Assisting in the Clinical Laboratory
Learning Objectives
1. Define, spell, and pronounce the terms listed in the vocabulary.
2. Apply critical thinking skills in performing the patient assessment and patient care.
5. Explain the three CLIA regulatory categories.
7. Compare and contrast the agencies that govern or influence practice in the clinical laboratory.
9. Describe the essential elements of a laboratory requisition.
10. Display sensitivity to patients’ rights and feelings in collecting specimens.
11. Explain chain of custody and illustrate why it is important.
12. Compare and contrast quality assurance and quality control.
13. Describe the differences between Greenwich time and military time.
14. Identify the Fahrenheit temperature and the Celsius temperature of common laboratory equipment.
15. Name the metric units used for measuring liquid volume, distance, and mass.
16. Describe the proper use of pipets.
17. Explain how dilutions are prepared.
18. Name the parts of a microscope, and describe their functions.
19. Summarize selected microscopy tests that can be performed in the ambulatory care setting.
20. Demonstrate proper use of the microscope.
21. Describe the safe use of a centrifuge.
22. Identify legal and ethical issues in the clinical laboratory setting.
Vocabulary
aliquot (a′-luh-kwaht) A portion of a well-mixed sample removed for testing.
analyte The substance or chemical being analyzed or detected in a specimen.
anticoagulants Chemicals added to a blood sample after collection to prevent clotting.
caustic (kos′-tik) Capable of burning, corroding, or damaging tissue by chemical action.
cytology (si-tah′-luh-je) The study of cells using microscopic methods.
diluent (dil-yuh′-wunt) A liquid used to dilute a specimen or reagent.
exudates (ek′-syu-dats) Fluids with high concentrations of protein and cellular debris that have escaped from the blood vessels and have been deposited in tissues or on tissue surfaces.
hemolyzed Term used to describe a blood sample in which the red blood cells have ruptured.
preservatives Substances added to a specimen to prevent deterioration of cells or chemicals.
specimen A sample of body fluid, waste product, or tissue that is collected for analysis.
Scenario
Marsha Rollins has been employed for 3 years as a certified medical assistant in a medical practice. The physicians have a medical laboratory on site, and Marsha has become experienced in collecting specimens, performing laboratory tests, and reporting results. Recently she was offered a position in a smaller practice closer to home; she has accepted the position, knowing that her experience will benefit the practice because the physicians would like to expand their on-site medical laboratory testing, and Marsha will be required to equip the laboratory.
While studying this chapter, think about the following questions:
• What regulations will guide the testing that will be performed in the lab?
• What equipment will she need and how will she ensure that it remains in good working order?
Laboratory medicine, or clinical pathology, is the medical discipline that applies clinical laboratory science and technology to the care of patients. The laboratory is the place in which a collected specimen is analyzed and evaluated. Tests are performed manually (by hand) or through automation (with the use of specialized instruments).
Role of the Clinical Laboratory in Patient Care
Personnel in the Clinical Laboratory
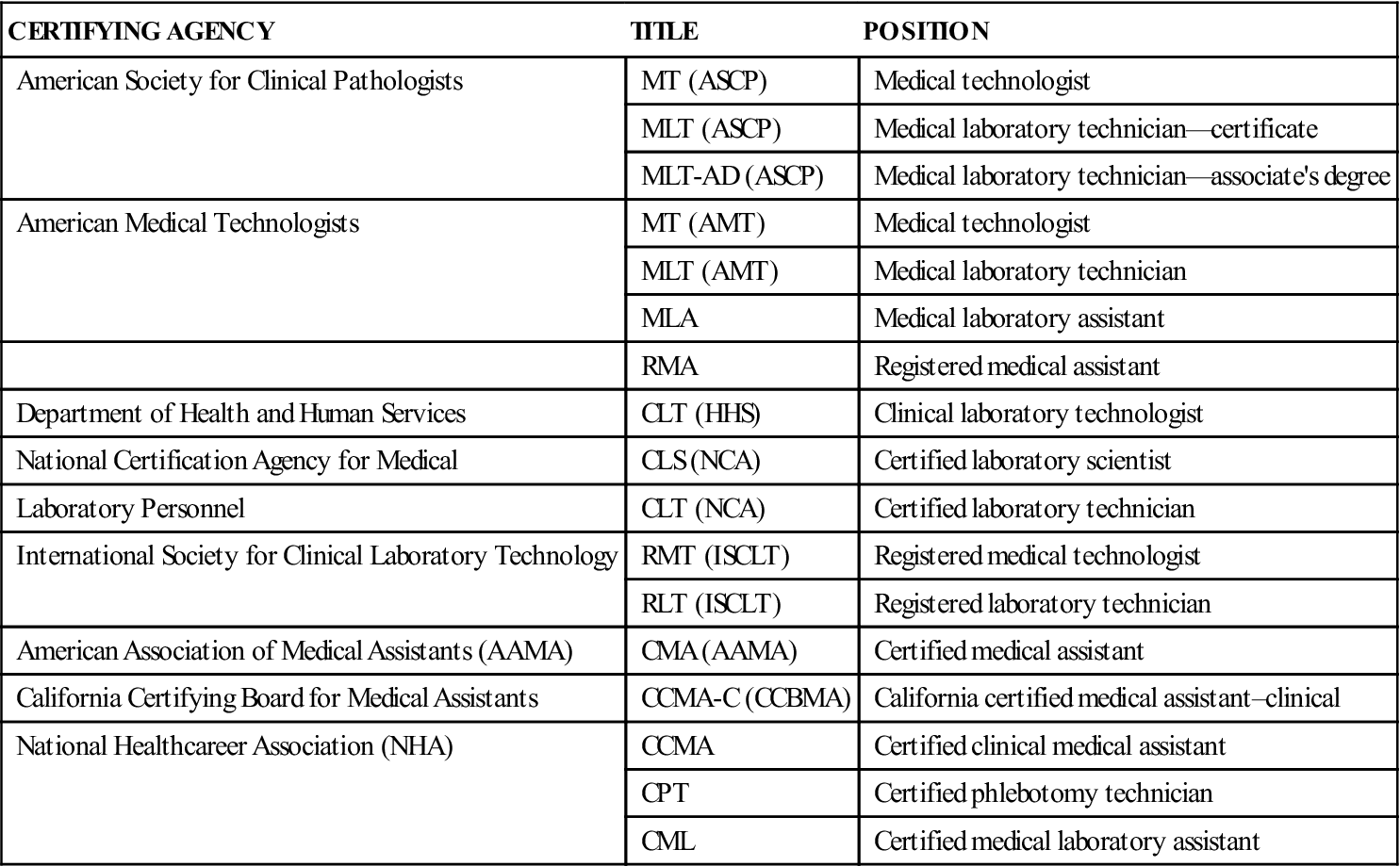
Medical laboratories are located in hospitals or in facilities such as physicians’ offices, clinics, public health departments, health maintenance organizations, and private referral laboratories. The director of a laboratory may be a pathologist, a physician specially trained in the nature and cause of disease, or a clinical laboratory scientist with a doctorate. The laboratory is staffed by various professionally trained individuals, including certified medical technologists (MTs), who have earned a baccalaureate degree, have had additional formal training, and have passed a national certification examination. Other personnel include certified medical laboratory technicians (MLTs) or medical laboratory assistants (MLAs) and certified medical assistants (CMAs). These employees have completed a 1- to 2-year specialized training program and have passed a registry examination. Laboratory assistants and phlebotomists, who have received specialized training in the collection and preparation of laboratory specimens, also work in laboratories. The agencies granting certifications and titles are described in Table 51-1.
TABLE 51-1
Certifying Agencies for Laboratory Personnel
| CERTIFYING AGENCY | TITLE | POSITION |
| American Society for Clinical Pathologists | MT (ASCP) | Medical technologist |
| MLT (ASCP) | Medical laboratory technician—certificate | |
| MLT-AD (ASCP) | Medical laboratory technician—associate’s degree | |
| American Medical Technologists | MT (AMT) | Medical technologist |
| MLT (AMT) | Medical laboratory technician | |
| MLA | Medical laboratory assistant | |
| RMA | Registered medical assistant | |
| Department of Health and Human Services | CLT (HHS) | Clinical laboratory technologist |
| National Certification Agency for Medical | CLS (NCA) | Certified laboratory scientist |
| Laboratory Personnel | CLT (NCA) | Certified laboratory technician |
| International Society for Clinical Laboratory Technology | RMT (ISCLT) | Registered medical technologist |
| RLT (ISCLT) | Registered laboratory technician | |
| American Association of Medical Assistants (AAMA) | CMA (AAMA) | Certified medical assistant |
| California Certifying Board for Medical Assistants | CCMA-C (CCBMA) | California certified medical assistant–clinical |
| National Healthcareer Association (NHA) | CCMA | Certified clinical medical assistant |
| CPT | Certified phlebotomy technician | |
| CML | Certified medical laboratory assistant |
The medical assistant is trained to perform certain testing procedures, as well as in methods of collecting specimens that are sent to outside reference laboratories for testing. Laboratory tests are an essential part of a medical diagnosis, and they help the physician determine the most appropriate treatment. In addition, they may be performed to help the physician decide which medication to prescribe and to monitor the effects of medications. Only healthcare practitioners may request laboratory testing for a patient. The medical assistant may be responsible for a number of these testing procedures. To assume this responsibility, the medical assistant must know proper patient preparation, the procedures for each test, and the normal range of results for the test. The medical assistant must carefully follow all laboratory instructions in obtaining and labeling specimens and sending them to the laboratory. Good communication among the patient, the office staff, and laboratory personnel is important. The medical assistant should make the patient feel at ease with these procedures and thus gain the patient’s cooperation.
Clinical Laboratory Testing
Clinical laboratory testing is used in conjunction with a thorough health history and physical examination to obtain essential data for the diagnosis and management of a patient’s condition. The body is considered to be healthy when a state of equilibrium exists in the internal environment. In this state, called homeostasis, the physical and chemical characteristics of body substances (e.g., fluids, secretions, excretions) are within a certain acceptable range, known as the normal or reference range. A change in homeostasis results in abnormal test values (i.e., outside the reference range). Abnormal values for a particular test may be seen with more than one pathologic condition. For example, a decrease in hemoglobin levels in red blood cells (RBCs) is seen in iron-deficiency anemia, but also in hyperthyroidism and cirrhosis of the liver. Therefore, physicians cannot rely solely on laboratory tests to make a diagnosis; they must use a combination of data obtained from the health history and physical examination, and a number of diagnostic and laboratory results.
Tests performed in a clinical laboratory range from simple screening tests to complex profile testing. A screening test examines a particular specimen for the presence of a substance that may indicate a disease state. These types of tests are not diagnostic for any particular disease, but rather indicate that the disease state may exist. Screening tests are done routinely on patients on the basis of their age, history, or gender. They often are qualitative in that a numeric value is not attached to the result; results may simply be reported as positive or negative. The fecal occult blood test for hidden or microscopic blood in the stool is an example of a screening test. Blood is not normally found in the stool, and its presence may indicate a cancerous lesion in the colon. A positive test result indicates that blood is present, but additional testing is required to determine the source of the blood. For example, further testing or examination may reveal that the patient had her menstrual period at the time of collection of the specimen, or that she had bleeding hemorrhoids.
In a quantitative test, units of measure are attached to numeric values. These values often are represented as the amount of analyte per given volume of specimen, and it is essential that the results be reported with the units of measure. For example, in a complete blood cell count for a healthy adult, the RBCs number 5 million per cubic millimeter (5 × 106/mm3), the hemoglobin value is 15 grams per deciliter (15 g/dL), and the hematocrit is 45%. Generally the units are printed on the laboratory report, but the medical assistant must always make sure that the values are consistent with the test performed.
Clinical Laboratory Improvement Amendments
In 1988 Congress passed the Clinical Laboratory Improvement Amendments (CLIA), establishing quality standards for all laboratory testing to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test is performed. A laboratory is defined as any facility that performs laboratory testing on specimens derived from humans for the purpose of providing information about the diagnosis, prevention, and treatment of disease or impairment in or assessment of health. The CLIA program is user-fee funded; therefore, all costs of administering the program must be covered by the regulated facilities. CLIA requires that all entities that perform even one test, including waived tests, must meet certain federal requirements and must register as a laboratory. An application must be submitted that reports information about a laboratory’s operation. The type of certificate to be issued and the fees to be assessed are determined from this information.
The CLIA categorization of commercially marketed in vitro diagnostic tests is now the responsibility of the U.S. Food and Drug Administration (FDA). The FDA has assumed primary responsibility for performing the CLIA complexity categorization functions, which include the process of assigning commercially marketed in vitro diagnostic test systems to one of three CLIA regulatory categories on the basis of their potential risk to public health: waived tests, moderate-complexity tests, and high-complexity tests.
Waived Tests
Waived tests (Table 51-2) include the following:
TABLE 51-2
CLIA-Waived Tests and Their Purposes
| CPT CODE(S) | TEST | PURPOSE |
| 81002, 81003QW | Dipstick or tablet reagent urinalysis (nonautomated) for bilirubin, glucose, hemoglobin, ketone, leukocytes, nitrite, pH, protein, specific gravity, urobilinogen | Urine screening to assess or diagnose diseases such as diabetes mellitus, kidney disease, and urinary tract infection |
| 81025 | Urine pregnancy tests: visual color comparison tests | Diagnosis of pregnancy |
| 81002 | Urine chemistry analyzer: automated urine dipstick analysis | Urine screening to assess or diagnose diseases such as diabetes mellitus, kidney disease, and urinary tract infection |
| 81002 | Urine chemistry analyzer for microalbumin and creatinine | Detection of kidney disease |
| 84830 | Ovulation tests: visual color comparison tests for luteinizing hormone | Detection of ovulation |
| 82270 82272 | Fecal occult blood | Colorectal screening to detect hidden blood in the stool |
| 85651 | Erythrocyte sedimentation rate, nonautomated | Diagnosis of inflammatory process; increases in presence of arthritis, infection, leukemia, and most cancers |
| 83026 | Hemoglobin-copper sulfate, nonautomated | Measurement of blood hemoglobin levels |
| 82947QW, 82950QW | HemoCue Hemoglobin System | Measurement of hemoglobin level in whole blood |
| 82962 | Blood glucose by glucose-monitoring devices cleared by the FDA specifically for home use | Monitoring of blood glucose levels |
| 85018QW | HemoCue B | Measurement of glucose levels in whole blood |
| 85013 | Spun microhematocrit | Measurement of blood count; screening for certain types of anemia |
| 82947QW, 82950QW, 82951QW | STAT-CRIT hematocrit | Screening for certain types of anemia |
| 83036QW | Hemoglobin and hemoglobin A1c by single analyte instruments with self-contained or component features to perform specimen-reagent interaction | Measurement of A1c levels to assess and manage long-term care of patients with diabetes |
| 82465QW | Cholestech LDX | Measurement of total blood cholesterol, triglycerides, HDL, and glucose levels |
| 86308QW | Blood mononucleosis antibodies | Rapid, qualitative test to detect antibodies to help diagnose infectious mononucleosis |
| 86318QW | Helicobacter pylori antibodies | Rapid whole-blood test to detect H. pylori antibodies to determine the cause of peptic ulcer |
| 86618QW | Borrelia burgdorferi antibodies | Rapid whole-blood test to detect B. burgdorferi antibodies to diagnose Lyme disease |
| 86701QW | Whole-blood OraSure HIV-1 test | Detection of HIV-1 in blood specimen |
| 87804QW | Nasal influenza A and B | Quick qualitative diagnosis of influenza antigens in nasal secretions or swab |
| 87449QW; 87880QW | Streptococcus A throat swab | Rapid strep test |
| 80047QW 82330QW 82374QW | Whole-Blood i-STAT Chem8+ Cartridge | Measures ionized calcium, carbon dioxide, chloride, creatinine, glucose, potassium, sodium, urea nitrogen, and hematocrit in whole blood |
| G0434QW | Urine and/or blood | Multiple tests for the presence of a variety of substance abuse agents |
| 83001QW | Urine fertility and menopause | Detects follicle-stimulating hormone in urine |
| 84443QW | Whole-blood thyroid-stimulating hormone (TSH) assay | Qualitative determination of TSH in whole blood |
From Centers for Medicare and Medicaid Services. http://www.cms.gov/CLIA/downloads/waivetbl.pdf.
Laboratory examinations and procedures that have been approved by the Food and Drug Administration for home use or that, as determined by the Secretary, are simple laboratory examinations and procedures that have an insignificant risk of an erroneous result, including those that (A) employ methodologies that are so simple and accurate to render the likelihood of erroneous results by the user negligible, or (B) the Secretary has determined pose no unreasonable risk of harm to the patient if performed incorrectly.
A CLIA database is available to the public on the Internet. This database contains the commercially marketed in vitro test systems categorized by the FDA since January 31, 2000, and tests categorized by the Centers for Disease Control and Prevention (CDC) before that date. The records can be searched by test system name, specialty or subspecialty, analyte, document number, qualifier, effective date, and complexity.
Moderate- and High-Complexity Tests
The CLIA program oversees the quality of nearly 200,000 different laboratory procedures. An estimated 10,000 different laboratory tests are performed in the United States every day; 75% of them are categorized by the FDA as moderate-complexity tests. Some of these tests are performed in physician’s office laboratories (POLs), including hematology and chemistry testing done on an automated analyzer, Gram staining, and microscopic analysis of urine sediment. High-complexity tests usually are not performed in a POL; these include Papanicolaou (Pap) smear analysis, blood typing and cross-matching, and cytologic testing.
Laboratories that perform moderate- to high-complexity testing must meet CLIA regulations and are subject to unannounced inspections every 2 years. Each laboratory that performs these tests must establish a system to maintain the integrity and identification of patients’ specimens throughout the testing process and to ensure accurate reporting of results. The laboratory also must have established and must follow written quality control (QC) and quality assurance (QA) procedures and must participate in proficiency testing, a form of external quality control. Three times a year, the laboratory must test samples provided by an approved proficiency testing agency using the same tests the laboratory would use to test a patient’s sample. Finally, CLIA regulations specify qualifications and responsibilities for personnel in the laboratory, from directors to testing personnel. Personnel requirements are most stringent for high-complexity testing.
Medical assistants may perform all CLIA-waived tests and some moderately complex tests, depending on the certification of the laboratory or POL in which they are employed. Although medical assistants may not perform high-complexity tests, they often are involved in collecting the specimens required, preparing the patient for the test, and recording the results in the medical record.
Divisions of the Clinical Laboratory
The laboratory is divided into various departments, which may include hematology, chemistry, microbiology, specimen collection and processing, blood bank, coagulation, serology, histology, cytology, toxicology, urinalysis, and special chemistry. The laboratory in the physician’s office usually performs procedures in urinalysis, hematology, chemistry, and microbiology.
Urinalysis
Urinalysis includes the physical, chemical, and microscopic examination of urine. In the physical examination, the color, clarity, and specific gravity are noted. Chemical analysis is performed to measure levels of such analytes as glucose, protein, ketones, blood, bilirubin, urobilinogen, nitrites, and pH. Microscopically the urine is examined for the presence of red, white, and epithelial cells, mucus, casts, crystals, yeasts, parasites, and bacteria. Additional quantitative tests may be performed in the urinalysis department to confirm routine screening tests.
Hematology
Tests performed in the hematology division may be qualitative or quantitative. Blood cell counts determine the exact number of RBCs or erythrocytes, white blood cells (WBCs, or leukocytes), or platelets (thrombocytes) either by manual or automated counting. Qualitative tests determine the characteristics of cells, such as size, shape, and maturity. In addition, the hematology department performs tests to determine the coagulating ability of blood components.
Chemistry
The clinical chemistry department analyzes blood, cerebrospinal fluid (CSF), urine, and joint fluid (synovial fluid). Procedures may include single tests or profiles, which include tests for a number of related analytes. Lipid profiles, for example, include assessments of total cholesterol, triglycerides, and low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol.
Microbiology
Microbiology involves the study of bacteria, fungi, yeasts, parasites, and viruses. In the microbiology laboratory, microorganisms are grown (cultured) from blood, urine, sputum, CSF, and wound specimens and are identified. Sensitivity testing then is performed on these organisms to determine the proper antibiotic therapy. Specimens for microbiology must be collected aseptically in sterile containers.
Laboratory Safety
The importance of safety in the laboratory cannot be overemphasized. Most laboratory accidents can be prevented through the use of proper techniques and common sense. Following safe practices in the laboratory requires a personal commitment and concern for others; an unsafe act may harm an innocent bystander without harming the person who performs the act.
Safety Standards and Governing Agencies
Safety standards for laboratories are initiated, regulated, and reviewed by several agencies or committees. These include the U.S. Department of Labor’s Occupational Safety and Health Administration (OSHA); the Clinical and Laboratory Standards Institute (CLSI, formerly the National Committee for Clinical Laboratory Standards), a nonprofit educational organization that provides a forum for the development, promotion, and use of national and international standards; the CDC, an agency of the U.S. Department of Health and Human Services; the College of American Pathologists (CAP), a leader in providing laboratory quality improvement programs; and the Environmental Protection Agency (EPA), a government agency charged with protecting human health and safeguarding the natural environment.
Through OSHA the government created a system of safeguards and regulations under the Occupational Safety and Health Act of 1970. This system affects nearly every worker in the United States, because the regulations apply to all businesses with one or more employees. (The regulations are discussed in detail in Chapter 27.) Two programs have been mandated by OSHA to ensure the safety of personnel working in clinical laboratories. One covers occupational exposure to chemical hazards; the other covers exposure to blood-borne pathogens. Both of these programs, as they relate to safety in the medical laboratory setting, are discussed later in this chapter.
Laboratory Hazards
Physical Hazards
Physical hazards in the laboratory can be classified as electrical, fire, and mechanical hazards. Electric shock is a threat when any electrical equipment is in use. It is imperative to keep all electrical equipment in proper repair and always to follow manufacturers’ instructions.
Use surge protectors, inspect all cords and plugs frequently, never use extension cords, and avoid overloading circuits. Unplug the electrical device before servicing, and never operate electrical instruments with wet hands. If a sink is nearby, make sure electrical cords do not come in contact with the water supply. Signs and labels should be placed on specific electrical hazards (Figure 51-1).
Open flames are rarely used in a laboratory, but the potential for fire still exists. Fires may be ignited by smoking, heating elements, and sparks. Flammable materials should not be stored near any source of ignition. All laboratory personnel should be familiar with the locations of fire extinguishers and fire safety blankets. Fire extinguishers should be the carbon dioxide (CO2), dry chemical, or halon type, known as the ABC type of extinguisher. ABC extinguishers can be used on all types of fires. These extinguishers should be inspected regularly by a licensed inspector and replaced or recharged if used. The medical assistant may be responsible for maintaining records on the care and maintenance of fire extinguishers.
Fire safety blankets should be used to smother flames on burning clothing. However, a victim should not be wrapped in a fire blanket, because this may intensify burns. Instead, the flames should be patted out or the victim directed to roll on the blanket.
Emergency phone numbers should be posted on the wall near the telephone, and all personnel should know the locations of fire alarms, the fire escape routes, and procedures to follow if exits are blocked. Periodic fire drills should be conducted, and hallways and exits should be kept free of clutter.
Mechanical hazards arise from the use of laboratory equipment. Special care should be exercised when using equipment with moving parts, such as centrifuges, and those that rely on pressure, including autoclaves. Centrifuges, devices that separate liquids from solids, present a hazard not only from moving parts but also from glassware that might break during centrifugation and from aerosols that might be created if tubes are not capped tightly. Pressurized types of equipment, such as autoclaves used in sterilization, present a danger if opened prematurely. Although centrifuges and autoclaves often have built-in safeguards, such as locks that prevent entry until the environment is safe, improper care of the equipment can result in failure of the safety measures.
Chemical Hazards
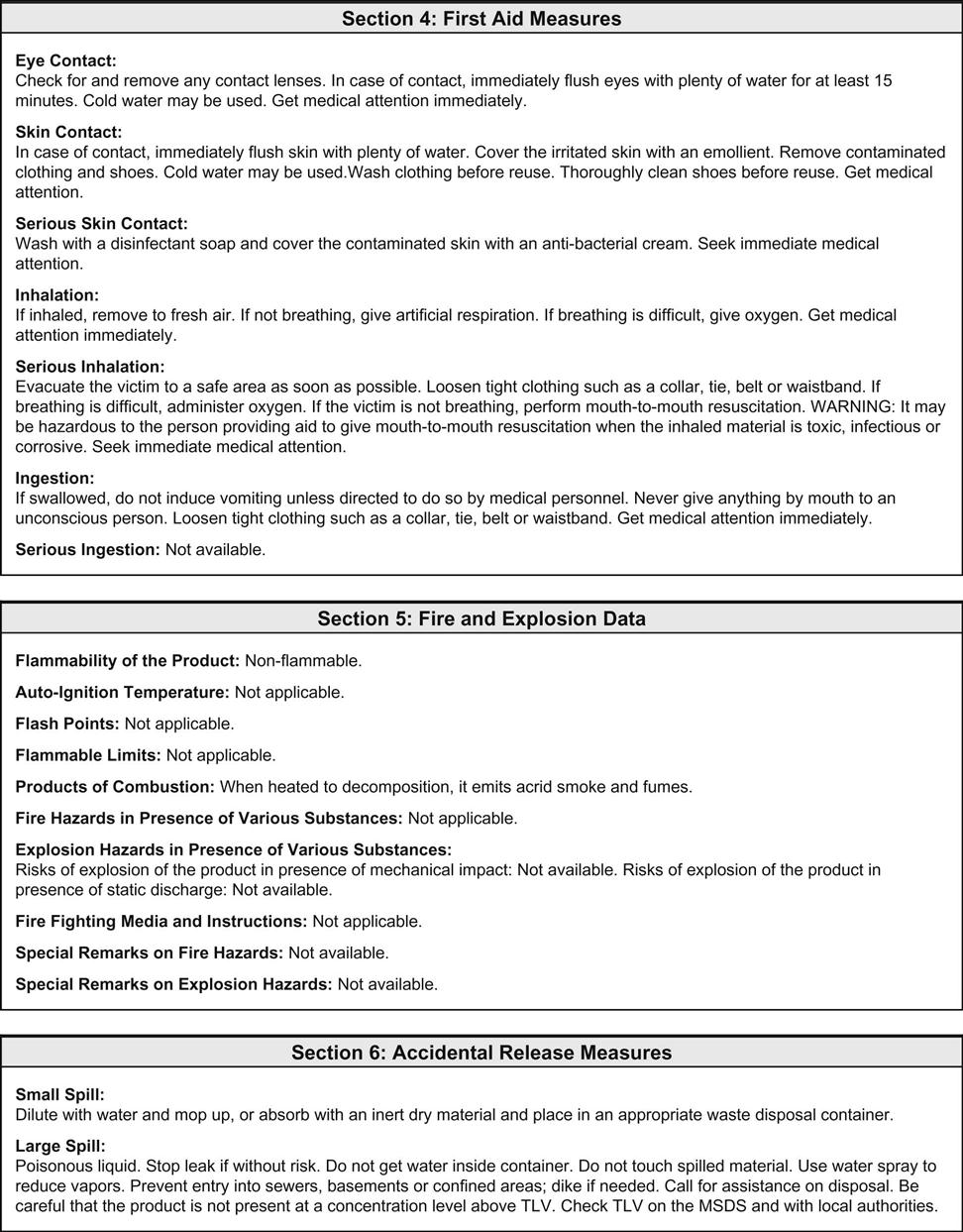
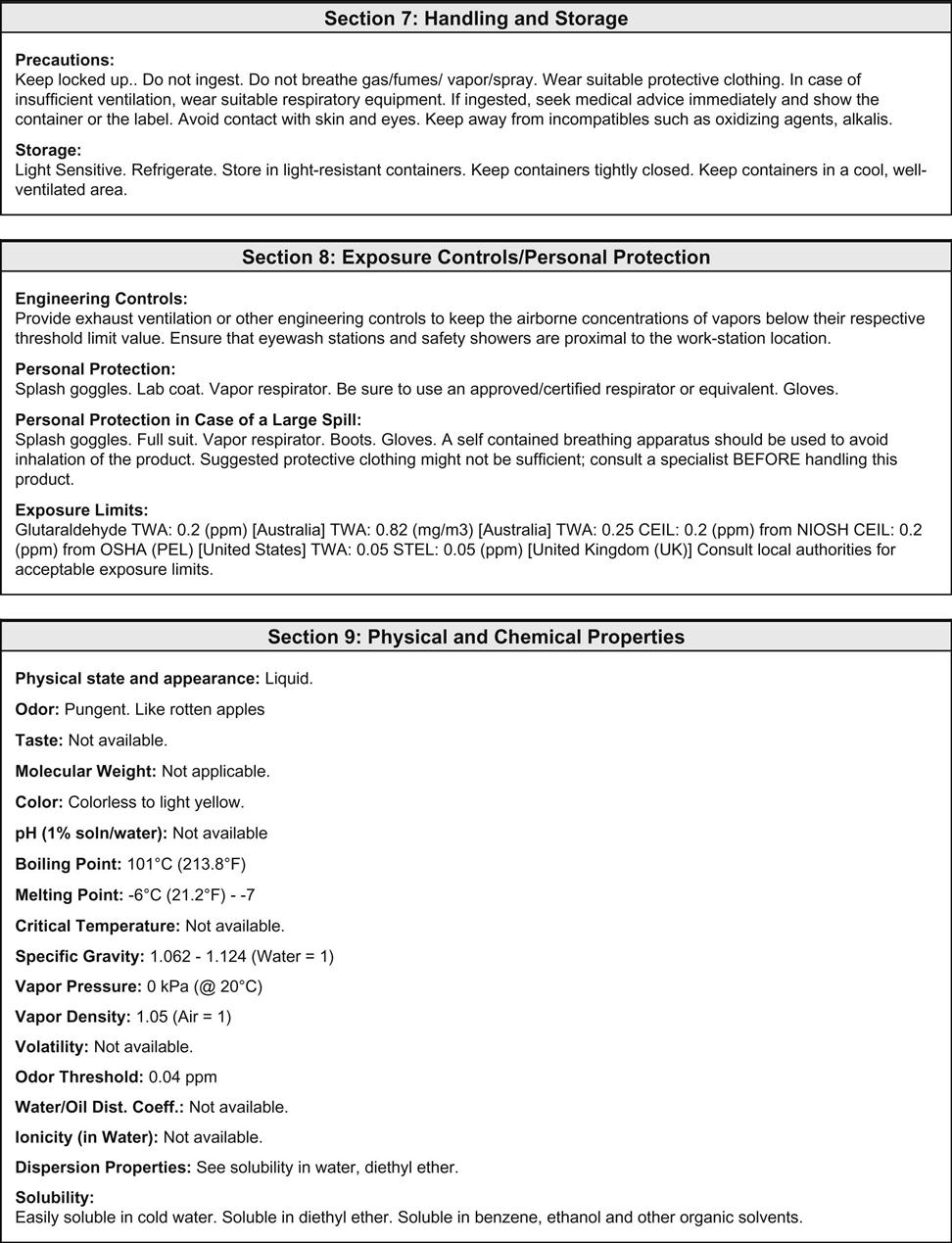
The clinical laboratory is home to chemicals that are flammable, caustic, poisonous, carcinogenic, and/or teratogenic. Exposure to these dangerous chemicals can occur through inhalation, direct absorption through the skin, ingestion, entry through a mucous membrane, or entry through a break in the skin. OSHA is involved in regulating the standards directed at minimizing occupational exposure to hazardous chemicals in laboratories. The OSHA hazard communication standard (known as the employee “right to know” rule) became law in 1991 and ensures that laboratory workers are fully aware of the hazards associated with their workplace. The law requires the development of a comprehensive plan to implement safe practice throughout the laboratory with regard to chemicals. This chemical hygiene plan must outline the specific work practices and procedures needed to protect workers from any health hazards that may arise from working with in-stock chemicals. All workers must be provided with information and training, and a material safety data sheet (MSDS) must be on file for all chemicals used in the laboratory. OSHA requires the manufacturer of the chemical to make these sheets available, usually as a package insert.
OSHA recommends that each MSDS sheet follow the 16-section format developed by the American National Standards Institute (ANSI). When this format is used, information about the hazardous material that affects the worker the most is identified at the beginning of the form and the more technical information about the product is given later. The 16-section MSDS template includes the following (Figure 51-2):
• Identification of the product
• Composition/information on ingredients
• Exposure controls/personal protection
• Physical and chemical properties
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree