Assisting in the Analysis of Blood
Learning Objectives
1. Define, spell, and pronounce the terms listed in the vocabulary.
2. Apply critical thinking skills in performing the patient assessment and patient care.
3. Name the main functions of blood.
4. Identify the role of the hematology laboratory in patient care.
5. Describe the appearance and function of erythrocytes.
6. Describe the appearance and function of granular and agranular leukocytes.
7. Differentiate between T cells and B cells.
8. Describe the appearance and function of thrombocytes.
9. Explain the process of clot formation.
10. Identify the anticoagulant of choice for hematology testing.
11. Explain the purpose of the microhematocrit test.
12. Perform a microhematocrit test.
13. Explain the role of hemoglobin in the body.
14. Perform a hemoglobin test.
15. Identify the tests included in a complete blood count (CBC) and their reference ranges.
16. Explain the process of automated blood cell counting.
17. Distinguish between normal and abnormal test results.
18. Describe the red blood cell (RBC) indices and how they are calculated.
19. Explain the reasons for performing a white blood cell (WBC) differential.
20. Discuss Wright’s stain sequence.
21. Describe the appearance of normal erythrocytes.
23. Cite the reasons for performing an erythrocyte sedimentation rate test.
24. Describe the sources of error for the erythrocyte sedimentation rate test.
25. Determine an erythrocyte sedimentation rate using a modified Westergren method.
26. Describe the tests performed to assess coagulation.
27. Differentiate between the ABO blood groupings and the Rh blood groupings.
28. Secure a capillary blood sample and determine the ABO and Rh groupings of the sample.
Vocabulary
anemia A condition marked by deficiency of red blood cells (RBCs).
centrifuge (sen′-truh-fuhj) An apparatus consisting essentially of a compartment that spins about a central axis to separate contained materials of different specific gravities or to separate colloidal particles suspended in a liquid.
enzymes Complex proteins produced by cells that act as catalysts in specific biochemical reactions.
polycythemia vera (pah-le-si-the′-me-uh/veh′-rah) A condition marked by an abnormally large number of red blood cells (RBCs) in the circulatory system.
type and cross-match Tests performed to assess the compatibility of blood to be transfused.
Scenario
Dana Cummings is a certified medical assistant working in the Westhills Family Practice Center. She is preparing to collect blood from Mr. Corrigan, who recently underwent renal transplantation because of complications from diabetes type 1. He has come to the office today for a routine examination. Dr. Fischbach suspects that Mr. Corrigan is anemic and orders an anemia panel in addition to a renal panel; a hemoglobin A1c level; a complete blood count, including hemoglobin, hematocrit, and differential; prothrombin time (PT); and alanine aminotransferase/aspartate aminotransferase (ALT/AST) testing.
While studying this chapter, think about the following questions:
• Why are so many tests being performed for Mr. Corrigan?
• Which of these tests probably will be completed today in the office laboratory?
The average body holds 10 to 12 pints of blood. The heart circulates the blood through the circulatory system more than 1,000 times every day. More than 70,000 miles of passageways, most of which are narrower than a human hair, carry blood throughout the body. The blood is contained in a closed system of vessels; the largest is the aorta, and the smallest are the capillaries. The capillaries are only one cell layer thick, and their thin, permeable walls allow certain substances to move back and forth between blood vessels and surrounding tissue. The circulating blood contains more than 25 trillion cells, and every second the body replaces 8 million old red blood cells (RBCs) with 8 million new RBCs.
Besides supplying body cells with nutrients and oxygen, the blood carries away carbon dioxide and urea, the waste products of normal cell activity. If the blood did not carry away these waste products, they would accumulate and damage the cell. Carbon dioxide is carried in the blood to the lungs, where it is exhaled as part of normal breathing. The blood carries urea to the kidneys, where it is excreted in the urine along with other body wastes. The blood also distributes enzymes, hormones, and other chemicals needed for control and regulation of body activities. In addition, the blood functions to maintain the body at a uniform temperature, to keep other body fluids in a state of pH balance, and to carry hormones from the secreting gland to the tissues where they are needed.
Blood testing is done routinely in the hematology, immunology (serology), immunohematology (blood banking), and chemistry sections of the laboratory. The degree of blood testing performed by medical assistants depends on the level of service offered by the physician’s office and the regulations established by the Clinical Laboratory Improvement Amendments (CLIA). As a medical assistant, you will not perform all the procedures described here. Some have been replaced by automated procedures, and others are considered highly complex by CLIA standards and therefore are not performed by medical assistants. Nevertheless, this chapter explains these procedures to provide background information critical to an understanding of the analysis of blood, from collection of the specimen, through testing, to recording of the results.
Hematology
The hematology section of a laboratory deals with counting RBCs, white blood cells (WBCs), and platelets; differentiating WBCs on stained blood smears; measuring the percentage of RBCs in the blood (hematocrit); and determining the oxygen-carrying capacity of the blood (hemoglobin).
The complete blood cell count (CBC) is the laboratory procedure most frequently ordered for blood specimens. It gives a fairly complete look at the components of blood and can provide a wealth of information about a patient’s condition. It routinely includes the following:
Whole blood is composed of formed elements suspended in a clear, yellow, liquid portion called plasma. Plasma makes up approximately 55% of blood by volume. The remaining 45% consists of formed cellular elements: erythrocytes (RBCs), leukocytes (WBCs), and thrombocytes (platelets). All of these cellular elements have special functions.
Erythrocytes
RBCs, or erythrocytes, are formed in the red bone marrow of the ribs, sternum, pelvis, and skull and in the ends of long bones in adults. The nucleus of the immature form of the RBC disintegrates as the cell matures. Loss of the nucleus results in the familiar shape of the RBC: a biconcave disk that is thicker at the rim than in the middle. Erythrocytes transport oxygen from the lungs to the body cells and carry carbon dioxide away from cells, back to the lungs to be exhaled. The main constituent is the red pigment hemoglobin, which is composed of iron and protein. Hemoglobin actually carries oxygen and some carbon dioxide throughout the body.
The life span of an erythrocyte is approximately 120 days. As the cell nears the end of its life, it becomes more fragile and eventually ruptures and breaks. The iron is reused for the formation of new RBCs, and the protein is converted into a bile pigment.
Leukocytes
WBCs, or leukocytes, have a nucleus and are larger than erythrocytes. The prime function of the leukocyte is to protect the body against infection and disease. The five types of leukocytes are classified as granular or agranular. Granular leukocytes, or polymorphonuclear leukocytes, include neutrophils, eosinophils, and basophils. They are characterized by their heavily granulated cytoplasm and segmented nuclei. Agranular leukocytes are the lymphocytes and monocytes, both of which have clear cytoplasm and a solid nucleus.
Granular leukocytes are phagocytic, that is, they engulf invading bacteria and viruses. Unlike erythrocytes, leukocytes function in the tissues. During inflammation, the blood carries the WBCs through dilated vessels to the site of injury. Capillary walls become more permeable, and granular cells squeeze through by ameboid motion. Once at the site of infection or injury, the cells engulf the invading microorganism, creating pus, which contains dead leukocytes, bacteria, and tissue cells.
Agranular leukocytes produce antibodies. The lymphocytes are classified as T cells or B cells, on the basis of their functional characteristics.
T Cells
T cells make up about 65% to 80% of circulating lymphocytes and have a life span of months to years. This is important for conferring long-lasting immunity to microbial infections. T cells mount the immune response to intracellular parasites, viruses, fungi, and bacteria. Delayed hypersensitivity reactions, such as the response to poison ivy, are controlled by T-cell defenses, as is organ transplant rejection. T cells are subdivided into several types, according to their function:
B Cells
B cells are formed in bone marrow and then migrate to other lymph organs, where they multiply and reside. When stimulated, B cells differentiate into plasma cells that produce specific antibodies to an antigen. Antibodies circulate in the plasma or are present in secretions. Some antibodies cause cells to clump and precipitate, whereas others activate the complement system. The complement system is a series of reactions between plasma proteins that amplifies the immunologic response to foreign molecules. Activation of the complement system leads to lysis of microorganisms or their phagocytosis by neutrophils.
Antibodies are protein molecules that attach to antigens. Very small antigens, such as toxins and viruses, can be directly neutralized by antibodies; larger antigens, such as bacteria, require the help of agranular leukocytes. Three steps are required to destroy these pathogens:
Thrombocytes
Thrombocytes are not true cells, but rather cytoplasmic fragments of a megakaryocyte, a large cell in the bone marrow. They are the smallest formed elements of the blood. They typically have a discoid shape; however, when activated, they become globular and form fingerlike cytoplasmic extensions called pseudopodia.
Clot Formation
In minor injuries, thrombocytes tend to collect and form plugs in blood vessel openings. To control bleeding from vessels larger than capillaries, a clot must form at the point of injury. Coagulation of the blood also is initiated by blood platelets. The platelets produce a substance that combines with calcium ions in the blood to form thromboplastin, which in turn converts the protein prothrombin into thrombin through a complex series of reactions. Thrombin, an enzyme, converts fibrinogen, a protein substance, into fibrin, an insoluble protein that forms an intricate network of minute, threadlike structures called fibrils, and causes the blood plasma to gel. The blood cells and plasma become enmeshed in the network of fibrils, forming a clot.
Blood clotting can be initiated by the extrinsic mechanism, in which substances from damaged tissues are mixed with the blood, or by the intrinsic mechanism, in which the blood itself is traumatized. More than 30 substances in blood have been found to affect clotting; whether blood will coagulate depends on a balance between the substances that promote coagulation (procoagulants) and those that inhibit it (anticoagulants). Coagulation of blood within blood vessels in the absence of injury can cause serious illness or death, especially when a clot forms in the coronary arteries (thrombosis) or in the cerebral arteries (stroke).
Hemophilia, a bleeding disorder, occurs when a person has a mutation in one of the clotting factor genes. It is a hereditary, gender-linked disorder that affects males of all races and ethnic groups. The mutated gene is on the X chromosome inherited from the mother. Approximately one in 4,000 males is born with the disorder; it is rare, but possible, for a female to have hemophilia. People with hemophilia are treated with intravenous (IV) purified clotting factor to prevent bleeding episodes. Internal bleeding, particularly in the joints, is a problem despite treatment and leads to painful arthritis.
Plasma
Plasma is a highly complex liquid that is the carrier for formed elements and other substances, such as proteins, carbohydrates, fats, hormones, enzymes, mineral salts, gases, and waste products. Plasma is composed of approximately 90% water, 9% protein, and 1% various other chemical substances. When plasma proteins and other components are used up during the clotting process, the remaining liquid is called serum.
Collection of Blood Specimens
For most hematology tests, an adequate blood sample can be obtained from capillaries by finger puncture. If a larger sample is required, blood can be obtained from a vein by venipuncture. For a CBC, venous blood is collected in a tube containing an anticoagulant that prevents clotting. Ethylenediaminetetraacetic acid (EDTA) is the anticoagulant of choice for hematology testing. It is important to prevent blood from being hemolyzed during collection for hematology testing.
Hematocrit
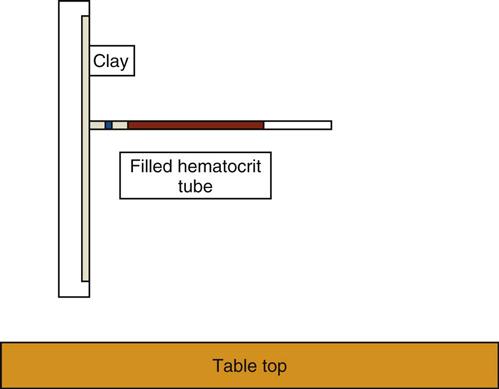
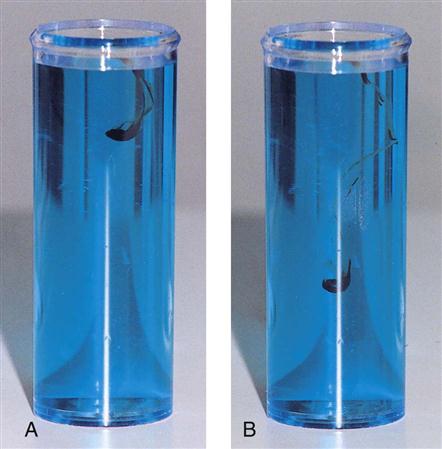
The hematocrit (Hct) is a measurement of the percentage of packed RBCs in a volume of blood. The spun microhematocrit test is based on the principle of separating the cellular elements from plasma by centrifugation (Procedures 54-1 and 54-2). Two or three drops of blood are collected from a capillary puncture in two capillary tubes and are placed in a specially designed microhematocrit centrifuge (Figure 54-1). Alternatively, the capillary tubes can be filled with EDTA-anticoagulated blood from a lavender-topped vacuum tube. Capillary tubes can either be preplugged or open and may be made of glass or plastic. If the tube is not plugged, it must be sealed with special clay before centrifugation.
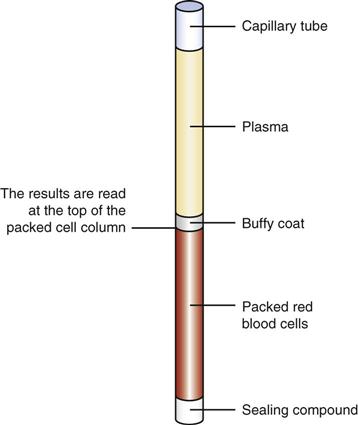
After centrifugation, packed RBCs are at the bottom of the tube, WBCs and platelets are in the center buffy coat, and plasma is on top (Figure 54-2). From this separation the microhematocrit is determined by comparing the concentration of RBCs with the total volume of the whole blood sample. The percentage is read by placing the tubes on a special microhematocrit reader. Some microhematocrit centrifuges have a built-in reading scale that reads calibrated capillary tubes. Microhematocrits should be performed in duplicate and the average of the two results reported.
Inverness Medical Professional Diagnostics (formerly Wampole, Princeton, New Jersey) manufactures a CLIA-waived instrument that uses electrical conductivity to determine the hematocrit. The STAT-CRIT Hct is a self-contained, portable unit that determines the hematocrit in 30 seconds. The testing method is based on the principle that blood is a conducting medium in which RBCs act as resistors. The plasma conducts electricity based on temperature. The greater the number of erythrocytes in a sample, the greater is the resistance recorded. A blood sample is introduced into the sample carrier, which has a thin, plastic membrane that permits rapid equilibration of the temperature of the blood and the measuring port. This instrument is best used with fresh blood obtained from a fingerstick; anticoagulants can interfere with conductivity, and heparin, not EDTA, is the anticoagulant of choice.
The hematocrit also can be calculated using RBC count and RBC size values from an automated cell counter.
Normal Hct values vary with gender and age (Table 54-1). They range from a low of 36% in women to a high of 52% in men. Low microhematocrit values can indicate anemia or the presence of bleeding; high values may be caused by dehydration or by a condition such as polycythemia vera. Values can be influenced by physiologic or pathologic factors and by collection techniques.
TABLE 54-1
Hematocrit (Hct) Reference Values
| AGE AND/OR GENDER | Hct VALUE (%) |
| Neonate | 44-64 |
| Infant | |
| 1 mo | 35-49 |
| 6 mo | 30-40 |
| Child, 1-10 yr | 35-41 |
| Adult | |
| Men | 42-52 |
| Women | 36-45 |
From Stepp CA, Woods MA: Laboratory procedures for medical office personnel, Philadelphia, 1998, Saunders.
The microhematocrit is a commonly performed test requested by physicians separately or as part of the CBC. Because it is a simple procedure that requires only a small amount of blood, it is an ideal screening test and often is part of a routine physical examination.
Hemoglobin
The hemoglobin (Hgb) determination is a rough measure of the oxygen-carrying capacity of blood. The hemoglobin concentration can be determined as part of the CBC or as an individual test. Many methods of determining the hemoglobin concentration have been used over the years. The earliest measures simply involved comparing the color of a drop of blood against a chart. Dark red blood has more hemoglobin than pale red blood. The international reference, or “gold standard,” method for hemoglobin determination is the hemiglobincyanide or cyanmethemoglobin (HiCN) method. A sample of whole blood is diluted in Drabkin’s reagent, which contains cyanide. The RBCs lyse, releasing hemoglobin, which reacts with cyanide to form hemiglobincyanide. Then the sample is placed in a colorimeter, and the amount of light absorbed by the sample at a 540-nm wavelength is determined.
A hemoglobinometer is a colorimeter that determines hemoglobin by measuring the amount of light absorbed by a sample of blood in which the hemoglobin has been released and chemically modified.
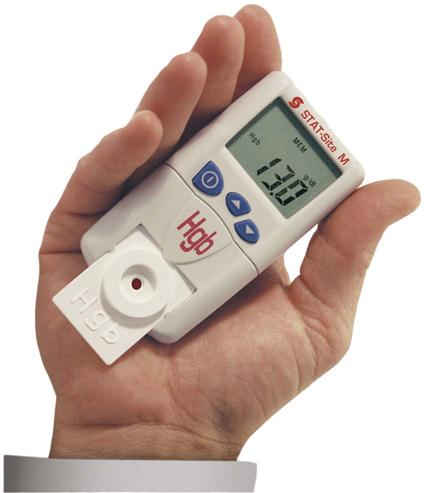
CLIA-waived methods include the STAT-Site M Hgb (Stanbio Laboratories, Boeme, Texas), a completely portable, battery-operated hemoglobin analyzer that fits in the palm of the hand (Figure 54-3), and the HemoCue (HemoCue AB, Angelgolm, Sweden) (Procedure 54-3). The HemoCue uses plastic cuvettes that contain sodium deoxycholate, sodium nitrite, and sodium azide. The sodium deoxycholate lyses the erythrocytes in the sample, releasing hemoglobin, which reacts with sodium nitrite to form methemoglobin. The methemoglobin reacts with the sodium azide to form azidemethemoglobin, which can be detected at two different wavelengths: 570 nm and 880 nm. Two wavelengths are used to compensate for possible turbidity in the sample. Capillary, venous, or arterial blood can be used in the cuvette, and cuvettes have a long shelf life.
The copper sulfate method is a CLIA-waived manual method of hemoglobin determination that is often used to screen blood donors. It is based on the principle of specific gravity; when a drop of blood from a patient with normal hemoglobin values is dropped into a copper sulfate solution, it falls rapidly to the bottom (Figure 54-4). If the drop falls slowly or not at all, hemoglobin levels are below reference range.
Normal hemoglobin values vary throughout life. They normally are quite high at birth, decline during childhood, and then increase through the teens until adult levels are reached (Table 54-2). Values range from a low of 12 g/dL in women to a high of 17.5 g/dL in men. The various factors that affect the hemoglobin level include age, gender, diet, altitude, and disease.
TABLE 54-2
Hemoglobin (Hgb) Reference Values
| AGE AND/OR GENDER | Hgb LEVEL (g/dL) |
| Neonate | 17-23 |
| Infant (2 mo) | 9-14 |
| Child | 11-16 |
| Female | 12-16 |
| Male | 15-17 |
From Stepp CA, Woods MA: Laboratory procedures for medical office personnel, Philadelphia, 1998, Saunders.
Hemoglobin and hematocrit tests often are performed together and are referred to as an “H&H.” A quick mental calculation should always be done before H&H results are reported: Hemoglobin value × 3 ± 3 should equal the hematocrit value. For example, if the hemoglobin is 15 g/dL, the hematocrit should be 42% to 48%.
Red Blood Cell Count
The RBC count is a commonly performed procedure and is part of the CBC (Table 54-3). It approximates the number of circulating RBCs. The function of RBCs is to transport oxygen to tissues. The condition in which the oxygen-carrying capacity of blood is below normal is called anemia. The RBC count often is decreased in anemia. Increases are found in people with dehydration, polycythemia vera, or severe burns, and in those who live at high altitudes, in whom it reflects an adaptation to the lower oxygen content of the air.
TABLE 54-3
Reference Ranges for Complete Blood Count Values
| TEST | NEONATES | INFANTS (6 mo) | CHILDREN | MEN | WOMEN |
| RBCs | 4.8-7.1 million/mm3 | 3.8-5.5 million/mm3 | 4.5-4.8 million/mm3 | 4.5-6 million/mm3 | 4-5.5 million/mm3 |
| Hematocrit (Hct) | 44%-64% | 30%-40% | 35%-41% | 42%-52% | 36%-45% |
| Hemoglobin (Hgb) | 17-23 g/dL | 9-14 g/dL | 11-16 g/dL | 15-17 g/dL | 12-16 g/dL |
| WBCs | 9,000-30,000/mm3 | 6,000-16,000/mm3 | 5,000-13,000/mm3 | 4,000-11,000/mm3 | |
| RBC Indices | |||||
| MCV | 96-108 fL | 82-99 fL | |||
| MCH | 32-34 pg | 26-34 pg | |||
| MCHC | 31-33 g/dL | 31-37 g/dL | |||
| WBC Differential | |||||
| Neutrophils | ≥45% by age 1 wk | 32% | 60% for children 2 yr or older | 50%-65% | |
| Bands | — | — | — | 0%-7% | |
| Eosinophils | — | — | 0%-3% | 1%-3% | |
| Basophils | — | — | 1%-3% | 0%-1% | |
| Monocytes | — | — | 4%-9% | 3%-9% | |
| Lymphocytes | ≥41% by age 1 wk | 61% | 59% for children 2 yr or older | 25%-40% | |
| Platelets | 140,000-300,000/mm3 | 200,000-473,000/mm3 | 150,000-450,000/mm3 | 150,000-400,000/mm3 | |
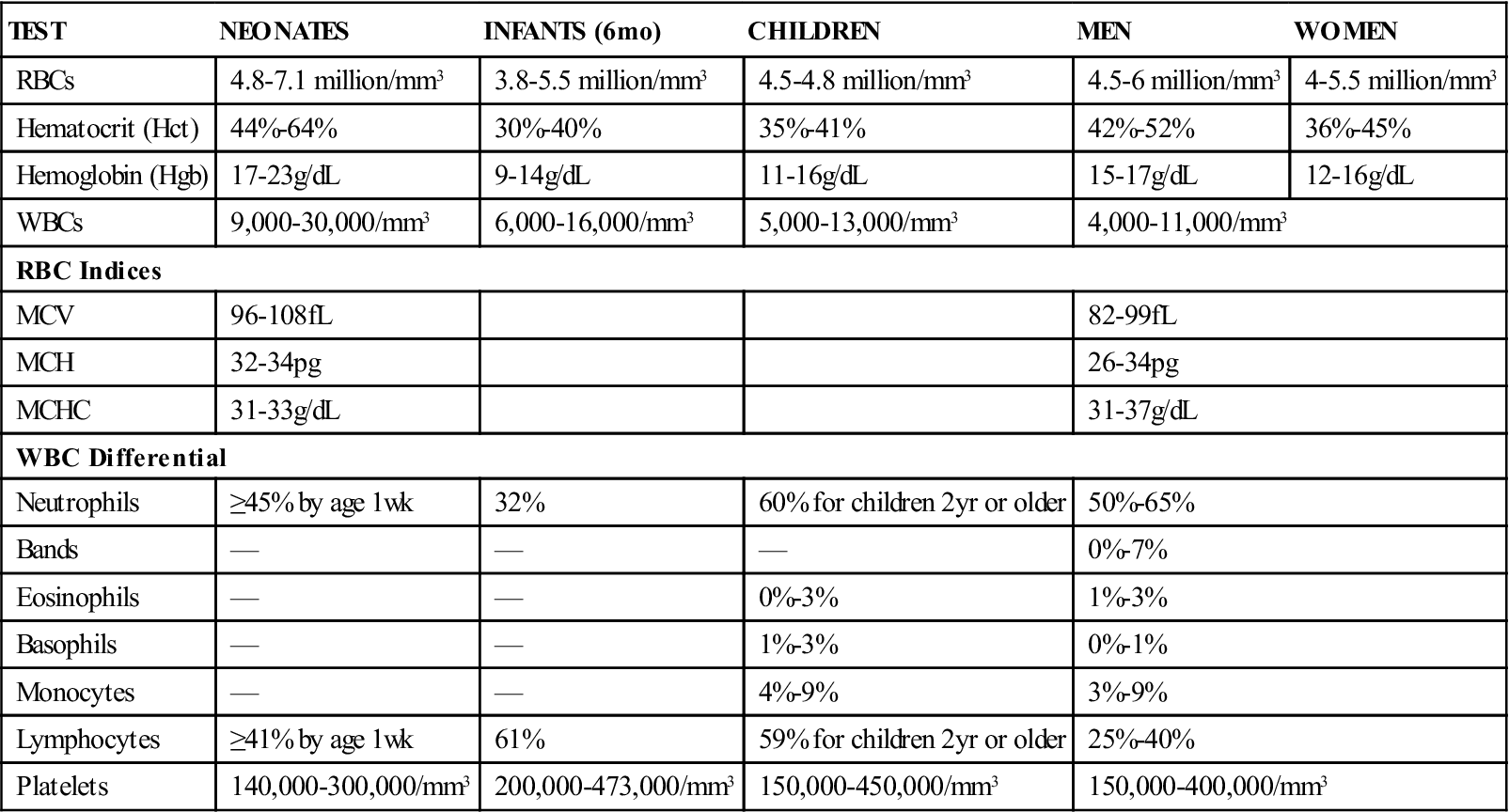
From Stepp CA, Woods MA: Laboratory procedures for medical office personnel, Philadelphia, 1998, Saunders.
Normal RBC values range from 4 million to 6 million cells/mm3. RBC counts usually are higher in males than in females.
White Blood Cell Count
The WBC count gives an approximation of the total number of leukocytes in circulating blood. The count is performed to help the physician determine whether an infection is present or to aid in the diagnosis of leukemia. It also may be used to follow the course of a disease and as an indication of whether the patient is responding to treatment.
The normal WBC count varies with age. It is higher in newborns and decreases throughout life. The average adult range is 4,000 to 11,000 cells/mm3. Many factors can affect the WBC count. An increase in the number of normal WBCs is a condition called leukocytosis.
Physiologic increases in the WBC count are seen with pregnancy, stress, anesthesia, exercise, and exposure to temperature extremes, and after treatment with corticosteroids. Pathologic causes of leukocytosis include many bacterial infections, leukemia, appendicitis, and pneumonia. A decrease in the WBC count is called leukopenia. This condition may be caused by viral infection or by exposure to radiation and certain chemicals and drugs.
Determining the Red Blood Cell and White Blood Cell Counts
In the past, blood counts were performed by diluting the blood with special pipets and diluting fluid, placing the sample on a counting slide called a hemacytometer, and manually counting the cells using a microscope. This method was time-consuming and often inconsistent and therefore has largely been replaced by automated cell counting.
The current availability of many different types of cell counters has made it possible for the physician’s office to become fully automated. Modern instruments range from relatively simple, inexpensive counters to very complex, expensive instruments. Automation improves the accuracy of cell counting and results in greater efficiency. In addition, it reduces the frequency of handling of individual blood specimens and the risk of exposure to blood-borne pathogens. The operation of the typical counters used in a physician’s office laboratory is considered moderately complex by CLIA standards. It is essential that strict standardization procedures and quality control methods be followed when automated instruments are used to perform blood cell counts.
Most automated cell counters operate first by diluting the cells in a fluid that conducts an electrical current. These diluted cells then pass through a special narrow opening in the instrument. The passing cells interrupt the flow of current, and each interruption is counted. Some instruments use a laser beam instead of an electrical current (Figure 54-5). Red cells and white cells are counted in separate diluting chambers or channels. In the white cell counting area, the red cells are first lysed, usually with acetic acid, to make the white cells easier to count. Platelets usually are counted in the same channel as red cells, and the cells are differentiated by size. All cells are reported in units per volume of whole blood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree