When the blood flow to the myocardium deteriorates, myocardial oxygen demands exceed those of supply and the myocardial cells begin an immediate process of demise. Within seconds, systolic contraction stops in the affected area. Following a brief period of ischaemia the myocardium will become injured. During this phase the effected myocardial cells cannot function, but with rapid treatment could be salvaged. Finally, after 15–30 minutes of loss of blood flow the myocardium will begin to infarct and the damage at this stage is irreversible (Conover, 2003) (see Table 7.1). Spreading from subendocardium to subepicardium, the time for full-thickness necrosis will be dependent on any collateral blood supply to the affected area (European Society of Cardiology, 2008).
Table 7.1 Stages of myocardial demise with associated ECG changes
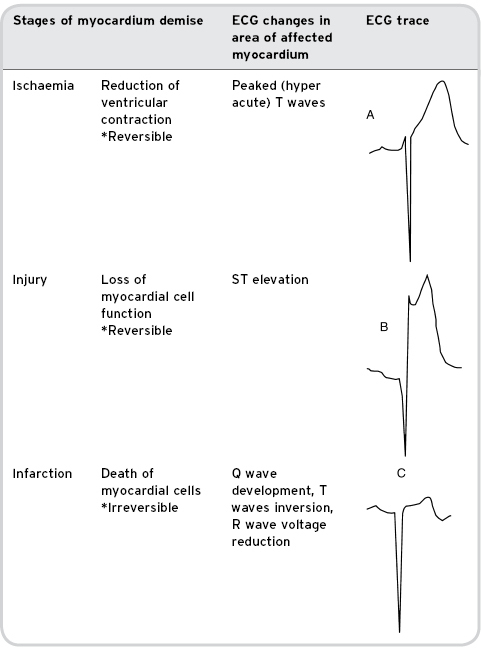
Rapid assessment, diagnosis and treatment in this patient group are essential in order to re-establish perfusion to the myocardium thus minimising infarct size and preserving myocardial function.
Presenting Features
Patients suffering from STEMI will present in a variety of ways depending on:
- the size and location of the infarct
- the degree of chest pain experienced
- the response of the autonomic nervous system to injury
- the patient’s co-morbidities and previous experience.
Generally patients will present with:
- chest pain
- nausea and possible vomiting
- dyspnoea (breathlessness)
- anxiety or a feeling of “impending doom”
- diaphoresis (sweating)
- pallor.
Most of the common presenting symptoms will result from the activation of the autonomic nervous system in response to injury, pain and anxiety. Sympathetic nervous response can lead to peripheral vasoconstriction causing the patient to look pale, and feel cool and clammy. As blood is moved away from the skin’s surface during vasoconstriction, a film of sweat may appear over the patient’s skin. This fluid would normally be invisible as it is evaporated by the warm blood flowing near to the skin’s surface. Inhibition of insulin production can lead to an increase in blood glucose (Richards, 2005). Heart rate, respiration rate and blood pressure may increase to varying degrees as part of the sympathetic nervous response.
Occasionally patients may experience a parasympathetic nervous response (more common in inferior and posterior infarcts; see below) and experience a low heart rate and blood pressure. Relaxation of the sphincters in the bladder and anus can lead to incontinence. Increased peristalsis and tone in the gastrointestinal tract can lead to nausea and vomiting (Gray et al., 2008).
In cases where cardiac output is reduced due to poor ventricular function, heart rate and respiration rate may increase to compensate for the reduction in oxygen supply to the tissues. The patient may feel breathless in the presence of pulmonary oedema (Gray et al., 2008).
Chest pain is the most common presenting feature, although it must be acknowledged that 20–30% of STEMI patients will present with little or no pain. Women, older adults and people with heart failure or diabetes are commonly found in this group (Moser and Riegel, 2008). Chest pain needs to be assessed to rule out differential diagnosis (see Chapter 3). Pain can accurately be assessed using the PQRST mnemonic (Table 7.2). When present, the pain secondary to STEMI may be described by the patient as:
- retrosternal
- “heavy”, “crushing”, “squeezing”, “vice-like”, “throbbing” or “like a band around the chest”
- radiating to anywhere from the umbilicus to the jaw and throat but commonly the left shoulder and ulner region of the left arm
- diffuse and difficult to pinpoint
- constant, and does not differ on movement or inspiration
- not being relieved by nitroglycerin
- coming on at rest and unrelieved by rest
- lasting in excess of 20 minutes (Thygesen et al., 2007).
Table 7.2 PQRST assessment of chest pain with indications for STEMI
| PQRST mnemonic for chest pain assessment | Findings associated with STEMI | |
| P | Precipitating or Palliating factors | Pain constant, can start at rest, unrelieved by rest, breathing or movement |
| Q | Qualitative factors | Tightness, heaviness, pressure, constriction, burning, difficult to pin point (clenched fist over central chest normally used to demonstrate – Levine’s sign) |
| R | Region and Radiation | Retrosternal, radiating to anywhere from the lower jaw to the epigastrium but commonly ulner aspect of the arms and hands |
| S | Severity and associated Symptoms | Ranges from “worse pain ever” to mild pain. Measure using a numerical rating scale from 1 to 10. Associated symptoms include sweating, dyspnoea, nausea, vomiting, weakness, anxiety, pallor |
| T | Timing | Lasts in excess of 20 minutes |
Care Priorities
Assessment and Diagnosis
Patients presenting with the clinical signs of STEMI need a rapid targeted assessment to confirm diagnosis (see Chapter 3 for full details). This will include the following.
Physiological Assessment Using the ABCDE Approach
This approach outlined in Wood and Rhodes (2003) provides a systematic way of identifying immediate or potentially life-threatening abnormalities. Assessment of airway, breathing, circulation, neurological deficit (disability) and exposure will help to prioritise care and provide diagnosis (see Chapter 4). It should be noted that during assessment of circulation, blood pressure and pulse should be checked in both arms (a deficit may indicate aortic dissection) (Jowett and Thompson, 2007).
Cardiac History and Physical Examination
This should focus on the cardiovascular system (as detailed in Chapter 3). This may not reveal any obvious clinical signs but will help to rule out differential diagnosis. This stage of the assessment should include:
- history taking, including account of the events leading up to admission
- the identification of any risk factors for cardiac disease (see Chapter 4)
- inspection, which may reveal hyperlipidemia, peripheral vascular disease, raised jugular venous pressure (JVP) in right ventricular infarct or existing heart failure
- palpation, which may reveal apex beat displaced outwards and valve abnormalities including mitral regurgitation secondary to papillary muscle dysfunction or rupture and tricuspid regurgitation in right ventricular infarction
- auscultation, which may reveal the presence of a new murmur. The presence of a significant new murmur could suggest valvular dysfunction, such as acute mitral regurgitation or even ventricular septal rupture.
Recording of a 12-Lead ECG
This should be performed immediately on arrival at hospital. ST elevation of 1 mm or more in two or more adjacent limb leads and 2 mm or more in two adjacent precordial (chest) leads is indicative of STEMI, as is a new conductive defect such as a new left bundle branch block (LBBB) (Conover, 2003). Posterior infarction often presents as ST depression in leads V1 and V2 (mirroring posterior wall ST elevation). A posterior lead ECG (utilising V7, V8 and V9) may be recorded to confirm diagnosis. A patient presenting with ST elevation in the inferior limb leads will require a V4R to identify right ventricular involvement (see below).
Blood Tests
These should include the following.
- Urea and electrolytes:
- abnormal potassium levels are associated with an increased risk of cardiac arrhythmias such as the life-threatening ventricular tachycardia and ventricular fibrillation and should be corrected as a priority in STEMI.
- Random glucose.
- Random cholesterol.
- Full blood count.
- Cardiac enzymes:
- sensitive and specific cardiac biomarkers such as troponin (I or T) or CK-MB are recommended to aid diagnosis. These proteins leak from damaged myocytes and can be detected in the blood. Rise in their levels coupled with evidence of ischemia indicates MI. Without evidence of ischemia the rise could be secondary to an alternative cause such as myocarditis, pulmonary embolism or aortic dissection (Thygesen et al., 2007)
- blood samples for both markers should be taken on admission and 6–9 hours later. A third sample at between 12 and 24 hours is sometimes taken if previous tests are inconclusive yet suspicion of MI remains high. Serum troponin levels peak at 12 hours and thus an undetectable 12-hour level is important to exclude myocardial events. The timing of blood samples will be guided by local protocol
- cardiac biomarkers should not be used to inform reperfusion decisions in order to prevent unnecessary delays in treatment.
- sensitive and specific cardiac biomarkers such as troponin (I or T) or CK-MB are recommended to aid diagnosis. These proteins leak from damaged myocytes and can be detected in the blood. Rise in their levels coupled with evidence of ischemia indicates MI. Without evidence of ischemia the rise could be secondary to an alternative cause such as myocarditis, pulmonary embolism or aortic dissection (Thygesen et al., 2007)
Chest X-Ray
Chest X-ray should be performed as routine during acute admission but should not delay treatment, unless differential diagnosis of the patient’s presenting symptoms is expected. In this case chest X-ray can assist in confirming an alternative cause.
Treatment Priorities
The primary aim of treatment for STEMI is to return myocardial perfusion and reduce infarct size. There has recently been a move away from thrombolysis towards primary percutaneous coronary intervention (PPCI) as the first-line treatment for AMI. This relies on local facilities and expertise, and where an acceptable PPCI service cannot be established, pre-hospital thrombolysis is recommended (DH, 2008). While reperfusion therapy is of paramount importance, other supportive treatments will help to maximise the amount of salvageable myocardium (this is explored in detail in Chapter 8 and in summary below; see Tables 7.3 and 7.4).
Table 7.3 Supportive pharmacology indicated in STEMI
| Supportive pharmacology | Indications and current debate |
| Aspirin 150–325 mg oral (ESC, 2008) | Platelet aggregation inhibitor |
| Oxygen therapy ESC (2008) BTS (2008) | Recommends administering oxygen therapy to those who are breathless or showing signs of heart failure Recommend that oxygen should not be used to treat breathlessness but hypoxaemia:
Nurses should give oxygen as prescribed and document clearly the rate of oxygen administered and oxygen saturations achieved |
| Pain relief with IV morphine (AHA, 2008). Diamorphine 2.5–5.0 mg IV is given at 1 mg/min followed by 2.5 mg doses until pain is relieved (Jowett and Thompson, 2007). | Comfort of the patient and reduced workload of the myocardium
These drugs need to be given by appropriately trained staff, clearly documented following local controlled drug policy and their effects monitored and documented to guide further treatment |
| Metoclopramide 5–10 mg or cyclizine 50 mg IV | Can be given with morphine to reduce the risk of nausea and vomiting (Cam, 2002) |
| Clopidogrel 300–600 mg loading dose followed by 75 mg daily thereafter | Patients undergoing PPCI There is conflicting guidance on whether clopidogrel should be given for STEMI patients receiving thrombolysis. The ESC (2008) recommends a loading dose of 300 mg if <75 years followed by 75 mg daily dose for all patients following STEMI for 12 months. However, NICE (2007) does not recommend the routine administration of clopidogrel and if clopidogrel is started during the acute phase it should be reviewed after four weeks. Health practitioners need to be guided by local policy |
| Beta blockade Commonly used beta-blockers include atenolol, bisoprolol, carvedilol, metoprolol, propranolol and sotalol | Use of routine IV beta blockade during the acute phase has been debated, but the use of oral therapy is still recommended where it is not contraindicated (e.g. heart failure, asthma or hypotension) IV administration is still indicated for patients who are hypertensive or tachycardic on admission (AHA, 2008; ESC, 2008) Long-term beta blockade for secondary prevention should be provided for all tolerant patients (NICE 2007). |
| Angiotensin-converting enzyme (ACE) inhibitor | ACE inhibitors given in the first 24 h post STEMI lead to a small but significant reduction in mortality ACE inhibitors are recommended strongly for patients with heart failure in the early phase and/or an ejection fraction <40%
Long-term therapy with ace inhibitors for all STEMI patients regardless of LV function for secondary prevention has been advised by NICE (2007), but their use is not recommended as mandatory for normotensive patients without reduced LV function by ESC (2008) |
Table 7.4 Supportive nursing investigations and care indicated in STEMI
| Supportive nursing care and interventions | Indications |
| Immediate bed rest | Reduce physical activity and myocardial oxygen demand |
| Insert a wide-bore IV cannula (e.g. 14 gauge) | To prepare for the urgent administration of treatment as required |
| Frequent observation of vital signs including heart rate, blood pressure, respiration rate, oxygen saturations and temperature | Physiological compromise can be sudden. The frequency of measurements will depend on the stability of the patient but should generally not be left longer than 30 min each time, moving to 4-hourly once the patient is stable (Moser and Riegel, 2008) |
| Continuous ECG monitoring | Life-threatening rhythms such as VT, VF and asystole are common in STEMI |
| Serial ECGs | To detect evolving infarction |
| Reassurance and clear communication | To reduce anxiety and promote patient autonomy and partnership in care |
Complications of STEMI
Complications post STEMI can include heart failure, ranging from mild failure to cardiogenic shock. This can be a consequence of arrhythmias or mechanical faults such as valve impairment, aneurysm, cardiac rupture or ventricular septal defect. Performing an ECG and echocardiography can help determine the cause. Other complications include pericarditis and continued ischemia. Dressler’s syndrome occurs in approximately 1–5% of infarcts and results from an auto immune reaction in the first few weeks following infarction. Dressler’s syndrome includes features such as malaise, pericarditis, fever, pleural effusion, anaemia and raised inflammatory markers. Treatment may have to be continued for several months and includes non-steroidal anti-inflammatory agents or steroids in severe cases (Swanton, 2003).
Patients should be assessed for risk of further re-infarction, late arrhythmias or death during the acute phase. This is becoming less urgent in the advent of PPCI as coronary investigation occurs at this point.
Territories Of STEMI: Special Considerations
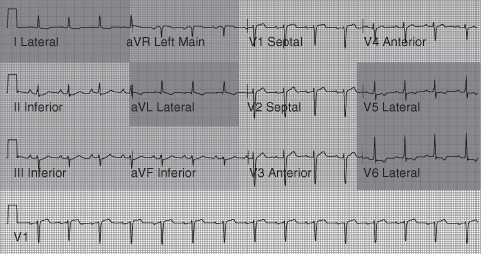
The area or territory of myocardial necrosis will depend on which coronary artery is occluded. See Table 7.5 and Figure 7.3 for infarct territories in relation to ST elevation on the 12-lead ECG.
Table 7.5 Regions of STEMI and associated ECG changes
| MI region | Artery occluded | Leads showing ECG changes |
| Anterior | LAD | V2–V5: anteroseptal infarction produces changes in one or more leads of V1–V4 (see Figure 7.4) |
| Inferior | Right (usually) | II, III, aVF “inferior leads” (see Figure 7.5) (Record right-sided ECG – leads V3R and V4R are clinically significant) (see Figures 7.6 and 7.7) |
| Posterior | Right or circumflex | Difficult to see. Posterior wall infarction causes dominant R wave (not q wave) in V1 with ST depression (see Figure 7.8) Often associated with inferior MI (posterior 12-lead ECG recommended – leads V7–V9 are clinically significant) |
| Lateral | Circumflex or diagonal branch of LAD | I, aVL, V5,6 “lateral leads” |
Figure 7.3 Diagrammatic representation of infarct territories in relation to the 12-lead ECG.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



