Anticonvulsants
Objectives
• Contrast the two international classifications of seizures with characteristics of each type.
• Differentiate between the types of seizures.
• Apply the nursing process to anticonvulsants including patient teaching.
Key Terms
anoxia, p. 304
anticonvulsants, p. 305
atonic seizure, p. 305
clonic seizure, p. 305
convulsion, p. 304
electroencephalogram, p. 304
gingival hyperplasia, p. 306
grand mal, p. 305
hydantoins, p. 306
idiopathic, p. 304
nystagmus, p. 306
petit mal, p. 305
psychomotor, p. 305
seizure, p. 304
seizure threshold, p. 306
sloughing, p. 306
status epilepticus, p. 306
teratogenic, p. 306
tonic seizure, p. 305
Millions of people in the United States have active epilepsy, a seizure disorder. The seizure associated with epilepsy results from abnormal electric discharges from the cerebral neurons and is characterized by a loss or disturbance of consciousness and usually by a convulsion (involuntary paroxysmal muscular contractions). The electroencephalogram (EEG), computed tomography (CT), and magnetic resonance imaging (MRI) are useful in diagnosing epilepsy. The EEG records abnormal electric discharges of the cerebral cortex. Of all seizure cases, 75% are considered to be primary, or idiopathic (of unknown cause), and the remaining are secondary to brain trauma, brain anoxia (absence of oxygen), infection, or cerebrovascular disorders (e.g., cerebrovascular accident, stroke). Epilepsy is a chronic, usually lifelong, disorder. The majority of persons with seizures had their first seizure before 20 years of age.
Seizures that are not associated with epilepsy could result from fever, hypoglycemic reaction, electrolyte imbalance (hyponatremia), metabolic imbalance (acidosis or alkalosis), and alcohol or drug use. When these conditions are corrected, the seizures cease. Recurrent seizures may result from birth and perinatal injuries, head trauma, congenital malformations, neoplasms (tumors), and idiopathic or unknown causes.
International Classification of Seizures
There are various types of seizures, such as grand mal (tonic-clonic), petit mal (absence), and psychomotor. The international classification of seizures (Table 22-1) describes two categories of seizure: generalized and partial. A person may also have mixed (more than one type) seizures.
TABLE 22-1
INTERNATIONAL CLASSIFICATION OF SEIZURES
| CATEGORY | CHARACTERISTICS |
| Generalized Seizures | Convulsive and nonconvulsive; involve both cerebral hemispheres of the brain |
| Tonic-clonic seizure | Also called grand mal seizure; most common form of seizure. In the tonic phase, skeletal muscles contract or tighten in a spasm lasting 3 to 5 seconds. In the clonic phase, there is a dysrhythmic muscular contraction, or jerkiness, of legs and arms lasting 2 to 4 minutes |
| Tonic seizure | Sustained muscle contraction |
| Clonic seizure | Dysrhythmic muscle contraction |
| Absence seizure | Also called petit mal seizure; brief loss of consciousness lasting less than 10 seconds; fewer than three spike waves on the electroencephalogram (EEG) printout; usually occurs in children |
| Myoclonic seizure | Isolated clonic contraction or jerks lasting 3 to 10 seconds; may be limited to one limb (focal myoclonic) or involve the entire body (massive myoclonic); may be secondary to a neurologic disorder such as encephalitis or Tay-Sachs disease |
| Atonic seizure | Head drop; loss of posture; sudden loss of muscle tone. If lower limbs are involved, patient could collapse |
| Infantile spasms | Muscle spasm |
| Partial Seizures | Involve one hemisphere of the brain. No loss of consciousness in simple partial seizures, but there is a loss of consciousness in complex partial seizures |
| Simple seizure | Occurs in motor, sensory, autonomic, and psychic forms; no loss of consciousness |
| Motor | Formerly called the Jacksonian seizure; involves spontaneous movement that spreads; can develop into a generalized seizure |
| Sensory | Visual, auditory, or taste hallucinations |
| Autonomic response | Paleness, flushing, sweating, or vomiting |
| Psychological | Personality changes |
| Complex seizure | Loss of consciousness. Patient does not recall behavior immediately before, during, and immediately after seizure |
| Psychomotor | Complex symptoms: automatisms (repetitive behavior such as chewing or swallowing motions), behavioral changes, and motor seizures |
| Cognitive | Confusion or memory impairment |
| Affective | Bizarre behavior |
| Compound | May lead to generalized seizures such as tonic-clonic, tonic |
Anticonvulsants
Drugs used for epileptic seizures are called anticonvulsants or antiepileptic drugs (AEDs). Anticonvulsant drugs stabilize nerve cell membranes and suppress the abnormal electric impulses in the cerebral cortex. These drugs prevent seizures but do not eliminate the cause or provide a cure. Anticonvulsants are classified as central nervous system (CNS) depressants.
With the use of anticonvulsants, seizures are controlled in approximately 70% of patients. Anticonvulsants are usually taken throughout the person’s lifetime. In some cases, the health care provider might discontinue the anticonvulsant if no seizures have occurred in the past 3 to 5 years.
There are many types of anticonvulsants used to treat seizures, including the hydantoins (phenytoin), long-acting barbiturates (phenobarbital, mephobarbital, primidone), succinimides (ethosuximide), benzodiazepines (diazepam, clonazepam), carbamazepine, and valproate (valproic acid). Anticonvulsants are not indicated for all types of seizures. For example, phenytoin is effective in treating grand mal (tonic-clonic) seizures and psychomotor seizures but is not effective in treating petit mal (absence) seizures.
Pharmacophysiology: Action of Anticonvulsants
The anticonvulsant drugs work in one of three ways: (1) by suppressing sodium influx through the drug binding to the sodium channel when it is inactivated, prolonging the channel inactivation and thereby preventing neuron firing; (2) by suppressing the calcium influx, preventing the electric current generated by the calcium ions to the T-type calcium channel; or (3) by increasing the action of gamma-aminobutyric acid (GABA), which inhibits neurotransmitter throughout the brain. The drugs that suppress sodium influx are phenytoin, fosphenytoin, carbamazepine, oxcarbazepine, valproic acid, topiramate, zonisamide, and lamotrigine. Valproic acid and ethosuximide are examples of drugs that suppress calcium influx. Examples of drug groups that enhance the action of GABA are barbiturates, benzodiazepines, and tiagabine. Gabapentin promotes GABA release.
Hydantoins
The first anticonvulsant used to treat seizures was phenytoin, a hydantoin discovered in 1938 that is still the most commonly used drug for controlling seizures. Hydantoins act by inhibiting sodium influx, stabilizing cell membranes, reducing repetitive neuronal firing, and limiting seizures. By increasing the electrical stimulation threshold in cardiac tissue, it also acts as an antidysrhythmic. It has a small effect on general sedation, and it is nonaddicting. However, this drug should not be used during pregnancy because it can have a teratogenic effect on the fetus.
Drug dosage for phenytoin and other anticonvulsants is age related. Newborns, persons with liver disease, and older adults require a lower dosage because of a decrease in metabolism resulting in more available drug. Conversely, individuals with an increased metabolic rate, such as children, may require an increased dosage. The drug dosage is adjusted according to the therapeutic plasma or serum level. Phenytoin has a narrow therapeutic range of 10 to 20 mcg/mL. The benefits of an anticonvulsant become apparent when the serum drug level is within the therapeutic range. Typically, if the drug level is below the desired range, the patient is not receiving the required drug dosage to control seizure activity. If the drug level is above the desired range, drug toxicity may result. Monitoring the therapeutic serum drug range is of utmost importance to ensure drug effectiveness. Prototype Drug Chart 22-1 lists the pharmacologic data associated with phenytoin.
Pharmacokinetics
Phenytoin is slowly absorbed from the small intestine. It is a highly protein-bound (90% to 95%) drug; a decrease in serum protein or albumin can increase the free phenytoin serum level. With a small to average drug dose, the half-life of phenytoin is approximately 24 hours, but the range can be from 7 to 42 hours. Phenytoin is metabolized to inactive metabolites, and this portion is excreted in the urine.
Pharmacodynamics
The pharmacodynamics of orally administered phenytoin include onset of action within 30 minutes to 2 hours, peak serum concentration in 1.5 to 6 hours, steady state of serum concentration in 7 to 10 days, and a duration of action dependent on the half-life of up to 45 hours. Oral phenytoin is most commonly ordered as a sustained-release (SR) capsule. The peak SR concentration time is 4 to 12 hours.
Intravenous (IV) infusion of phenytoin should be administered by direct injection into a large vein via a central line or peripherally inserted central catheter. The drug may be diluted in saline solution; however, dextrose solution should be avoided because of drug precipitation. The manufacturer recommends administration of phenytoin via Y-tube or three-way stopcock rather than continuous IV infusion to avoid precipitation. IV phenytoin 50 mg, or a fraction thereof, should be administered over 1 minute for adults and at a rate of 25 mg/min when the patient is older. Infusion rates of more than 50 mg/min may cause hypotension or cardiac dysrhythmias, especially with older and debilitated patients. Local irritation at the injection site may be noted, and sloughing (formation of dead tissue that separates from living tissue) may occur. The IV line should always be flushed with saline before and after each dose to reduce venous irritation. Intramuscular (IM) injection of phenytoin irritates tissues and may cause damage. For this reason and because of its erratic absorption rate, phenytoin is not given by the IM route.
Side Effects and Adverse Reactions
The severe side effects of hydantoins include neurologic and psychiatric effects (i.e., slurred speech, confusion, depression), thrombocytopenia (low platelet count), leukopenia (low white blood cell count), and gingival hyperplasia (overgrowth of the gum tissues or reddened gums that bleed easily). Patients on hydantoins for long periods might have an elevated blood sugar (hyperglycemia) that results from the drug inhibiting the release of insulin. Less severe side effects include nausea, vomiting, constipation, drowsiness, headaches, alopecia, hirsutism, and nystagmus (constant, involuntary, cyclical movement of the eyeball).
Drug-Drug Interactions
Drug interaction is common with hydantoins, because they are highly protein-bound. Hydantoins compete with other drugs (e.g., anticoagulants, aspirin) for plasma protein-binding sites. The hydantoins displace anticoagulants and aspirin, causing more free-drug availability and increasing their activity. Barbiturates, rifampin, and a chronic ingestion of ethanol increase hydantoin metabolism. Drugs like sulfonamides and cimetidine (Tagamet) can increase the action of hydantoins by inhibiting liver metabolism, which is necessary for drug excretion. Antacids, calcium preparations, sucralfate (Carafate), and antineoplastic drugs also decrease the absorption of hydantoins. Antipsychotics and certain herbs can lower the seizure threshold (level at which seizure may be induced) and increase seizure activity (Herbal Alert 22-1). The patient should be closely monitored for seizure occurrence.
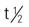 : 7-42 h; average: 24 h
: 7-42 h; average: 24 h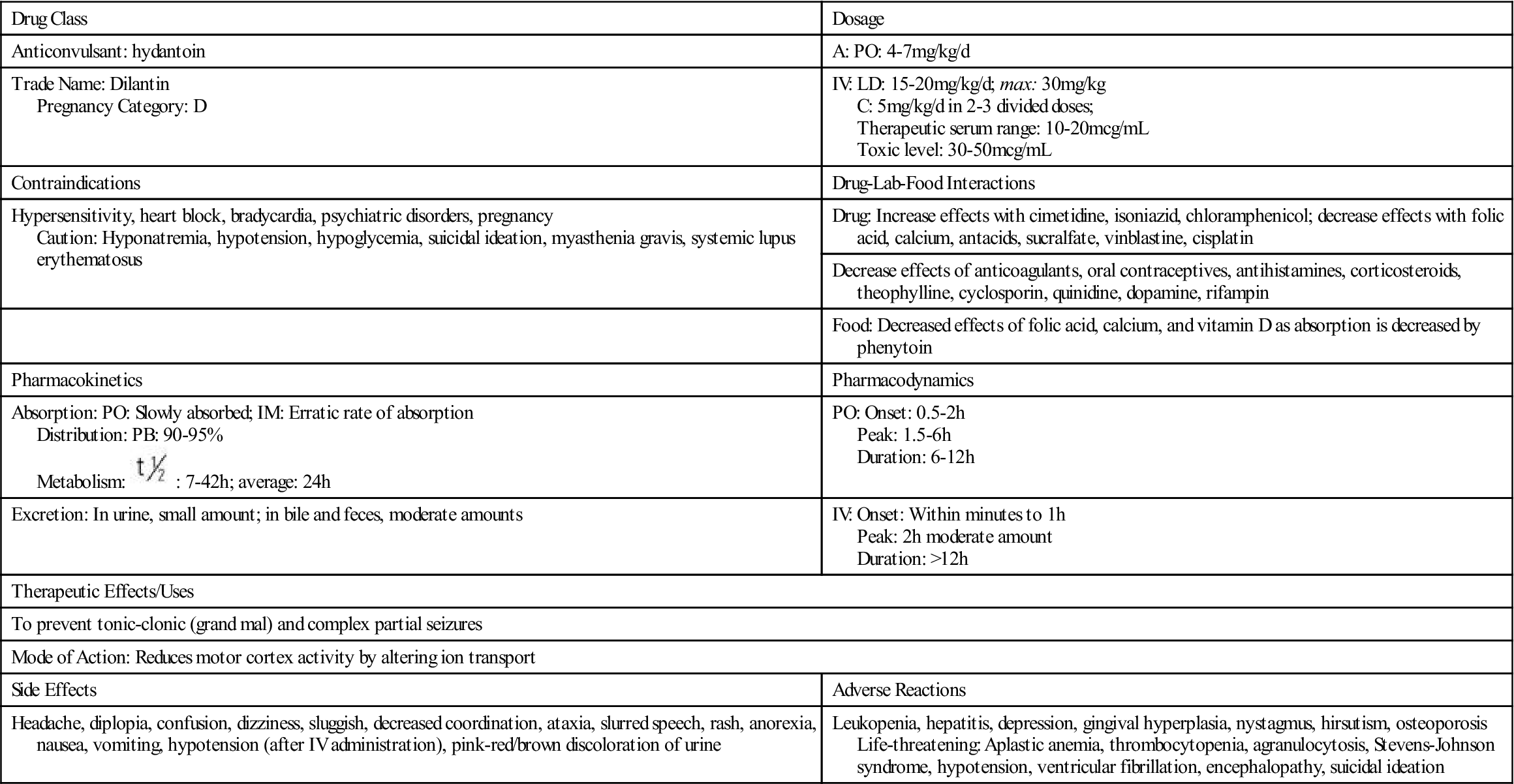
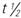 , half-life; t.i.d., three times a day; >, greater than; <, less than.
, half-life; t.i.d., three times a day; >, greater than; <, less than.


