Anticancer Drugs
Objectives
• Differentiate between cell cycle–specific and cell cycle–nonspecific anticancer drugs.
• Discuss ways the nurse can avoid exposure to chemotherapeutic agents.
• Apply patient-centered collaborative care, including teaching, related to anticancer drugs.
• Develop a focused teaching plan about the uses and side effects of anticancer drugs.
Key Terms
adjuvant therapy, p. 505
alkylating agents, p. 507
antimetabolites, p. 513
antineoplastic drugs, p. 503
antitumor antibiotics, p. 518
apoptosis, p. 503
cell cycle–nonspecific drugs, p. 504
cell cycle–specific drugs, p. 504
combination chemotherapy, p. 503
cytoprotectant (chemoprotectant), p. 510
cytotoxic therapy, p. 503
doubling time, p. 504
growth fraction, p. 504
hormones, p. 524
leucovorin rescue, p. 513
metastasis, p. 505
myelosuppression, p. 505
nadir, p. 505
neoadjuvant chemotherapy, p. 505
palliative chemotherapy, p. 505
protocol, p. 505
taxanes, p. 521
vesicant, p. 510
vesication, p. 518
vinca alkaloids, p. 521
![]() http://evolve.elsevier.com/KeeHayes/pharmacology/
http://evolve.elsevier.com/KeeHayes/pharmacology/
The authors gratefully acknowledge the work of Carolee A. Polek, who updated this chapter for the eighth edition.
Cancer-related deaths rank second only to heart disease in the United States. Even though cancer-related mortality has decreased since the early 1990s, 1 in 3 women and 1 in 2 men are projected to develop cancer over their lifetime. Excluding skin cancers, the highest incidence rates in men are prostate, lung, and colorectal cancer. In women, breast, lung, and colorectal cancers occur with the highest frequency. Lung cancer remains the leading cause of cancer-related death regardless of gender.
The incidence and mortality rates of cancer differ by ethnicity. African-American men have higher incidence and death rates from cancer than do white men. African-American women have a lower incidence of cancer than white women, but a higher mortality rate. Latinos have a lower incidence than non-Latinos, except for cancer of the stomach, liver, and cervix. Cancer among Asian Americans has traditionally been lower than other ethnic groups, but incidence increases as immigrants adopt a more westernized lifestyle. The incidence of liver and stomach cancer is higher among Asian immigrants, secondary to higher rates of chronic hepatitis B in this group.
Cancer is a group of diseases in which abnormal cells grow out of control and may spread to other areas of the body. Deoxyribonucleic acid (DNA) is the genetic substance in the body cells that transfers information necessary for the production of enzymes and protein synthesis. In most cases, cancer is caused by damage to the DNA within the cell. Although some cancers are inherited, most develop when genes in a normal cell become damaged or lost (mutation). More than one mutation is required before a malignancy can develop. Therefore the development of cancer is a multistep process that may take years to complete.
Pharmaceuticals are often used to destroy cancer cells and are called by different names, including anticancer drugs, cancer chemotherapeutic agents, antineoplastic drugs, or cytotoxic therapy. In the 1970s, the use of two or more chemotherapy agents (combination chemotherapy) to treat cancer was adopted and led to improved response rates and increased survival times. Chemotherapy may be used as the sole treatment of cancer or in conjunction with other modalities (e.g., radiation, surgery, biologic response modifiers). Combination chemotherapy has proved to be effective in curing some cancers. When cancer cannot be cured, anticancer drugs may be given to control the disease for a period of months to years. If cancer can no longer be controlled, chemotherapy may be used to relieve disease-related symptoms or improve quality of life. This is called palliative treatment.
Genetic, Infective, Environmental, and Dietary Influences
Cancer is a genetic disease. Genes provide the instructions for the production and function of cellular proteins that are essential for normal cellular activities. Genetic defects may occur in a variety of ways, including deletion, translocation, duplication, inversion, or insertion of genetic material. When these defects cannot be effectively repaired, cells exhibit abnormal characteristics and unregulated growth. More than 2000 genes have been causally implicated in the formation of cancer. Cancers that have a proven genetic influence include breast, ovarian, prostate, endometrial, colon, pancreatic, and lung cancers; retinoblastoma; and malignant melanoma. Many more genetic influences are expected to be found. Environment, lifestyle, viruses, and diet can influence the development of these and other types of cancers. Box 37-1 gives examples of types of environmental products, viruses, and foods that have a carcinogenic effect on cancer development in humans.
Genes can cause cells to become cancerous in several ways. Proto-oncogenes are normal genes that are involved in the controlled growth, division, and death (apoptosis) of cells. An oncogene is a mutation in a proto-oncogene. An abnormal oncogene can effect cellular growth-control proteins and trigger unregulated cell division. Tumor-suppressor (TS) genes (anti-oncogenes) signal a cell to cease multiplying and act to stop the action of oncogenes. If TS genes become lost or dysfunctional, cells could reproduce uncontrollably. Other genes repair damage to DNA. If these DNA-repair genes are damaged, mutations are not mended and are subsequently passed on to the next generation of daughter cells. It may take a long time before sufficient cell mutations take place and cause cancer to develop. As a result, cancers more commonly occur in older individuals.
A number of viruses are associated with the development of cancer. The human papillomavirus (HPV) has been found in most women with invasive cervical cancer. Individuals with human immunodeficiency virus (HIV) may develop lymphomas and anal or genital cancers. The Epstein-Barr virus is found in almost all people with Burkitt’s lymphoma in central Africa. This virus has been implicated in the development of nasopharyngeal cancer. Hepatocellular carcinoma (liver cancer) is linked to the hepatitis B or C virus. Other viruses that have a link to the development of cancer include human T-cell lymphotropic virus, type 1 (HTLV-1); human T-cell lymphotropic virus, type 2 (HTLV-2); and Kaposi sarcoma–associated herpes virus.
Bacteria can play a role in the development of cancer. The presence of Helicobacter pylori in the stomach is associated with an increased risk of developing gastric cancer. Some reports have indicated a link between certain bacteria and cancer of the gallbladder, colon, and lung. However, evidence that supports an association between bacterial infection and other cancers is unclear.
Environmental factors associated with the development of cancer include tobacco use, diet, infectious agents, chemicals, excessive sun exposure, and radiation. According to the American Cancer Society (ACS), the use of tobacco, an unhealthy diet, and inadequate physical activity account for 75% of cancer cases and deaths in the United States.
Cell Cycle–Nonspecific and Cell Cycle–Specific Anticancer Drugs
The cell cycle for normal and cancer cells, growth fraction, and doubling time are discussed in Unit XII (see Figure XII-1). See the discussion at the beginning of Unit XII for clarification of the cell cycle and definitions.
Anticancer drugs cause cell death by interfering with cell replication and are classified according to their action on the cell. Cell cycle–nonspecific (CCNS) drugs (phase-nonspecific drugs) act during any phase of the cell cycle, including the G0 phase. Cell cycle–specific (CCS) drugs (phase-specific drugs) exert their influence during a specific phase or phases of the cell cycle. CCS agents are most effective against rapidly growing cancer cells. In general, the CCNS drugs include the alkylating drugs (although some alkylating agents are CCS), antitumor antibiotics, and hormones. The CCS drugs include antimetabolites and vinca alkaloids. Figure 37-1 shows selected types of anticancer drugs and the phase of the cell cycle in which they are most effective.
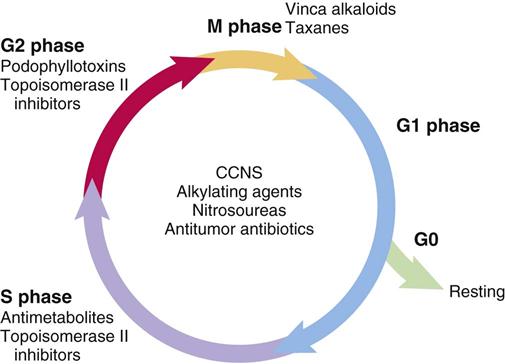
Growth fraction and doubling time are two factors that play a major role in the response of cancer cells to anticancer drugs. Anticancer drugs are more effective against neoplastic cells that have a high growth fraction (i.e., a high percentage of actively dividing cells). Leukemias and some lymphomas have high growth fractions and thus respond well to anticancer drug therapy.
Solid tumors have a large percentage of their cell mass in the G0 phase, so they generally have a low growth fraction and are less sensitive to anticancer drugs. High-dose chemotherapy results in better tumoricidal (tumor-killing) effects. Depending on the type of cancer, malignant cell growth is usually faster in the earlier stages of tumor development. As the tumor grows, the blood supply decreases, thereby slowing the growth rate. Anticancer agents are more effective against small, fast growing tumors with sufficient blood supply. As the tumor enlarges, its growth fraction decreases and its doubling time increases, reducing the effectiveness of anticancer therapy. The vascularization in solid tumors can be inconsistent. Some areas of tumor may have an adequate blood supply, while other areas are poorly perfused. This characteristic may make some large tumors resistant to anticancer drugs and therefore difficult to treat.
Cancer Chemotherapy
Anticancer drugs are not selective, so both cancer cells and normal cells are affected. The side effects of chemotherapy are largely related to the toxic effects on normal cells. Antineoplastic agents are effective because normal cells are able to repair themselves and continue to grow, whereas cancer cells are less able to do so; thus, the side effects of chemotherapy are most often temporary. Chemotherapy is usually administered systemically for cancer that has spread to other parts of the body, for tumors in multiple sites, or for tumors that are too large to be removed through other means (e.g., surgery). The most common route of chemotherapy administration is via intravenous (IV) infusion, although other routes may be used, including oral, intramuscular, subcutaneous, intraperitoneal, intraventricular (intrathecal), intrapleural, intravesicular, intra-arterial, or topical.
Some types of cancer can be cured with chemotherapy (e.g., Hodgkin’s disease, Burkitt’s lymphoma, testicular cancer). Other types of cancer (e.g., breast cancer, colon cancer) may be treated with surgery first, followed by chemotherapy to eliminate any residual tumor cells (microscopic metastases) that may remain in the body. This is referred to as adjuvant therapy. Sometimes neoadjuvant chemotherapy may be given first to help shrink a large tumor, so that it can be surgically removed. Palliative chemotherapy is used to relieve symptoms associated with advanced disease (e.g., pain, shortness of breath) and improve quality of life.
Chemotherapy administration is guided by specific protocols that were developed based on the results of controlled research studies. The length of treatment is determined by the type and extent of the malignancy, type of chemotherapy given, expected side effects of these drugs, and the amount of time that normal cells need to recover. Chemotherapy is usually given in cycles to improve the likelihood that cancer cells will be destroyed and that normal cells can recover. The duration, frequency, and number of cycles of chemotherapy are based on the type and size of the tumor, whether the disease has spread to other areas of the body (metastasis), and the condition of the patient. Chemotherapy treatment may consist of one agent or a combination of agents. Combination chemotherapy may be administered on one day or spread out over several days. The duration of each treatment varies from minutes to days and may be repeated weekly, biweekly, or monthly, based on the protocol being followed. Selected anticancer/antineoplastic drugs are listed in Box 37-2 according to classification.
Combination Chemotherapy
Single-agent drug therapy is not usually used to treat cancer, because combinations of anticancer agents have demonstrated more effective tumoricidal activity. Chemotherapy is most effective when it is able to kill cells in all phases of the cell cycle. Using two or more chemotherapy drugs at a time (combination chemotherapy) makes this more likely to occur.
To maximize cell death, CCS and CCNS drugs are often combined. Each individual chemotherapy agent used in combination therapy should have proven tumoricidal activity. Using two or more drugs together may have a synergistic effect. In addition, each drug should have a different mode of action and different dose-limiting toxicities. The use of a combination of antineoplastic agents has the advantage of decreasing drug resistance and increasing destruction of cancer cells. Some of the combinations of anticancer drugs used in cancer treatment are presented in Table 37-1.
TABLE 37-1
SELECTED COMBINATIONS OF ANTICANCER DRUGS
| GENERIC (BRAND) | ACRONYM* | SELECTED USES |
| doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velban), dacarbazine (DTIC) | ABVD | Hodgkin’s lymphoma |
| fluorouracil (5-FU, Adrucil), doxorubicin/hydroxydoxorubicin (Adriamycin), cyclophosphamide (Cytoxan) | FAC* | Breast cancer, prostate cancer |
| cyclophosphamide (Cytoxan), doxorubicin/hydroxydoxorubicin (Adriamycin), methotrexate (Rheumatrex, Trexall) | CAM | Prostate cancer |
| cyclophosphamide (Cytoxan), epirubicin (Pharmorubicin), fluorouracil (5-FU, Adrucil) | CEF | Breast cancer |
| cyclophosphamide (Cytoxan), doxorubicin/hydroxydoxorubicin (Adriamycin), vincristine (Oncovin), prednisone, rituximab (Rituxan) | CHOP + rituximab (CHOP+R) | Non-Hodgkin’s lymphoma |
| etoposide (VePesid, VP-16), leucovorin, fluorouracil (5-FU, Adrucil) | ELF | Esophageal cancer, stomach cancer |
| folinic acid (leucovorin), fluorouracil (5-FU, Adrucil), irinotecan (Camptosar) | FOLFIRI | Colorectal cancer |
| folinic acid (leucovorin), fluorouracil (5-FU, Adrucil), oxaliplatin (Eloxatin) | FOLFOX | Colorectal cancer |
| gemcitabine (Gemzar), capecitabine (Xeloda) | GEMCAP | Cancer of the pancreas |
| idarubicin (Idamycin), cytarabine (ARA-C, Cytosar), etoposide (VP-16, VePesid) | ICE | Acute myelogenous leukemia |
| mechlorethamine (Mustargen), vincristine (Oncovin), procarbazine (Matulane), prednisone | MOPP | Hodgkin’s disease |
| melphalan (Alkeran), prednisone | MP | Multiple myeloma |
| mitomycin C (Mutamycin), vinblastine (Velban), cisplatin (Platinol) | MVP | Small cell lung cancer |
| paclitaxel (Taxol), carboplatin (Paraplatin) | PC | Non–small cell lung cancer |
| docetaxel (Taxotere), doxorubicin (Adriamycin), cyclophosphamide (Cytoxan) | TAC | Recurrent breast cancer |
| vinblastine (Velban), bleomycin (Blenoxane), cisplatin (Platinol) | VBP | Testicular cancer |
*Acronyms are based on the name of the chemotherapy agents used in a specific protocol (e.g., ABVD [Adriamycin, bleomycin, vinblastine, dacarbazine], FAC [fluorouracil, Adriamycin, cyclophosphamide]). Both generic and trade names are used in acronyms.
General Side Effects and Adverse Reactions
Anticancer drugs exert adverse effects on rapidly growing normal cells (e.g., skin, hair). These drugs can also affect cells in the gastrointestinal (GI) tract, mucous membranes, bone marrow, and reproductive system. When there is a significant decrease in the cells in the bone marrow (white blood cells, platelets, red blood cells) the condition is called myelosuppression. Following chemotherapy administration, the time at which the blood count is at the lowest is called the nadir and typically occurs 7 to 10 days after treatment. Table 37-2 lists the general adverse reactions to anticancer drugs on the fast-growing cells of the body. Selected nursing measures and considerations are included.
TABLE 37-2
GENERAL ADVERSE REACTIONS TO ANTICANCER DRUGS
| ADVERSE REACTIONS | NURSING MEASURES AND CONSIDERATIONS |
| Bone Marrow Suppression, Myelosuppression | |
| Low RBC count (anemia) | Assess for fatigue, shortness of breath, low blood pressure, increased heart rate, increased respiratory rate, and oliguria. Assess for cyanosis. Plan rest periods. Administer oxygen as prescribed. Elevate head of bed to facilitate breathing. Provide pain mediation if pain is increasing oxygen consumption. Provide assistance to bathroom. Monitor for mental status changes. Anemia may be treated with ferrous sulfate or infusions of RBCs. Erythropoietin may be administered to stimulate RBC production. |
| Low WBC count (neutropenia) | Susceptibility to infection increases as WBCs decrease. Visitors with colds or infections should take precautions (e.g., wear mask) or avoid visiting the patient. Report fever, chills, upper respiratory infections, or sore throat to health care provider. Health care providers and visitors should wash hands before and after contact with the patient. Neutrophils are the primary WBCs that fight infections. Usual signs of infection (pain, swelling, redness, warmth, pus) may be absent or greatly reduced in neutropenic patients. Monitor for increase (or decrease) in body temperature. Elevated temperature is considered a sign of infection. Avoid medications that may mask temperature. Immediately report temperatures of 101° F (38.3° C) or above to health care provider. Appropriate cultures (e.g., blood, urine, sputum) are collected, and an antibiotic regimen is initiated. Assess for localized infections. Auscultate breath sounds. Monitor WBC count. Colony-stimulating factors (e.g., filgrastim) may be administered to stimulate WBC production. |
| Low platelet count (thrombocytopenia) | Petechiae, bruising, bleeding of gums, and nosebleeds are signs of a low platelet count; report these signs to health care provider. Assess for bleeding, petechiae, and ecchymosis. Assess for occult bleeding in urine, feces, and emesis. Monitor platelet counts and bleeding time. Apply pressure to injection sites. Platelet transfusions may be needed. Avoid medications that may promote bleeding (e.g., aspirin). Avoid invasive procedures (e.g., injections, indwelling urinary catheters, rectal temperature). |
| GI Disturbances | |
| Anorexia | Loss of appetite may be related to anemia, pain, fatigue, or bitter taste caused by some chemotherapy agents. Provide small, frequent meals that are high in calories and protein. Plan rest periods. Address issues of pain control. Hard candy or ice chips may help relieve bitter taste. |
| Nausea and vomiting | Antineoplastic drugs often stimulate the chemoreceptor trigger zone (CTZ), leading to nausea and vomiting. Nausea and vomiting (N/V) may be caused by irritation of GI tract; effects of radiation to chest, abdomen, or brain; anxiety; constipation; pain; electrolyte imbalances; or other medications. Grading scales are useful to assess severity. Provide antiemetics before, during, and after chemotherapy. Assess for GI upset, and medicate appropriately. Minimize noise, stimulation, and odors. Frequent mouth care is recommended. |
| Diarrhea | Diarrhea may be one of three types: osmotic (absorption defects), secretory (bacterial infection, neoplasm), or exudative (secondary to chemotherapy). Chemotherapeutic agents most commonly associated with diarrhea are alkylating agents, antitumor antibiotics, and antimetabolites. Treatment (e.g., medications, diet changes) will depend on cause. |
| Diarrhea may be caused by other medications (e.g., antibiotics); comorbid conditions (e.g., Crohn’s disease); or enteral feedings (e.g., tube feeding). Assess normal bowel habits; monitor for electrolyte imbalances and dehydration. Administer appropriate antidiarrheal medications (e.g., antibiotics, anticholinergics, antispasmodics, psyllium, kaolin and pectin, octreotide acetate). Teach patient to eat small, frequent meals; follow a low-residue diet; limit spicy, fatty foods; limit intake of salty foods, whole grains, fresh fruits and vegetables; limit caffeine and carbonated drinks; and avoid very hot or very cold foods (may stimulate peristalsis). Monitor intake and output. A grading scale may be useful to assess severity. | |
| Mucositis (stomatitis) | Many antineoplastic agents cause changes in oral mucosa; generally occur 2 to 14 days after initiation of therapy. Assess for taste changes, tissue swelling, redness, pain, dry mouth, white patches, or a white coating on the oral mucosa. Mucositis ranges from mild to severe. Symptomatic treatment may include frequent mouth rinses, topical anesthetics, antibiotics, antifungal medication, saliva substitutes, and pain medication. Patient should avoid commercial mouthwashes that contain alcohol. A soft toothbrush is recommended. Offer ice chips or ice pops to help relieve pain. Assess intake and output. Evaluate caloric needs. A grading scale may be useful to assess severity. |
| Other | Not all chemotherapeutic agents cause hair loss. Hair thinning, patchy baldness, or complete alopecia may occur, depending on drug. Hair on all areas of the body is affected. Hair loss may be gradual (progressing with each cycle of chemotherapy) or rapid. Hair regrowth usually occurs once chemotherapy is completed; texture may be somewhat changed. Before therapy, discuss potential hair loss and ways to address problem (wigs, scarves, hats, turbans). Assess for body image changes, concerns. |
| Alopecia | |
| Fatigue | Fatigue may be caused by chemotherapy, sleep disturbances, emotional distress, depression, bone marrow suppression, infection, pain, or electrolyte imbalances. |
| Assess fatigue using a visual analogue scale (0 = no fatigue; 10 = worst fatigue). Address conditions that might be contributing to fatigue (e.g., lack of sleep, pain, depression). Plan ways to help patient conserve energy. Plan a well-balanced diet. Encourage patient to participate in regular (but not strenuous) exercise. Encourage stress-reduction measures (e.g., relaxation, guided imagery). A grading scale may be useful in assessing fatigue. | |
| Infertility | If infertility occurs, it may be permanent. Pretreatment counseling is advised. |
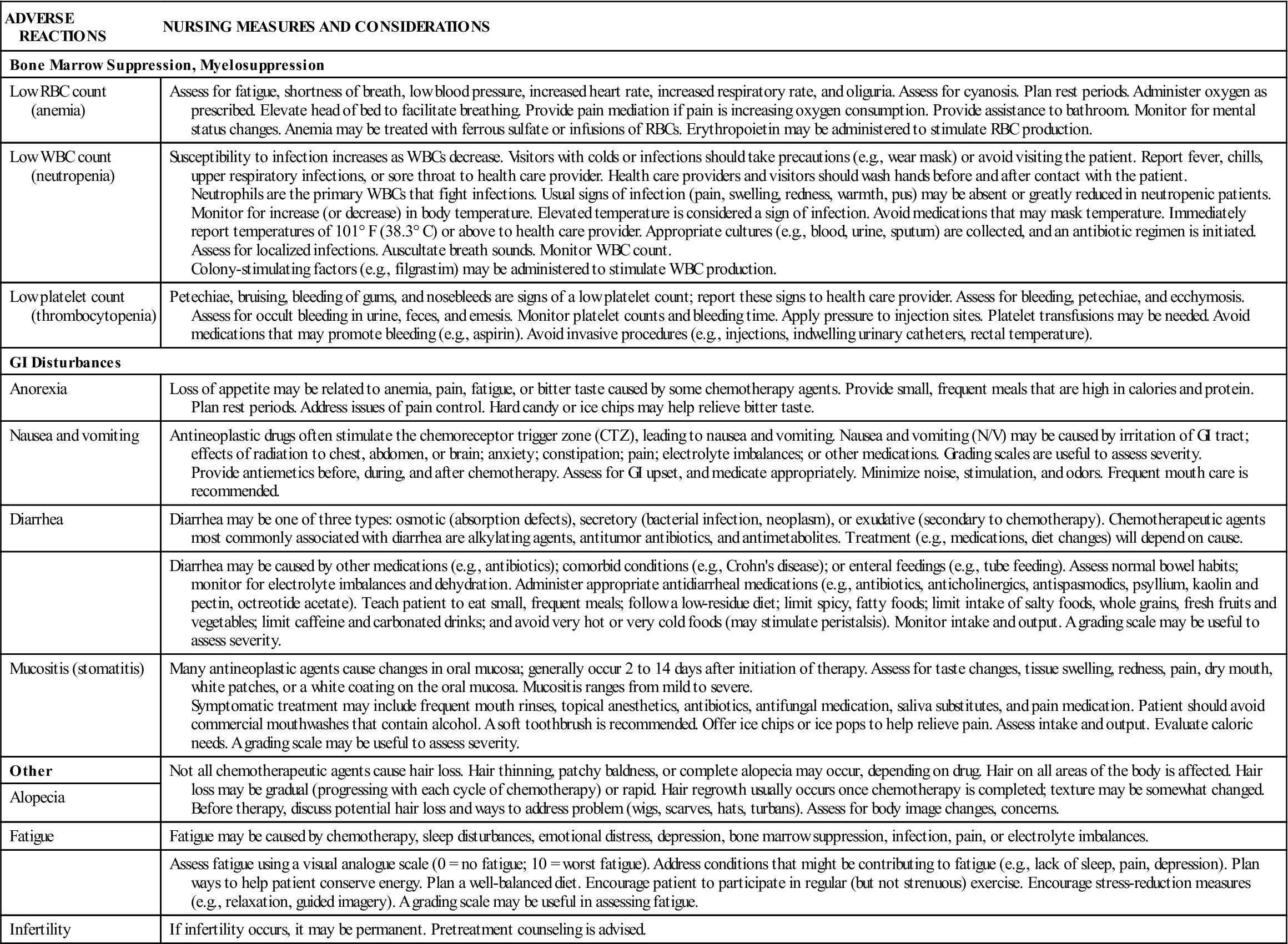
GI, Gastrointestinal; RBC, red blood cell; WBC, white blood cell.
Anticancer Therapy in Outpatient Settings and in the Home
The administration of anticancer drugs in outpatient settings is cost-effective and convenient. Although chemotherapy regimens have become increasingly aggressive, most patients are not hospitalized unless they require close monitoring or are very ill. Some chemotherapy agents are administered in the home. Patients receiving highly potent drugs at home may need to be closely monitored for severe adverse reactions or to assure adequate hydration. When a chemotherapy drug is given in the home, a health care provider qualified to administer anticancer agents follows the policies provided by the oncologist and the home health care agency. To reduce the nurse’s exposure to chemotherapy drugs (hazardous drugs, or HD) during intravenous (IV) administration, the following precautions should be followed to minimize occupational exposure:
• Wear gowns (disposable, impermeable, lint-free) during the administration of IV chemotherapy.
• Change gloves after chemotherapy administration and if they become contaminated or punctured.
• Cytotoxic drugs can be accidentally absorbed by inhalation, contact with skin or mucous membranes, and ingestion. The following guidelines should be followed:
2. Wash hands before and after administration of chemotherapy.
3. Avoid hand-to-mouth or hand-to-eye contact while working with chemotherapy.
4. Refer to agency policy for priming IV tubing and disconnecting tubing after administration.
6. Refer to agency policy for disposal of used equipment.
7. Refer to agency policy for chemotherapy spills or exposure.
Alkylating Drugs
One of the largest groups of anticancer drugs is the alkylating compounds. Alkylating agents cause cross-linking of DNA strands, abnormal base pairing, or DNA strand breaks, thus preventing the cell from dividing. Drugs in this group belong to the CCNS category and kill cells in various and multiple phases of the cell cycle. However, they are most effective against cells in the G0 phase. Alkylating agents are effective against many types of cancer, including acute and chronic leukemias, lymphomas, multiple myeloma, and solid tumors (e.g., breast, ovary, uterus, lung, bladder, and stomach). Drugs in this category are classified into several groups: mustard gas derivatives (e.g., cyclophosphamide [Cytoxan]), ethylenimines (e.g., thiotepa [Thioplex]), alkylsulfonates (e.g., busulfan [Myleran]), triazines (e.g., dacarbazine [DTIC]), nitrosoureas (e.g., carmustine [BiCNU]), and metal salts (e.g., cisplatin [Platinol]). Nitrosoureas are unique because they can cross the blood-brain barrier, making them useful in the treatment of brain cancer. Table 37-3 lists the alkylating drugs, uses, and considerations.
TABLE 37-3
ANTINEOPLASTICS: ALKYLATING DRUGS
| DRUG TYPE AND NAME | ROUTE AND DOSAGE | USES AND CONSIDERATIONS |
| Nitrogen Mustards | ||
| chlorambucil (Leukeran) | PO: 0.1-0.2 mg/kg/d (equals 4-8 mg/m2/d) for 3-6 wk | Lymphocytic leukemia, lymphomas, and cancer of the breast and ovaries. Side effects include nausea, vomiting, anorexia, diarrhea, abdominal upset, and leukopenia. Pregnancy category: D; PB: 99%; 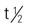 : 1.5 h : 1.5 h |
| cyclophosphamide (Cytoxan) | See Prototype Drug Chart 37-1. | |
| ifosfamide (Ifex) | IV: 1.2-2 g/m2/d for 5 d in combination with mesna | Testicular cancer, lymphoma, lung cancer, and sarcomas. Mesna, an uroprotective agent, is added to prevent hemorrhagic cystitis. Pregnancy category: D; PB: 69%-75%; 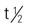 : 7-15 h (high dose) : 7-15 h (high dose) |
| mechlorethamine HCl (Mustargen) | IV: 0.4 mg/kg, or 12-16 mg/m2 Intracavitary: 0.2-0.4 mg/kg Topical: Dilute 10 g in 60 mL sterile water, apply with gloves (use precautions) | Hodgkin’s disease, solid tumors, and pleural effusion caused by cancer of the lung. Similar side effects as chlorambucil. Pregnancy category: D; PB: UK; 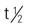 : 1 min : 1 min |
| melphalan (Alkeran) | For multiple myeloma: 0.25 mg/kg/d; repeat every 6 wk For bone marrow transplant: IV 50-60 mg/m2 | Multiple myeloma, melanoma, and cancers of the breast, ovary, and testes. Pregnancy category: D; PB: 30%; 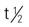 : 1.5 h : 1.5 h |
| temozolomide (Temodar) | PO: 150/mg/m2/d; repeat every 28 d | Refractory anaplastic astrocytoma. Pregnancy category: D; PB: 15%, 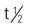 : UK : UK |
| Nitrosoureas | ||
| carmustine (BiCNU, BCNU) | IV: 75-100 mg/m2/d for 2 d | Hodgkin’s disease, multiple myeloma, melanoma, and brain tumors. May be used for cancer of the breast and lung. Nausea, vomiting, and stomatitis may occur. Pregnancy category: D; PB: UK; 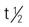 : 15-30 min : 15-30 min |
| lomustine (CeeNu, CCNU) | PO: 130 mg/m2 every 6 wk | Advanced Hodgkin’s disease and brain tumors. Pregnancy category: D; PB: 50%;  : 1-2 d : 1-2 d |
| streptozocin (Zanosar) | IV: 500 mg/m2/d for 5 d, repeat every 3-4 wk | Pancreatic islet cell tumor and cancer of the lung. May also be used for Hodgkin’s disease and colorectal cancer. Nausea, vomiting, diarrhea, and leukopenia may occur. Pregnancy category: C; PB: UK;  : 30-45 min : 30-45 min |
| Alkyl Sulfonates | ||
| busulfan (Myleran, Busulfex) | PO: 4-8 mg/d for 2-3 wk initially; then maintenance dose IV: 0.8 mg/kg | Myelocytic leukemia. Monitor WBCs closely. May be used as preparation agent in bone marrow transplant. Pregnancy category: D; PB: UK;  : 2.5 h : 2.5 h |
| Alkylating-Like Drugs | ||
| altretamine (Hexalen) | PO: 260 mg/m2/d in 4 equally divided doses for 14 d or 21 d | Ovarian cancer. Also used for breast, cervix, colon, endometrial, head/neck, and lung cancers; lymphomas. Nausea and vomiting and peripheral neuropathy may occur. Pregnancy category: D; PB: 6%;  : 13 h : 13 h |
| carboplatin (Paraplatin) | IV: 360 mg/m2 every 4 wk Intraperitoneal: 200-650 mg/m2 in 2 L For bone marrow transplant 1600 mg/m2 IV in divided doses over 4 d | Recurrent ovarian cancer. May be used as preparation agent in bone marrow transplant. Pregnancy category: D; PB: 0%; 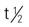 : 2-6 h : 2-6 h |
| cisplatin (Platinol) | IV: 50-100 mg/m2 every 3-4 wk For ovarian cancer: intraperitoneal: 100 mg/m2 every 3 wk | Ovarian and testicular cancer. Used as adjunctive treatment. Has been used for cancer of the bladder, head and neck, and endometrium. Nausea, vomiting, peripheral neuropathy, stomatitis, tinnitus, and blurred vision may occur. Ototoxicity occurs in 30% of patients. Pregnancy category: D; PB: >90%; 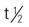 : 58-75 h : 58-75 h |
| oxaliplatin (Eloxatin) | IV: 85-130 mg/m2 infusion in 200-250 mL 5% dextrose in water | Metastatic colorectal cancer. Used with 5-FU and leucovorin. Ovarian cancer, head and neck cancer, and malignant melanoma. Pregnancy category: D; PB: >90%; 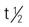 : 20-40 d : 20-40 d |
| dacarbazine (DTIC) | IV: 375 mg/m2 every 2 wk | Metastatic malignant melanoma, sarcomas, neuroblastoma, and refractory Hodgkin’s disease. May be given as an IV bolus (push) or by IV infusion. Common side effects are anorexia, nausea, and vomiting. Pregnancy category: C; PB: 5%-10%; 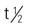 : 5 h : 5 h |
| thiotepa (Thioplex) | IV: 0.3-0.4 mg/kg IV once every 1-4 wk Intracavitary: Bladder: 60 mg in 30-60 mL of sterile water weekly for 3-4 wk Effusions: 0.6-0.8 mg/kg every 1-4 wk | Palliative therapy, especially for breast and ovarian cancer. Pregnancy category: D; PB: UK; 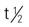 : 1.5-2 h : 1.5-2 h |
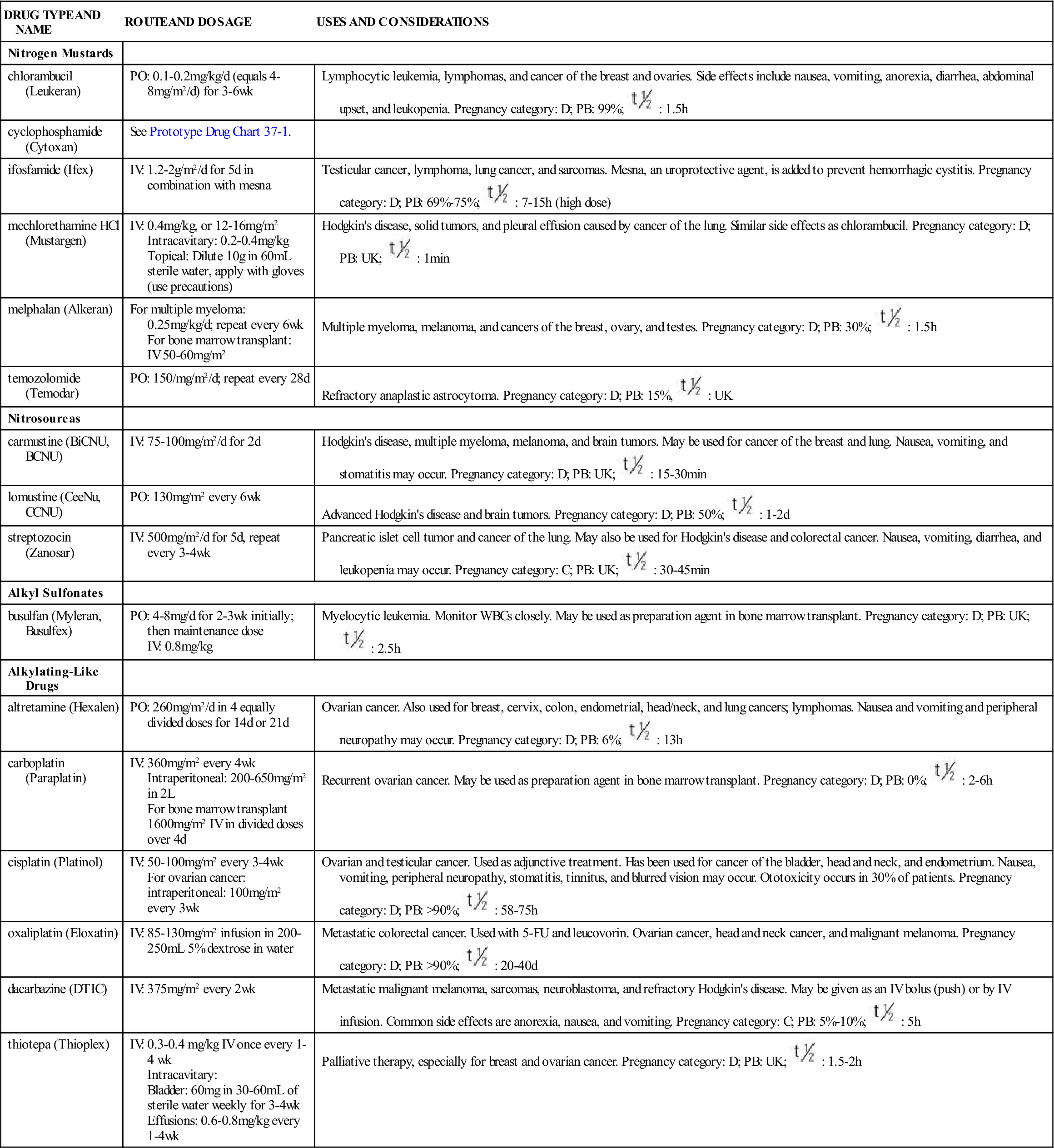
Note: Chemotherapeutic doses and schedules will vary depending on protocol, body surface area (m2), age, functional status, and comorbid conditions. For a full discussion of body surface area in dosage calculation, see Chapter 14.
5-FU, Fluorouracil; d, day; FDA, U.S. Food and Drug Administration; GI, gastrointestinal; h, hour; IV, intravenous; min, minute; PB, protein-binding; 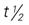 , half-life; UK, unknown; WBC, white blood cell; wk, week; >, greater than; <, less than.
, half-life; UK, unknown; WBC, white blood cell; wk, week; >, greater than; <, less than.
The side effects for alkylating drugs include nausea, vomiting, hemorrhagic cystitis, alopecia, anemia, leukopenia, thrombocytopenia, bone marrow suppression (anemia, leukopenia, thrombocytopenia), secondary malignancies, and sterility. Major dose-limiting toxicities may occur in the blood cell line (red blood cell, white blood cell, platelet) and urinary systems. General adverse reactions to chemotherapeutic drugs are listed in Table 37-2.
Cyclophosphamide
Mechlorethamine (nitrogen mustard, [Mustargen]), the first alkylating drug introduced for cancer treatment, became available for clinical use during World War II. Mechlorethamine is administered as part of a chemotherapy regimen to treat Hodgkin’s disease, especially if the disease is resistant to other drug combinations. This drug is a severe vesicant that can cause tissue necrosis if it infiltrates into the tissues. Cyclophosphamide (Cytoxan), an analogue of nitrogen mustard, may be prescribed orally, intrapleurally, or IV. The patient should be well hydrated while taking this drug to prevent hemorrhagic cystitis (bleeding as a result of severe bladder inflammation). MESNA (2-mercaptoethane sulphonate sodium [MESNEX]) is a cytoprotectant (chemoprotectant) drug that is often given with high-dose cyclophosphamide to inactivate urotoxic metabolites in the bladder and minimize damage to this organ.
Pharmacokinetics
Cyclophosphamide is well absorbed from the GI tract. Its half-life is moderate, and it is moderately protein-bound (<60%). The drug is metabolized by the liver, and less than 50% is excreted unchanged in the urine.
Pharmacodynamics
Cyclophosphamide, an early antineoplastic drug, is still prevalent in chemotherapy protocols to treat breast cancer, leukemia, lymphoma, multiple myeloma, ovarian cancer, retinoblastoma, and neuroblastoma. The onset of action begins in 2 to 3 hours; however, therapeutic effect may take several days. It is one of the anticancer drugs that can be administered orally.
Several drug interactions may occur with cyclophosphamide. Patients should report all medications they are taking, including over-the-counter (OTC) medicines and herbal supplements. Serious drug interactions can occur when taking cyclophosphamide and aspirin, the gout medication allopurinol (Lopurin, Zyloprim), phenobarbital (Luminal), warfarin (Coumadin), thiazide diuretics, and some psychiatric medications. Herbal Alert 37-1 lists herbal supplements that may also interact with cyclophosphamide.
Side Effects and Adverse Reactions
The side effects of cyclophosphamide reflect those seen in this general class of antineoplastic drugs. Hemorrhagic cystitis is a serious problem that can arise when high doses of cyclophosphamide are given. Patients who receive a high dose should be assessed for cardiomyopathy and syndrome of inappropriate antidiuretic hormone secretion (SIADH) during treatment with this drug. In addition, cyclophosphamide may cause a change (darkening) in the skin or fingernails. Prototype Drug Chart 37-1 details the pharmacologic behavior of cyclophosphamide.






























