characteristic of the disease.
Diagnosis can be established based on clinical findings; additional examinations may include visualization of grains or filaments in discharge or biopsy and tissue culture. On ultrasonographic evaluation, the mycetoma grains, capsules, and the resulting inflammatory granulomas have characteristic appearances.27 Treatment is difficult. Surgical excision, commonly in combination with systemic therapy, may be effective. Sulfonamides, tetracycline, aminoglycosides, rifampin, ciprofloxacin, amoxicillin, clavulanate, and oral azoles may be used depending on sensitivities of the etiologic agent. Recent preliminary evidence suggests that linezolid, imipenem, and the newer triazoles voriconazole and posaconazole also may be efficacious.28
Vibrio Vulnificus Infection
Vibrio vulnificus, a bacterium, is found widely in raw shellfish in Atlantic Coast waters.29 It produces extracellular proteolytic and elastolytic enzymes and collagenases that favor tissue invasiveness. Wound infection with V. vulnificus occurs when contaminated seawater enters the body through a break in the epidermal barrier, commonly during fishing or water sport activities. Breaks in the skin relating to opening oysters is a common method of inoculation. Pustular lesions, lymphangitis, lymphadenitis, and cellulitis may ensue; in some cases, rapid progression to myositis and skin necrosis follows. Treatment of V. vulnificus wound infections consists of antibiotics, such as the combination of minocycline or doxycycline and ceftazidime, and wound care.30
Primary septicemia from V. vulnificus occurs 24 to 48 hours after the ingestion of raw oysters, especially in patients with hepatic cirrhosis, diabetes, renal failure, or immunosuppression. Clinically, fever and hypotension may be present, along with the development of bullous cellulitis and necrotic skin ulcers.
Necrotizing Fasciitis
Necrotizing fasciitis (NF) is an uncommon but life-threatening soft tissue infection characterized by rapidly spreading inflammation and necrosis of the skin, subcutaneous fat, and fascia31 (Fig. 20-5). Mortality rates have exceeded 70% in the past,32 although recent data have shown a dramatic decrease, with mortality rates below 10%.33 Rapid, early intervention, including surgical debridement and antimicrobial therapy, is imperative to reduce morbidity and mortality.
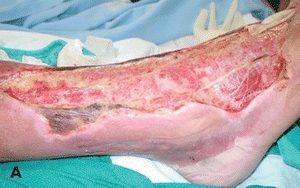
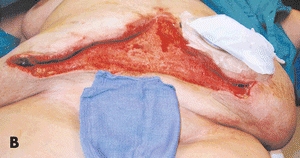
Figure 20-5. (A) Necrotizing fasciitis of the foot. (B) Necrotizing fasciitis of the abdomen.
The incidence of NF has been reported to be 0.40 cases per 100,000 population.33 Although it is rare, certain conditions can predispose patients to developing the disease, including immunocompromised states such as diabetes mellitus (the most common), acquired immunodeficiency syndrome (AIDS), malignancy, obesity, peripheral vascular disease,34 and trauma, such as burns, lacerations, or minor trauma (Table 20-8).
Table 20-8 Conditions Leading to Necrotizing Fasciitis

NF spreads quickly along the fascia due to the relatively poor blood supply to this area. Thus, initially, overlying tissue may appear healthy even though the infection is rapidly spreading. NF is categorized as type 1, type 2, or type 3 depending on which organisms are cultured. Type 1 NF is caused by a polymicrobial infection from aerobic and anaerobic bacteria such as Clostridium and Bacteroides species. Type 2 NF consists of group A Streptococcus (Streptococcus pyogenes) with or without a coexisting staphylococcal infection. Type 3 NF is caused by a Vibrio infection from a puncture wound due to fish or marine insects.
NF can affect any area of the body, but it most commonly occurs on the extremities. Involvement of the genitalia is referred to as Fournier’s gangrene and usually results from a polymicrobial infection.35 There is a higher mortality rate when the head, neck, chest, and abdomen are involved because those areas tend to be refractory and, as such, are more difficult to treat.
Early on, patients generally present with a clinical picture similar to cellulitis. As with less aggressive cases of cellulitis, redness and edema can be seen at the site, with a spreading, diffuse inflammatory reaction that blends into the surrounding tissue. The overlying skin is shiny and tense without any clear lines of demarcation. However, patient complaints of severe pain are usually out of proportion to the clinical lesion. This characteristic clue of NF may be the only hint of a deeper, more aggressive infection. It is critical to be alert for this sign because the earlier the diagnosis is made, the earlier treatment can be instituted and the better the chance of survival will be. Treatment involves immediate surgical intervention to remove infected and necrotic tissue in addition to systemic antibiotic therapy.36
Over time, frank cutaneous gangrene may extend beyond the skin and into the subcutaneous fat and fascial planes below. Separation of the necrotic tissue along the fascial planes with suppuration may occur. Myonecrosis develops in the underlying muscle. Lymphadenitis, lymphangitis, crepitation, and venous thrombosis are seen less often.
Metastatic abscesses have been reported in the liver, lung, spleen, brain, and pericardium, but these are rare. In addition to cutaneous manifestations of NF, there are systemic findings as well as those associated with progression of disease. Patients usually will appear toxic with high fever, chills, and constitutional symptoms. In fulminant cases, multiorgan system failure occurs.
Vasculopathies
A heterogeneous group of disorders is classified under this category. Vasculopathy is characterized by occlusion of small vessels within the skin due to thrombi or emboli, which leads to tissue hypoxia and the clinical manifestations of purpura, livedo reticularis, and painful ulcers. Cryofibrinogenemia, monoclonal cryoglobulinemia, and antiphospholipid antibody syndrome (APS) are among the causes of vasculopathies that commonly present as atypical skin ulcerations of the lower extremities.
Cryofibrinogenemia
Cryofibrinogenemia occurs as a primary (idiopathic) disorder or in association with underlying diseases, such as infectious processes, malignancy, or collagen, vascular, or thromboembolic disease. The clinical presentation is painful cutaneous ulcerations located on the leg and foot; these lesions are usually unresponsive to treatment (Fig. 20-6). Other cutaneous findings include livedo reticularis (a netlike erythema), purpura, ecchymoses, and gangrene. The pathogenesis of the lesions is related to the in vivo occlusion of small blood vessels initiated in the distal extremities by the abnormal precipitate. This hypothesis is corroborated by the pathology findings of cryofibrinogen, consisting of thrombi within superficial dermal vessels due in part to protein deposition. Cryofibrinogen is a circulating complex of fibrin, fibrinogen, and fibronectin along with albumin, cold-insoluble globulin, and factor VIII. The complex is soluble at 98.6°F (37°C) but forms a cryoprecipitate at 39.2°F (4°C).37 Additionally, this complex can be made to clot with thrombin. The mechanism by which cryofibrinogen is produced is not well understood.
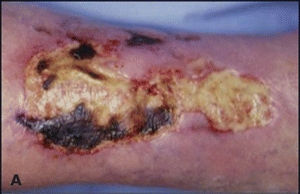
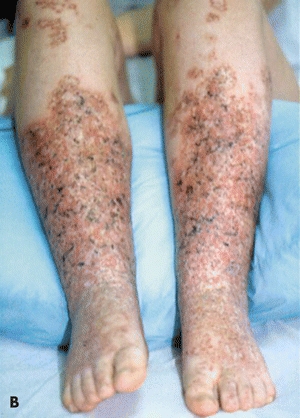
Figure 20-6. (A) Cryofibrinogenemia. (B) This patient has painful punctate ulcers on the feet and legs secondary to cryofibrinogenemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


