Carotid Pulse
The carotid pulse is not normally visible when looking at the neck. It can be palpated medial to and below the angle of the jaw. It is important not to palpate both sides simultaneously; this may decrease blood flow to the brain and cause the patient to have syncope (Bickley and Szilagyi, 2008). Listen over both carotid arteries with the stethoscope for the presence of bruits, a murmur-like or “whooshing” sound caused by constriction or altered flow. Ask the patient to hold a breath for a few seconds to make listening easier.
Examination of the Precordium
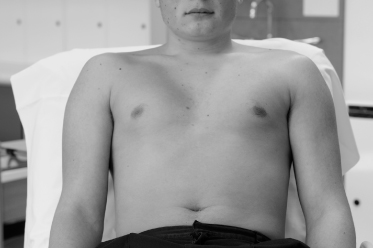
Inspection, palpation and auscultation are used to examine the precordium (Figure 3.2). The exam is usually done with the patient at 30°. Other positions needed are turning to the left side and sitting upright.
Inspection
- Look at the precordium for deformities of the chest wall such as pectus excavatum (a depression in the lower part of the sternum) or kyphoscoliosis (an abnormal curvature of the spine) which may lead to compression of large vessels and cause an ejection systolic murmur (Kumar and Clark, 2005), also note any visible scars (sternotomy, lateral thoracotomy).
- The apical impulse may be visible at approximately the midclavicular line, 5th intercostal space and is best observed when the patient is sitting up and the heart is closer to the chest wall. This is easily obscured by obesity, large breasts or a muscular chest, and doesn’t necessarily present an abnormal finding.
- A readily visible and bounding apical impulse suggests a large left ventricle.
- Right ventricular hypertrophy or atrial enlargement may cause a heave over the left parasternal area.
Palpation
- Palpate the apical beat or point of maximal impulse (PMI). This is usual at 5th intercostal space, midclavicular line in adults. It may shift slightly to the 6th intercostal space, just left of the midclavicular line in older adults – its radius is usually no more than 1 cm
- Ask the patient to lean forward or lay on the left side if the PMI is difficult to find. Apical impulses are usually impalpable in emphysema, obesity and effusions (pericardial or pleural)
- Assess the character of the impulse:
- more vigorous than expected is described as a heave or lift
- forceful or widely distributed – may indicate increased cardiac output or ventricular hypertrophy
- thrusting displaced apical beat – suggests volume overload, possibly from mitral or aortic valve incompetence, left-to-right shunt, or cardiomyopathy
- tapping apex beat – occurs in mitral stenosis.
- more vigorous than expected is described as a heave or lift
- Palpate the left sternal border and the base. A palpable murmur that feels like a fine, rushing vibration is described as a thrill.
Auscultation
- The key is to use a systematic approach listening and describing what is heard. There are four valves (mitral, tricuspid, pulmonary and aortic) and each has an auscultatory area. These areas reflect sounds transmitted in the direction of blood flow as it passes through the valves, not surface markings of the valves. For this reason, there are five key listening areas:
- aortic valve area – 2nd right intercostal space, right sternal border
- pulmonary valve area – 2nd left intercostal space, left sternal border
- second pulmonic area (Erb’s point) – 3rd intercostal space, left sternal border
- tricuspid area – 4th left intercostal space, lower left sternal border
- mitral (apical) area – apex of the heart, 5th left intercostal space, midclavicular line.
- aortic valve area – 2nd right intercostal space, right sternal border
- It is useful to listen over each main area with the bell and diaphragm of the stethoscope. The diaphragm is able to pick up relatively high-pitched sounds (S1, S2) while the bell transmits softer and lower pitched sounds (S3, S4, mitral stenosis murmur). Listen with the patient supine and sitting up, leaning slightly forward. Take time to listen carefully for each heart sound, isolating each component of the cardiac cycle. It may be helpful to palpate the carotid pulse to assist in determining systole and diastole, particularly when an irregular rhythm such as atrial fibrillation is present. Note any added sounds then listen for murmurs (Bickley and Szilagyi, 2008).
- Heart sounds are described by pitch, intensity, duration and timing in the cardiac cycle. Sounds produced are generally low pitched. S1 and S2 result from valve closure and are the most distinct sounds and provide useful clues about heart function:
- S1 – mitral and tricuspid valve closure, indicates the beginning of systole, best heard towards the apex
- S2 – aortic and pulmonic valve closure, indicates the end of systole, best heard in aortic and pulmonic areas, higher-pitched and shorter than S1
- splitting of S2 – S2 is made up of two sounds that merge during expiration. Aortic valve closure (A2) contributes most of the S2 sound when heard in the aortic and pulmonic areas and tends to override the sound from pulmonic valve closure (P2). During inspiration, P2 occurs a little later and gives S2 two phases or a split S2. Listening while asking the patient to take a deep breath may reveal a split S2. Normal or physiological splitting is common in children and young adults. In older adults a delayed closure of P2 may be associated with right ventricular hypertrophy or pulmonary hypertension
- S3 – first phase of rapid ventricular filling in early diastole; increased with exercise, fast heart rate, elevation of legs and increased venous return. Often described as a ventricular gallop and heard best at the apex
- S4 – second phase of ventricular filling; atrial contraction causing ventricular filling towards the end of diastole, heard just before S1. A physiological S4 may be heard in middle-aged adults, particularly after exercise. In older adults suspect hypertensive disease, coronary artery disease, myocardial ischaemia, infarction or congestive heart failure. Often described as an atrial gallop.
- S1 – mitral and tricuspid valve closure, indicates the beginning of systole, best heard towards the apex
- Extra heart sounds – heart valves usually open without making a noise unless thickened or roughened. Extra heart sounds are described as:
- ejection clicks are heard when deformed but mobile aortic or pulmonic valves open. This sound is heard early in systole
- opening snaps are associated with an abnormal mitral or tricuspid valve and are best heard in diastole
- a pericardial friction rub may be heard when inflammation of the pericardial sac causes the parietal and visceral surfaces to become rough. This produces a rubbing or grating sound heard with the stethoscope. Pericardial friction rub is often heard loudest of the apex and usual covers systole and diastole (Seidel et al., 2003)
- prosthetic valves produce loud clicks during opening and closure.
- ejection clicks are heard when deformed but mobile aortic or pulmonic valves open. This sound is heard early in systole
- Murmurs – palpation of the precordium may have provided some clues about the heart that can be considered during auscultation. A forceful left ventricular beat may be because of aortic or mitral valve disease so listening carefully for a murmur is important. Murmurs are generally caused by some disruption in blood flow into, through or out of the heart. Diseased valves that either do not open or close normally are the most common causes of murmurs. Other reasons for murmurs may include high output states (thyrotoxicosis, pregnancy), structural defects, altered blood flow in major vessels near the heart, obstructive disease in cervical arteries and vigorous left ventricular ejection (Seidel et al., 2003). A full examination as well as other diagnostic testing is useful in accurate diagnosis of a murmur. Murmurs are classified according to timing (systole or diastole), pitch, intensity, pattern, quality, location, radiation and relationship to respiration (Tables 3.1 and 3.2).
Table 3.1 Gradation of murmurs
Adapted from Bickley (2004). Reproduced with permission from John Wiley & Sons Ltd.
| Grade 1 | Very faint, may be barely audible with a stethoscope in a quiet room |
| Grade 2 | Quiet, but heard when the stethoscope is placed over the heart |
| Grade 3 | Moderately loud |
| Grade 4 | Loud, with palpable thrill |
| Grade 5 | Very loud, thrill palpated easily |
| Grade 6 | Audible when stethoscope not in contact with chest wall, thrill palpated easily |
Table 3.2 Timing of murmurs
Adapted from Kumar and Clark (2005). Reproduced with permission from John Wiley & Sons Ltd.
| Timing | Likely causes |
Midsystolic  | Innocent murmurs (no cardiovascular abnormality) Physiologic murmur (pregnancy, sepsis, anaemia) Aortic stenosis or aortic sclerosis Pulmonic stenosis Hypertrophic cardiomyopathy |
Pansystolic 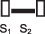 | Mitral regurgitation Tricuspid regurgitation Ventricular septal defect |
Late systolic  | Mitral valve prolapse Hypertrophic cardiomyopathy Coarctation of aorta |
Early diastolic  | Aortic regurgitation Pulmonary regurgitation |
Mid-late diastolic 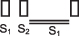 | Mitral stenosis Tricuspid stenosis |
Peripheral Arteries and Veins
Palpation of radial and carotid pulses, measurement of blood pressure, and examination of JVP has been discussed above. A full examination of the vascular system should be completed, even if there were no obvious initial signs, to gain a clear picture of the state of the cardiovascular system (Munro and Campbell, 2003). This part of the examination should be performed in a systematic way that is comfortable for the examiner.
- Peripheral pulses – palpate pulses and compare with opposite side:
- brachial – medial to biceps tendon
- radial – medial and ventral side of the wrist
- femoral – may be difficult to palpate in obese patients but helpful to identify any potential problems if this route is to be used for access in coronary angiography
- popliteal – popliteal fossa – helpful if the patient is prone with knee flexed
- dorsalis pedis – medial side of the dorsum of the foot with foot slightly dorsiflexed
- posterior tibial – behind and just inferior to medial malleolus of the ankle
- abdominal aorta – press on both sides of the aorta, feeling for any lateral pulsation suggestive of an aneurysm.
- brachial – medial to biceps tendon
- Auscultation – bruits are caused by turbulent blood flow due to constriction or altered flow. Use a stethoscope to listen over major blood vessels for the presence of a bruit. Sites to auscultate include:
- abdominal aorta – if there are clinical signs of aortic aneurysm
- femoral arteries – if there are signs of peripheral arterial disease in lower limb.
- abdominal aorta – if there are clinical signs of aortic aneurysm
Chest Pain Assessment
Chest pain is a common reason for people to seek healthcare advice within the primary and secondary care setting (Cayley, 2005). It is a symptom most commonly associated with heart disease, but may also be present in many different disease processes (Kumar and Clark, 2005). Chest pain may be present in acute coronary syndromes (ACS), but patients with pulmonary or gastrointestinal problems may also present with chest pain, as well as those with trauma or soft tissue injury (Table 3.3). A thorough history and clinical examination will help the practitioner make a correct diagnosis.
Table 3.3 Chest pain
Adapted from Bickley and Szilagyi (2008).
| Differential diagnosis of chest pain | Common signs presentation |
| Acute coronary syndromes |
|
| Aortic aneurysm/dissection |
|
| Pericarditis/myocarditis |
|
| Pleurisy |
|
| Oesophagogastric disorders |
|
| Chest wall pain (costochondritis) |
|
History Taking
The history will help to clearly establish the character of the symptoms in order to distinguish between chest pain from myocardial ischaemia and non-ischaemic or non-cardiac chest pain. There are five factors that may help in determining which patients are having pain related to an acute coronary syndrome: the nature of the pain, a prior history of heart disease, gender, age and the number of traditional risk factors present (Braunwald et al., 2002). Initial questions should be broad, such as “Do you have pain or discomfort in your chest?” Further questions should elicit the attributes of the pain (Bickley and Szilagyi. 2009).
- Onset – “When did you first notice the pain?”
- Provocation – “What kinds of activities bring on the pain?” “Do you have other symptoms such as shortness of breath, sweating, nausea or fast heart beat?”
- Quality – “What is it like?” Ask patients to use their own words to describe the pain.
- Radiation – “Where is it?” “Does it radiate?” Chest pain symptoms are varied and may be poorly localised. Ask the patient to point to the area of discomfort.
- Relief – “What do you do to make it better?”
- Severity – “How bad is it?” A pain scale may be helpful.
- Timing – “When does (did) it start?” “How long does it last?” “How often does it occur?” Patients having an ACS may have severe discomfort that comes on quickly or gradually and usually lasts for more than 20 minutes (Wallentin et al., 2002).
Risk factors should be established for all patients presenting with chest pain. This will help in making a diagnosis. Major risk factors for cardiac disease include personal history of ischaemic heart disease, family history of ischaemic heart disease, smoking, diabetes, hypertension and hypercholesterolaemia. The more major risk factors that are present, the more likely the disease becomes (this is discussed further in Chapter 4).
Clinical Examination
The immediate examination of any patient presenting with chest pain is focused on determining if symptoms are life-threatening (Erhardt et al., 2002). Myocardial ischaemia and infarction may quickly lead to cardiac arrest. Recognising and responding to early signs and symptoms of cardiac problems may prevent a cardiac arrest (ILCOR, 2005). The following approach in parallel with the history and cardiac examination may be used to quickly assess the patient (Smith et al., 2002).
- Airway – assess for any signs of airway obstruction and treat airway obstruction as an emergency. Administer oxygen at a high concentration as soon as possible.
- Breathing – look, listen and feel for signs of breathing problems. An increased breathing rate may be the first physiological observation to alter in the deteriorating patient (Smith et al., 2002). Count the respiratory rate while assessing the depth and pattern of breathing. Life-threatening conditions (acute severe asthma, pneumothorax and pulmonary oedema) must be treated immediately. Provide breaths with a pocket mask or bag-mask to any patient who has stopped breathing or has inadequate breathing.
- Circulation – do a rapid general examination of the patient noting colour of hands and feet and temperature of the hands. Measure CRT. Take a blood pressure and look for other signs of decreased cardiac output such as a change in level of consciousness. Any patient with a suspected ACS should be treated initially with oxygen, aspirin, nitroglycerine and morphine. A 12-lead ECG should be recorded.
- Disability – assess the patient’s conscious level quickly using AVPU: Alert, responds to Vocal stimuli, responds to Painful stimuli or Unresponsive. Hypoxia, hypercapnia and cerebral hypoperfusion are common causes of unconsciousness.
- Exposure – look at the patient exposed from head to toe and consider anything else that may be causing chest pain (e.g. injury, trauma).
Once the patient is stabilised a full cardiovascular examination as described above can be completed. In some cases, the initial exam will be unremarkable and a diagnosis is made based on the reported history. In many cases, further testing will be required. The following sections will discuss electrocardiography, laboratory testing and diagnostic procedures.
Electrocardiography
The 12-lead ECG is used frequently in primary and secondary care settings. It is a cheap, non-invasive and rapid investigation that is widely used for patients presenting with chest pain. Acute coronary syndromes, pulmonary embolism, pericarditis and aortic dissection may cause ECG changes. However, the ECG may also be normal in these conditions so the reading must be interpreted along with the history and clinical examination (ECG recording and interpretation is discussed in detail in Chapter 10).
The 12-lead ECG should be recorded as soon as possible during the initial assessment of the patient with chest pain. It is a crucial part of risk assessment and planning of treatment. The main focus of 12-lead ECG interpretation during the initial assessment is to diagnose the underlying rhythm using the approach described above and determine if there are changes related to myocardial ischaemia. Where in the myocardium the ischaemia is happening will determine which leads on the 12-lead ECG show changes.
- Acute ST segment elevation in a patient with a history and clinical findings of an acute myocardial infarction is an indication for immediate treatment to try to re-open an occluded coronary artery (coronary reperfusion). This will be with percutaneous coronary intervention or thrombolytic therapy. The diagnosis is ST elevation myocardial infarction (STEMI).
- ST segment depression indicates that ischaemia is occurring, but the patient is not likely to benefit from immediate reperfusion therapy. The diagnosis is either unstable angina or non-ST elevation myocardial infarction (NSTEMI). Higher-risk patients may need immediate medical treatment (e.g. low molecular weight heparin, aspirin, clopidogrel, beta blockade, glycoprotein IIb/IIIa inhibitor), prompt investigation with coronary angiography and in some cases PCI or coronary bypass surgery.
Laboratory Tests
There is a range of blood tests commonly done in primary and secondary care settings to evaluate patients with chest pain. Common tests include a full blood count (FBC), urea and electrolytes (U&Es), lipid profiles and cardiac markers. The results of most testing will not alter the immediate clinical management with the exception of cardiac markers.
Cardiac Markers
Serum cardiac troponin (I and T) is a very useful tool for determining the presence and extent of myocardial ischaemia. The clinical history, 12-lead ECG and cardiac troponin results are considered to be the key markers in diagnosing an acute coronary syndrome (Bertrand et al., 2002).
- Cardiac troponin is found in myocardial cells and can be detected in the serum within 4 hours of ischaemic injury. They may remain elevated for up to one week, but troponin levels are most reliable 8–12 hours after onset of the patient’s worst pain (James et al., 2005).
- Elevated troponin is also useful in predicting adverse outcomes. The higher the level of troponin, the greater the risk of death and re-infarction (Antman et al., 2004).
- Normal troponin levels are determined by local laboratories.
- Troponin may be elevated in significant pulmonary embolism, cardiac contusion, heart failure or renal failure (Thygesen et al., 2007). The results should always be interpreted in light of clinical history and examination findings.
- Other cardiac biochemical markers may be used and include creatine kinase (CK), CK-MB (an isoenzyme of CK) and myoglobin.
Diagnostic Procedures
Chest X-Ray
Chest radiography is often done if the patient presents to the hospital with chest pain. Patients with ACS generally have a normal chest X-ray. Abnormal radiographic findings such as pulmonary oedema or increased cardiac ratio from left ventricular dilatation may provide further diagnostic information for patients presenting with cardiac symptoms. A chest X-ray is also important for patients presenting with traumatic chest pain to identify a fracture, haemothorax, pneumothorax, pericardial effusion, mediastinal injury or diaphragmatic rupture.
Echocardiography
An echocardiagram (ECHO) is useful for identifying abnormalities in the myocardial wall that occur from ischaemia and necrosis. There is limited usefulness for an ECHO in the acute phase of an acute coronary syndrome. However, the ECHO may be valuable in detecting an aortic dissection or pulmonary embolus.
Exercise Tolerance Testing
The exercise tolerance test is generally used for patients who present with chest pain but do not have:
- pain during the period of observation
- ST segment depression or ST segment elevation
- elevated troponin (or other cardiac biochemical markers).
These patients are considered to be at low risk for developing adverse events such as myocardial infarction or death (Bertrand et al., 2002). The exercise test is useful for determining whether the underlying cause of chest pain is related to cardiac disease. The Bruce protocol is the most widely adopted protocol and consists of seven stages of three minutes each (Hill and Timmis, 2003). The modified Bruce protocol may be used for patients following a myocardial infarction or who have difficulty with mobility. A highly abnormal exercise test result is an indication for urgent further investigation (SIGN, 2007).
Myocardial Perfusion Scintigraphy (MPS)
Myocardial perfusion scintigraphy with exercise or pharmacologic stress is another non-invasive investigation that can detect the presence of coronary heart disease (SIGN, 2007). It is particularly useful for patients who have pre-existing ECG abnormalities, unusual patterns in the electrical activity in the heart or those who are not able to exercise.
Coronary Angiography
Coronary angiography is an invasive procedure to view the coronary arteries and determine the nature, anatomy and severity of coronary artery disease. As well as providing diagnostic imaging of the coronary arteries, the valvular and left ventricular function can also be determined. SIGN (2007) recommends that angiography should be considered in patients who are at high risk or where a diagnosis remains unclear after non-invasive testing.
Summary
Clinical examination skills are essential when considering patients with cardiac problems, the emphasis is upon using a systematic approach to ensure a thorough and timely examination and assessment. General techniques of inspection, palpation, percussion and auscultation need skill and understanding to perfect and perform with confidence, but are essential to the nurse working within cardiac care.
References
Antman EM, Anbe DT, Armstrong PW et al. (2004) ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College or Cardiology/American Heart Association Task Force on Practice Guidelines. Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction. Journal of the American College of Cardiology 44(3): 671–719.
Bertrand ME, Simoons ML and Fox KAA (2002) Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal 23: 1809–40.
Bickley L and Szilagyi P (2008) Bates’ Guide to Physical Examination and History Taking. Philadelphia, Lippincott Williams and Wilkins.
Braunwald E, Antman EM, Beasley JW, Califf RM and Cheitlin MD (2002) ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction. Journal of the American College of Cardiology 40: 1366–74.
Cayley WE (2005) Diagnosing the cause of chest pain. American Family Physician 72(10): 2012–21.
Cox C (2004) Physical Assessment for Nurses. Oxford, Blackwell.
Erhardt L, Herlitz J, Bossart L et al. (2002) Task Force report on the management of chest pain. European Heart Journal 23(15): 1153–76.
Guyton AC and Hall JE (2000) Textbook of Medical Physiology. Philadelphia, W B Saunders.
Hill J and Timmis A (2003) Exercise tolerance testing. In: Morris F, Edhouse J, Brady WJ and Camm J (eds) ABC of Clinical Electrocardiography, pp. 41–5. London, BMJ Publishing Group.
International Liaison Committee on Resuscitation (2005) International consensus on cardiopulmonary resuscitation and ememrgency cardiovascular science with treatment recommendations. Resuscitation 67: 187–211.
James SK, Lindahl B and Wallentin LC (2005) Biomarkers for risk stratification in non-ST segment elevation acute coronary syndromes: what is their relation to classical clinical characteristics? In: Ferrari R and Hearse DJ (eds) Dialogues in Cardiovascular Medicine – Acute Coronary Syndromes 10(3): 153–62.
Kumar P and Clark M (2005) Clinical Medicine. Oxford, Elsevier Saunders.
Longmore M, Wilkinson I, Turmezei T and Cheung C (2007) Oxford Handbook of Clinical Medicine. Oxford, Oxford University Press.
Moule P and Albarran J (2009) Practical Resuscitation for Healthcare Professionals. Oxford, Wiley-Blackwell.
Munro J and Campbell I (2003) Macleod’s Clinical Examination. London, Churchill Livingstone.
Scottish Intercollegiate Guideline Network (SIGN) (2007) Cardiac Rehabilitation. A quick reference guide. www.sign.ac.uk (accessed September 2009).
Seidel H, Ball J, Dains J and Benedict GW (2003) Mosby’s Guide to Clinical Examination. St Louis, Mosby.
Smith G, Osgood V and Crane S (2002) ALERT – a multiprofessional training course in the care of the acutely ill adult patient. Resuscitation 52: 281–6.
Thygesen K, Alpert JS and White HD (2007) Universal definition of myocardial infarction. European Heart Journal 28(20): 2525–38.
Wallentin L, Bertil L and Siegbahn A (2002) Unstable coronary artery disease Section 2. In: Crawford MH and DiMarco JP (eds) Cardiology, pp. 13.1–13.19. London, Mosby.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree