Antidiabetics
Objectives
• Compare type 1 and type 2 diabetes mellitus.
• Describe the symptoms of diabetes mellitus.
• Differentiate symptoms of hypoglycemic reaction and hyperglycemia.
• Compare the action of oral antidiabetic drugs and their side effects.
• Differentiate between the action of insulin, oral antidiabetic agents, and glucagon.
• Apply the nursing process to the patient taking insulin and oral antidiabetic agents.
Key Terms
diabetes mellitus, p. 759
hypoglycemic reaction, p. 762
insulin, p. 758
insulin shock, p. 762
ketoacidosis, p. 763
lipodystrophy, p. 760
oral antidiabetic drugs, p. 758
oral hypoglycemic drugs, p. 758
polydipsia, p. 759
polyphagia, p. 759
polyuria, p. 759
type 1 diabetes mellitus, p. 759
type 2 diabetes mellitus, p. 759
Antidiabetic drugs are used primarily to control diabetes mellitus, a chronic disease that affects carbohydrate metabolism. There are two groups of antidiabetic agents: (1) insulin and (2) oral hypoglycemic (antidiabetic) drugs. Insulin, a protein secreted from the beta cells of the pancreas, is necessary for carbohydrate metabolism and plays an important role in protein and fat metabolism. The beta cells make up 75% of the pancreas, and the alpha cells that secrete glucagon, a hyperglycemic substance, occupy approximately 20% of the pancreas. Oral hypoglycemic drugs, also known as oral antidiabetic drugs (to avoid confusion with the term hypoglycemic reaction), are synthetic preparations that stimulate insulin release or otherwise alter the metabolic response to hyperglycemia.
Diabetes Mellitus
Diabetes mellitus, a chronic disease resulting from deficient glucose metabolism, is caused by insufficient insulin secretion from the beta cells. This results in high blood glucose (hyperglycemia). Diabetes mellitus is characterized by the three p‘s: polyuria (increased urine output), polydipsia (increased thirst), and polyphagia (increased hunger). Diabetes mellitus is a disorder of the pancreas, whereas diabetes insipidus is a disorder of the posterior pituitary gland, discussed in detail in Chapter 51.
The four types of diabetes are presented in Table 52-1.Viral infections, environmental conditions, and genetic factors contribute to the onset of type 1 diabetes mellitus. Type 2 diabetes mellitus is the most common type of diabetes. Some sources suggest that heredity and obesity are the major factors that cause type 2 diabetes. With type 2 diabetes, there is some beta-cell function with varying amounts of insulin secretion. Hyperglycemia may be controlled for some type 2 diabetes patients with oral antidiabetic (hypoglycemic) drugs and a diet prescribed by the American Diabetic Association; however, about one third of patients with type 2 diabetes need insulin. Patients with type 2 diabetes who use one or two oral antidiabetic drugs may become insulin-dependent years later.
TABLE 52-1
TYPES AND OCCURRENCES OF DIABETES MELLITUS
| TYPES OF DIABETES MELLITUS | PERCENTAGE OF OCCURRENCES |
| Type 1 | 10%-12% |
| Type 2 | 85%-90% |
| Secondary diabetes (medications, hormonal changes) | 2%-3% |
| GDM | 1% (2%-5% of all pregnancies) |
Certain drugs increase blood glucose and can cause hyperglycemia in prediabetic persons. These include glucocorticoids (cortisone, prednisone), thiazide diuretics (hydrochlorothiazide [HydroDIURIL]), and epinephrine. Usually the blood glucose level returns to normal after the drug is discontinued.
During the second and third trimesters of pregnancy, the levels of the hormones progesterone, cortisol, and human placental lactogen (hPL) increase. These increased hormone levels can inhibit insulin usage. This is a contributing factor for the occurrence of gestational diabetes mellitus (GDM) during pregnancy. Glucose is then mobilized from the tissue and lipid storage sites. After pregnancy, the blood glucose level may decrease; however, some patients may develop diabetes mellitus, whereas others may develop type 2 diabetes in later years.
Insulin
Insulin is released from the beta cells of the islets of Langerhans in response to an increase in blood glucose. Oral glucose load is more effective in raising the serum insulin level than an intravenous (IV) glucose load. Insulin promotes the uptake of glucose, amino acids, and fatty acids and converts them to substances that are stored in body cells. Glucose is converted to glycogen for future glucose needs in the liver and muscle, thereby lowering the blood glucose level. The normal range for blood glucose is 60 to 100 mg/dL and 70 to 110 mg/dL for serum (or plasma) glucose. When the blood glucose level is greater than 180 mg/dL, glycosuria (glucose in the urine) can occur. Increased blood glucose acts as an osmotic diuretic, causing polyuria. When blood glucose remains elevated (>200 mg/dL), diabetes mellitus occurs.
In 2009, the American Diabetes Association (ADA) revised its recommendations to include hemoglobin A1c (HbA1c) for the diagnosis of diabetes. Previously, this test had been used to monitor treatment. HbA1c is a derivative of the interaction of glucose with hemoglobin in red blood cells (RBCs). Because RBCs have a life span of approximately 120 days, the HbA1c level reflects the average glucose level for up to 3 months. In monitoring treatment, the goal is to keep the diabetic patient’s HbA1c below 7%. For diagnostic purposes, an HbA1c level of ≤5% indicates that the patient does not have diabetes, 5.7% to 6.4% indicates prediabetes, and ≥6.5% indicates a diagnosis of diabetes mellitus.
Beta Cell Secretion of Insulin
The beta cells in the pancreas secrete approximately 0.2 to 0.5 units/kg/day. A patient weighing 70 kg (154 pounds) secretes 14 to 35 units of insulin per day. More insulin secretion may occur if the person consumes a greater caloric intake. A patient with diabetes mellitus may require 0.2 to 1 units/kg/day. The higher range may be because of obesity, stress, or tissue insulin resistance.
Commercially Prepared Insulin
Insulins are all currently manufactured by using deoxyribonucleic acid (DNA) technology. Human insulin (Humulin) was introduced in 1983 and duplicates insulin produced by the pancreas of the human body. Examples of human insulin include Humulin R and Novolin N. The use of Humulin insulin has a low incidence of allergic effects and insulin resistance. Human insulin analogues are modifications of human insulin with alterations in onset and duration of action. Insulin lispro (Humalog) and insulin aspart (Novolog) are examples of human insulin analogues.
Insulins are usually administered subcutaneously (subQ). Abdominal injections of insulin are absorbed faster than other body sites and found to be more consistent. Newly diagnosed patients with insulin-dependent diabetes are usually prescribed human insulin. In addition, patients in whom hyperglycemia develops during pregnancy or who already have diabetes and become pregnant are usually prescribed human insulin.
The concentration of insulin is 100 units/mL or 500 units/mL (U100/mL or U500/mL, respectively), and the insulin is packaged in a 10-mL vial. Insulin 500 units is only available in short-acting regular insulin (Humulin R and Novolin R). Insulin 500 units is seldom used except in emergencies and for patients with serious insulin resistance (>200 units/day). Insulin 40 units is no longer used in the United States, although it is still used in other countries. Insulin syringes are typically marked in units of 100 units per 1 mL or 50 units per 0.5 mL for insulin U100. Insulin syringes must be used for accurate dosing. To prevent dosage errors, the nurse must be certain that there is a match of the insulin concentration with the calibration of units on the insulin syringe. Before use, the patient or nurse must roll, not shake, cloudy insulin bottles to ensure that the insulin and its ingredients are well mixed. Shaking a bottle of insulin can cause bubbles and an inaccurate dose. Insulin requirements vary; usually less insulin is needed with increased exercise, and more insulin is needed with infections and high fever.
Administration of Insulin
Insulin is a protein and cannot be administered orally because gastrointestinal (GI) secretions destroy the insulin structure. It is administered subQ, at a 45- to 90-degree angle. The 90-degree angle is made by raising the skin and fatty tissue; the insulin is injected into the pocket between the fat and the muscle. In a thin person with little fatty tissue, the 45- to 60-degree angle is used. Regular insulin is the only type that can be administered IV.
The site and depth of insulin injection affect absorption. Insulin absorption is greater when given in the abdominal areas than when given in the thigh and buttock areas. Heat and massage could increase subQ absorption. Cooling the subQ area can decrease absorption.
Insulin is usually given in the morning before breakfast. It can be given several times a day. Insulin injection sites should be rotated to prevent lipodystrophy (tissue atrophy or hypertrophy), which can interfere with insulin absorption. Lipoatrophy (tissue atrophy) is a depression under the skin surface that primarily occurs in women and children. Lipohypertrophy (tissue hypertrophy) is a raised lump or knot on the skin surface that is more common in men. It is frequently caused by repeated injections into the same subQ site. The patient needs to develop a “site rotation pattern” to avoid lipodystrophy and to promote insulin absorption. There are various insulin rotation programs, such as an 8-day rotation schedule (insulin is given at a different site each day). The ADA suggests that insulin be injected daily at a chosen site for 1 week. Injections should be 1.5 inches apart (a knuckle length) at a site area each day. When a patient requires two insulin injections a day (morning and evening), one site should be chosen on the right side (morning) and one site chosen on the left side (evening). Figure 52-1 illustrates the sites for insulin injections. A record of injection area sites and dates administered should be kept.
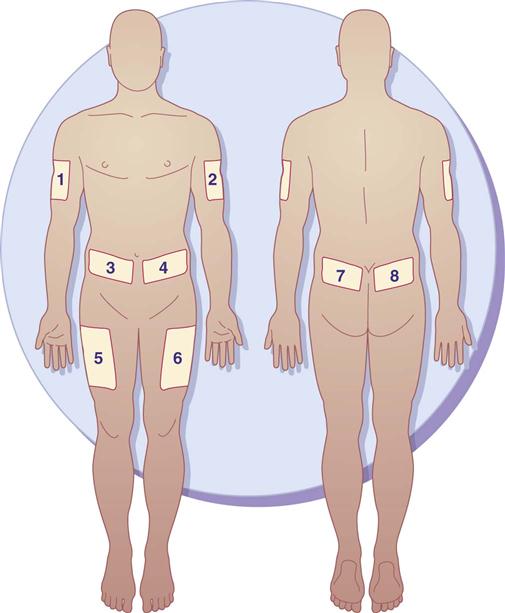
Illness and stress increase the need for insulin. Insulin doses should not be withheld during illness, including infections and stress. Hyperglycemia and ketoacidosis may result from insufficient insulin.
Types of Insulin
There are several standard types of insulin: rapid acting, short acting, intermediate acting, long acting, and combinations. Rapid- and short-acting insulins are in a clear solution without any added substance to prolong insulin action. Intermediate-acting insulins are cloudy and may contain protamine, a protein that prolongs the action of insulin, or zinc, which also slows the onset of action and prolongs the duration of activity.
Rapid-acting insulins include insulin lispro (human analog) (Humalog) and human insulin aspart (rDNA origin) (NovoLog). Insulin lispro is formed by reversing two amino acids in human regular insulin (Humulin). Insulin aspart (rDNA origin) is another human insulin analogue in which a single amino acid (proline) has been substituted with aspartic acid to help prevent the molecules from clumping together to allow quicker entry into blood circulation. Insulin lispro and insulin aspart act faster than regular insulin, so they must not be administered more than 5 minutes before mealtime (food should be present before administering these insulins). Patients who are insulin-dependent and take rapid-acting insulin usually require intermediate-acting insulin as well.
Short-acting insulin has an onset of action in 30 minutes to 1 hour. The peak action occurs in 2.5 to 5 hours, and the duration of action is 6 to 8 hours. Regular (unmodified, crystalline) insulin is short-acting insulin that can be administered IV and subQ. Regular insulin is generally given 30 minutes before meals.
Intermediate-acting insulins include neutral-protamine-Hagedorn (NPH), Lente, and Humulin N. Isophane insulins like NPH and Humulin N contain protamine, a protein that prolongs the action of insulin. Humulin L insulin contains zinc, which also prolongs the insulin action time. The onset of intermediate-acting insulin is 1 to 2 hours, peak action occurs in 6 to 12 hours, and the duration of action is 18 to 24 hours.
Insulin glargine (Lantus) is long-acting insulin with an onset of 1 hour. It is evenly distributed over a 24-hour duration of action; thus it is administered once a day, usually at bedtime. Incidence of nocturnal hypoglycemia is not as common as with other insulins because of its continuous sustained release. Insulin detmir (Levemir) is another long-acting insulin that peaks in 6 to 8 hours and lasts for 12 to 24 hours. These two insulins are analogues of human insulin. Approved by the FDA in 2000, Glargine was the first long-acting recombinant DNA (rDNA origin) human insulin for patients with types 1 and 2 diabetes. Glargine and detmir are available in a prefilled 3-mL cartridge for the “OptiPen One” insulin pen device. Some patients complain of more pain at the injection site with the administration of glargine than with NPH insulin.
Combination insulins are commercially premixed. These include Humulin 70/30, Novolin 70/30, Humulin 50/50, and Humalog Mix 75/25. These combinations are widely used. The Humulin 70/30 vials or prefilled disposable pens contain 70% of human insulin isophane (intermediate-acting insulin, NPH) and 30% regular (fast-acting) insulin. The exterior of an insulin pen resembles a fountain pen. The Humulin 50/50 vial or pen contains 50% isophane (NPH) insulin and 50% regular insulin. The Humalog 75/25 mix is available in vials or prefilled disposable pens and contains 75% lispro protamine insulin and 25% lispro “rapid” insulin. Humalog 75/25 helps prevent hypoglycemia, which could occur with the 70/30 or 50/50 combination insulins, and helps control hyperglycemia more effectively. The 75/25 pen contains 300 units of insulin and does not require refrigeration after first use. It can be stored at room temperature for up to 10 days. With these combinations of insulin, the patient does not have to mix regular and NPH insulins as long as one of these combinations is effective. However, some patients need less than 25% or 30% regular insulin and more intermediate-acting insulin. These patients needs to mix the two insulins in the prescribed proportions.
Regular insulin can be mixed with protamine or zinc insulin in the same syringe. However, mixing insulin can alter the absorption rate.
Insulin Resistance
Antibodies develop over time in persons taking animal insulin. This can slow the onset of insulin action and extend its duration of action. Antibody development can cause insulin resistance and insulin allergy. Obesity can also be a causative factor for insulin resistance. Skin tests with different insulin preparations may be performed to determine whether there is an allergic effect. Human and regular insulins produce fewer allergens.
Storage of Insulin
Unopened insulin vials are refrigerated until needed. Once an insulin vial has been opened, it may be kept (1) at room temperature for 1 month or (2) in the refrigerator for 3 months. Insulin is less irritating to the tissues when injected at room temperature. Insulin vials should not be put in the freezer. In addition, insulin vials should not be placed in direct sunlight or in a high-temperature area. Prefilled syringes should be stored in the refrigerator and should be used within 1 to 2 weeks. Opened insulin vials lose their strength after approximately 3 months.
Prototype Drug Chart 52-1 lists the pharmacologic data for the different types of insulin.
Pharmacokinetics
All insulins can be administered subQ, but only regular insulin can be given IV. The half-life varies. Insulin is metabolized by the liver and muscle and is excreted in the urine.
Pharmacodynamics
Insulin lowers blood sugar by promoting the use of glucose by the body’s cells. Insulin is also active in the storage of glucose as glycogen in muscles. The onset of action of rapid-acting insulin given subQ is 5 to 15 minutes. The onset of action of regular insulin is 30 minutes to 1 hour given subQ and 10 to 30 minutes given IV. The onset of action of intermediate-acting insulin is 1 to 2 hours. The peak action of insulins is important because of the possibility of hypoglycemic reaction (insulin shock) occurring during that time. The peak action for rapid-acting insulin is 30 minutes to 3 hours. The peak time for regular insulin is 2.5 to 5 hours; for intermediate-acting insulin, it is 6 to 12 hours. The nurse needs to assess for signs and symptoms of hypoglycemic reaction, such as nervousness, tremors, confusion, sweating, and increased pulse rate. Orange juice, sugar-sweetened beverages, or hard candy should be kept available and given if a reaction occurs. If patient is unable to ingest fast acting carbohydrates, glucagon may be given. Glucagon promotes the breakdown of glycogen in the liver, raising blood glucose within 10 minutes.
Regular insulin can be given several times a day, especially during the regulation of insulin dosage. Intermediate- and long-acting insulins are usually administered once a day. Regular insulin can be mixed with intermediate-acting insulin (Humulin N), especially if rapid onset of action is needed. When switching from one type of insulin to another, the patient may require a dose adjustment because human insulin has a shorter duration of action.
Sliding-Scale Insulin Coverage
Insulin may be administered in adjusted doses that depend on individual blood glucose test results. When the diabetic patient has extreme variances in insulin requirements (e.g., stress from hospitalization, surgery, illness, or infection), adjusted dosing or sliding-scale insulin coverage provides a more constant blood glucose level. Blood glucose testing is performed several times a day at specified intervals (usually before meals). A preset scale usually involves directions for the administration of rapid- or short-acting insulin.
Drug Interactions.
Drugs such as thiazide diuretics, glucocorticoids (cortisone preparations), thyroid agents, and estrogen increase the blood sugar; therefore the insulin dosage may need adjustment. Drugs that decrease insulin needs are tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), aspirin products, and oral anticoagulants.
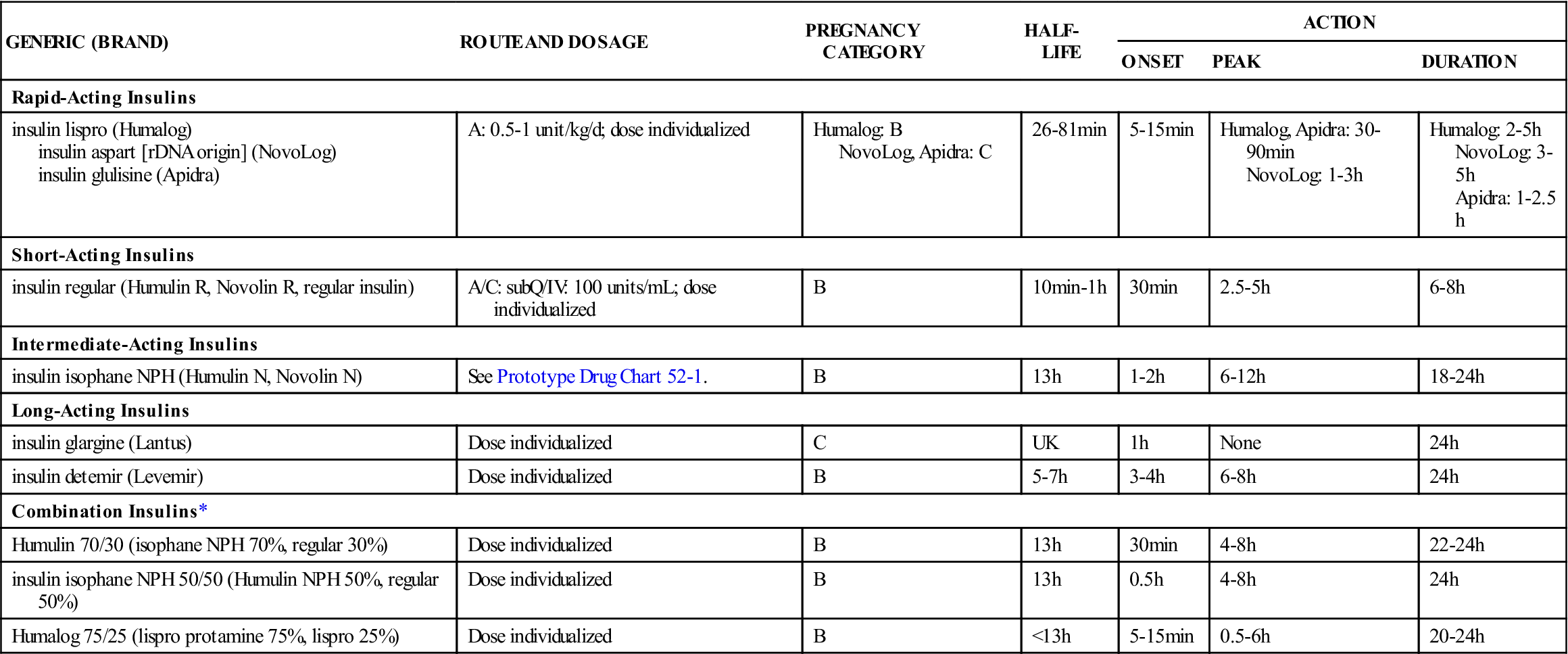
Table 52-2 lists the rapid-acting, short-acting, intermediate-acting, long-acting, and combination insulins with their dosages, uses, and considerations.
![]() TABLE 52-2
TABLE 52-2
| GENERIC (BRAND) | ROUTE AND DOSAGE | PREGNANCY CATEGORY | HALF-LIFE | ACTION | ||
| ONSET | PEAK | DURATION | ||||
| Rapid-Acting Insulins | ||||||
| insulin lispro (Humalog) insulin aspart [rDNA origin] (NovoLog) insulin glulisine (Apidra) | A: 0.5-1 unit/kg/d; dose individualized | Humalog: B NovoLog, Apidra: C | 26-81 min | 5-15 min | Humalog, Apidra: 30-90 min NovoLog: 1-3 h | Humalog: 2-5 h NovoLog: 3-5 h Apidra: 1-2.5 h |
| Short-Acting Insulins | ||||||
| insulin regular (Humulin R, Novolin R, regular insulin) | A/C: subQ/IV: 100 units/mL; dose individualized | B | 10 min-1 h | 30 min | 2.5-5 h | 6-8 h |
| Intermediate-Acting Insulins | ||||||
| insulin isophane NPH (Humulin N, Novolin N) | See Prototype Drug Chart 52-1. | B | 13 h | 1-2 h | 6-12 h | 18-24 h |
| Long-Acting Insulins | ||||||
| insulin glargine (Lantus) | Dose individualized | C | UK | 1 h | None | 24 h |
| insulin detemir (Levemir) | Dose individualized | B | 5-7 h | 3-4 h | 6-8 h | 24 h |
| Combination Insulins* | ||||||
| Humulin 70/30 (isophane NPH 70%, regular 30%) | Dose individualized | B | 13 h | 30 min | 4-8 h | 22-24 h |
| insulin isophane NPH 50/50 (Humulin NPH 50%, regular 50%) | Dose individualized | B | 13 h | 0.5 h | 4-8 h | 24 h |
| Humalog 75/25 (lispro protamine 75%, lispro 25%) | Dose individualized | B | <13 h | 5-15 min | 0.5-6 h | 20-24 h |
Side Effects and Adverse Reactions: Hypoglycemic Reactions and Ketoacidosis.
When more insulin is administered than needed for glucose metabolism, a hypoglycemic reaction, or insulin shock, occurs. The person may exhibit nervousness, trembling, and lack of coordination; have cold and clammy skin; and complain of a headache. Some patients become combative and incoherent. Giving sugar orally or IV increases the use of insulin, and the symptoms disappear immediately.
In response to an excessive dose of insulin, diabetic patients may develop the Somogyi effect. This hypoglycemic condition usually occurs in the predawn hours of 2:00 AM to 4:00 AM. A rapid decrease in blood glucose during the nighttime hours stimulates a release of hormones (e.g., cortisol, glucagon, epinephrine) to increase blood glucose by lipolysis, gluconeogenesis, and glycogenolysis, thus creating the Somogyi effect. Management of the Somogyi effect involves monitoring blood glucose between 2:00 AM and 4:00 AM and reducing the bedtime insulin dosage.
Hyperglycemia on awakening is known as the dawn phenomenon. The patient usually awakens with a headache and reports night sweats and nightmares. Management of the dawn phenomenon involves increasing the bedtime dose of insulin.
With an inadequate amount of insulin, sugar cannot be metabolized, and fat catabolism occurs. The use of fatty acids (ketones) for energy causes ketoacidosis (diabetic acidosis or diabetic coma). Table 52-3 lists the signs and symptoms of hypoglycemic reaction and ketoacidosis.
TABLE 52-3
HYPOGLYCEMIC REACTION AND DIABETIC KETOACIDOSIS
| REACTION | SIGNS AND SYMPTOMS |
| Hypoglycemic reaction (insulin shock) | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
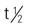 : varies with type of insulin
: varies with type of insulin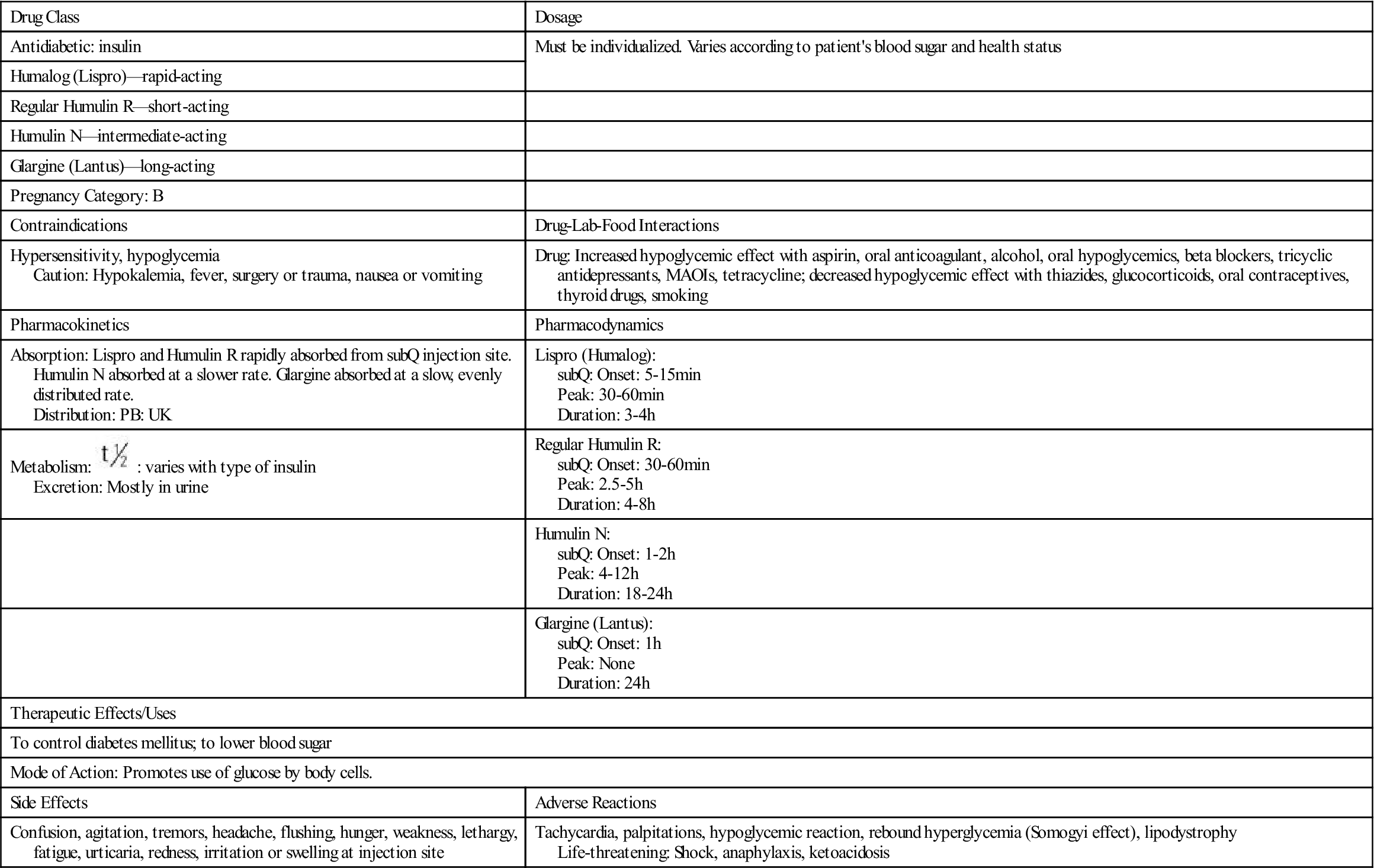
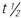 , half-life; UK, unknown.
, half-life; UK, unknown.