Keith Harding, MB, ChB, FRCGP, FRCP, FRCS
Joyce K. Stechmiller, PhD, ACNP-BC, FAAN
Gregory Schultz, PhD
Objectives
After completing this chapter, you’ll be able to:
- describe the physiology of normal and abnormal wound healing
- discuss the cascade of wound healing events
- compare and contrast acute and chronic wound healing
- discuss the effects of biofilms on wound healing
- explain the principles of wound bed preparation and TIME in the management of patients with wounds.
Wound Healing Events
When a patient experiences tissue injury, it’s essential that hemostasis is rapidly achieved and tissue is repaired to prevent invasion by pathogens and restore tissue function. The process of wound healing is a complex sequence of events that starts when the injury occurs and ends with complete wound closure and successful, functional scar tissue organization. Although tissue repair is commonly described as a series of stages, in reality it’s a continuous process during which cells undergo a number of complicated biological changes to facilitate hemostasis, combat infection, migrate into the wound space, deposit a matrix, form new blood vessels, and contract to close the defect.
However, wound closure isn’t a marker of healing completion; the wound continues to change, in a process called remodeling, for up to 18 months postclosure. During this prolonged phase of remodeling and maturation, the closed wound is still quite vulnerable.
 Patient Teaching
Patient Teaching
Remind your patient that the process of wound healing can take up to 18 months. Although the wound may appear to be closed, changes are occurring in the underlying tissue. This means that the wound is still vulnerable to damage. Tell the patient to seek professional advice if he or she has any concerns about the wound.
Wound Healing Cascade
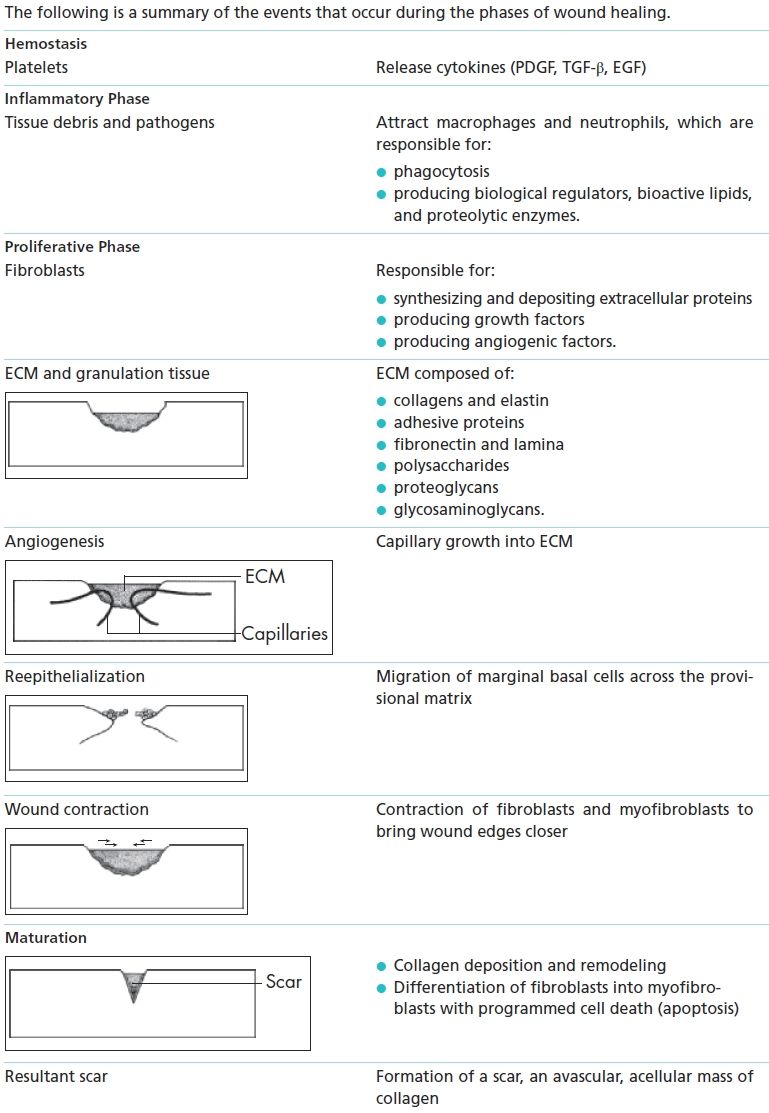
The process of healing is usually divided into four phases—hemostasis, inflammation, proliferation/ repair, and maturation/remodeling—each of which overlaps the others while remaining distinct in terms of time after injury (Fig. 5-1).
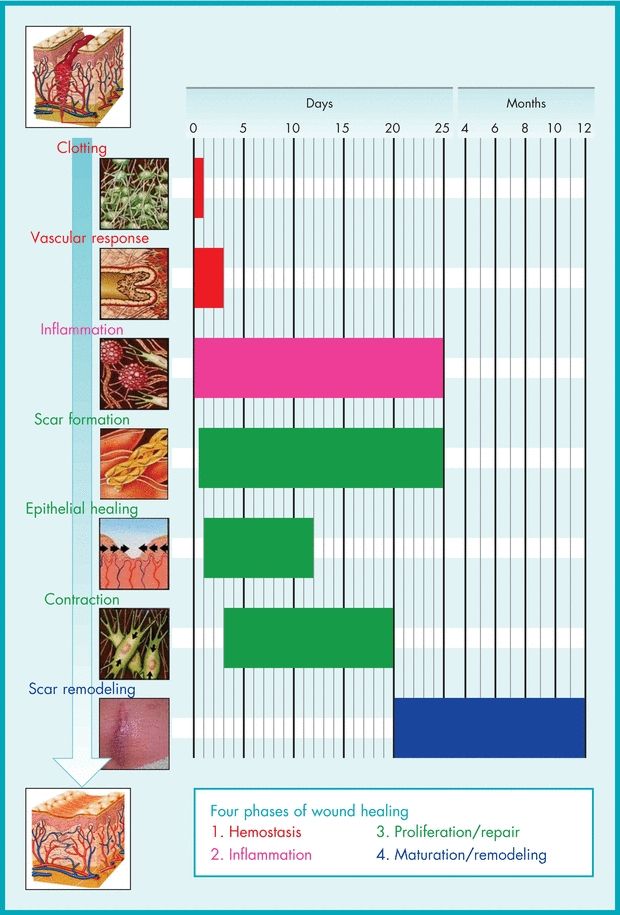
Figure 5-1. Sequence of molecular and cellular events in skin wound healing.
 Patient Teaching
Patient Teaching
Inform your patient that the process of healing is often separated into four different phases. When a wound is being assessed, the clinician is looking for signs of progress as well as deterioration.
Hemostasis
The disruption of tissue following injury causes hemorrhage, which initially fills the wound and exposes the blood to various components of the extracellular matrix (ECM).1 Platelets aggregate and degranulate, which activates factor XII (Hageman factor), resulting in clot formation and hemostasis. Hemostasis stops hemorrhage at the site of blood vessel damage. This is essential as it preserves the integrity of the closed and high-pressure circulatory system to limit blood loss. A fibrinous clot forms during coagulation, acting as a preliminary matrix within the wound space into which cells can migrate.
After the fibrin clot forms, another mechanism is activated as part of the body’s defense system—fibrinolysis—in which the fibrin clot starts to break down. This process prevents clot extension and dissolves the fibrin clot to allow ease of further cell migration into the wound space,2 allowing the next stage of healing to proceed.
Inflammatory Phase
As the fibrin clot is degraded, the capillaries dilate and become permeable, allowing fluid into the injury site and activating the complement system. The complement system is composed of a series of interacting, soluble proteins found in serum and extracellular fluid that induce lysis and the destruction of target cells. C3b, a complement molecule, helps bind (opsonize) neutrophils to bacteria, facilitating phagocytosis and subsequent bacterial destruction.
Cytokines and some proteolytic fragments that are hemoattractive are also found in the wound space.2 Their abundance and accumulation at the site of injury initiate a massive influx of other cells. The two main inflammatory cells—neutrophils and macrophages—are attracted to the wound space to mount an acute inflammatory response.3
Neutrophils appear in a wound shortly after injury and reach their peak number within 24 to 48 hours; their main function is to destroy bacteria by the process of phagocytosis. Neutrophils have a very short life span: After 3 days without infection, their numbers reduce rapidly.
Tissue macrophages are derived from blood monocytes and arrive approximately 2 to 3 days after injury, followed by lymphocytes. Like neutrophils, macrophages also destroy bacteria and debris through phagocytosis; however, macrophages are also a rich source of biological regulators, including cytokines and growth factors, bioactive lipid products, and proteolytic enzymes, which are also essential for the normal healing process.2,4
Cytokines, Growth Factors, and Chemotaxis
Cytokine is a broad term that includes such molecules as growth factors, interleukins, tumor necrosis factors, and interferons. These molecules act on a variety of cells by exerting a wide range of biological functions by means of their specific receptors on target cells or proteins. Pathogens, endotoxins, tissue degradation products, and hypoxia are all factors that stimulate cells to produce cytokines following injury. The main cellular sources for these cytokines are platelets, fibroblasts, monocytes and macrophages, and endothelial cells. These cells are involved in physiological as well as pathological conditions (e.g., tumors), although in wound healing they play an important role as mediators. Cytokines regulate cell proliferation, migration, matrix synthesis, deposition and degradation, and inflammatory responses in the repair process (Table 5-1).
Table 5-1 Major Cytokines Involved in Wound Healing
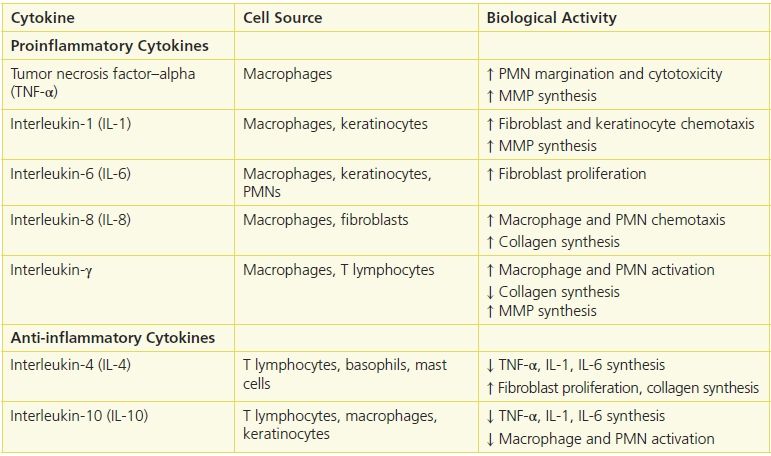
Immediately after injury, platelet degranulation releases numerous cytokines, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), and epidermal growth factor (EGF). These cytokines, together with other chemotactic agents, such as tissue debris and pathogenic materials, attract neutrophils and, later, macrophages. In time, these cells contribute to a larger number and variety of cytokines, which participate in the healing process.3 (See Patient Teaching: Normal and abnormal signs of the inflammatory process.)
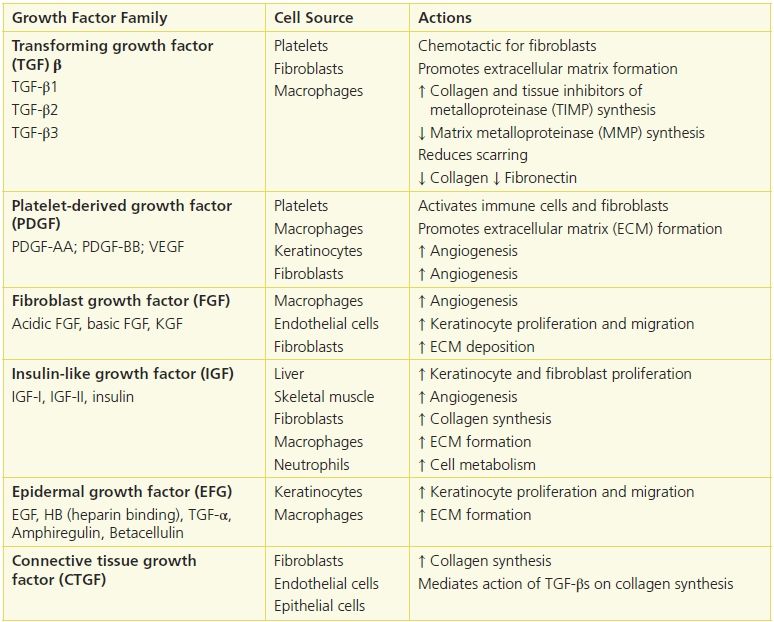
Cytokines have diverse effects on the healing process, interacting in additive, synergistic, or inhibitory ways (Table 5-2). For example, keratinocyte growth factor enhances the stimulation of collagenase synthesis exerted by insulin-like growth factor. TGF is inhibitory to fibroblast growth in the presence of EGF but stimulates cell division when PDGF is present.
Table 5-2 Major Growth Factor Families
Proliferation Phase
The proliferation phase usually begins 3 days after an injury and lasts for a few weeks. This phase is characterized by the formation of granulation tissue in the wound space. The new tissue consists of a matrix of fibrin, fibronectin, collagens, proteoglycans, glycosaminoglycans (GAGs), and other glycoproteins.5 Fibroblasts move into the wound space and proliferate. Because the type III collagen in the wound has decreased tensile strength, the patient is at risk for such abnormalities as wound dehiscence or opening of wound edges in a previously closed wound that healed by primary intention. If organs are protruding from the now opened wound, it’s called evisceration, which is a medical emergency that requires immediate surgery.
 Patient Teaching
Patient Teaching
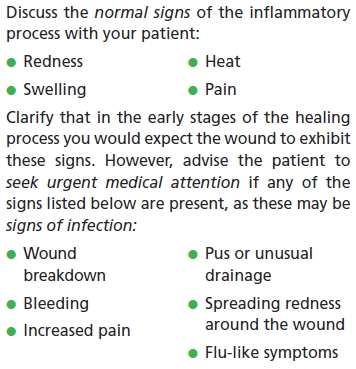
Normal and abnormal signs of the inflammatory process
 Practice Point
Practice Point
Keep in mind that the induration, heat, discomfort, redness, and swelling experienced during the inflammatory phase are part of the normal wound healing processes and aren’t, at this stage, likely to be due to wound infection. Remember to share this information with your patients.
 Practice Point
Practice Point
During the first 3 weeks after surgery, the patient is at high risk for wound dehiscence and evisceration. Advise the patient that he or she should follow any postsurgical advice very carefully as the repaired tissue will not regain its full strength. The wound is at risk of breakdown if undue pressure is exerted on the area. For instance, patients who have undergone an abdominal procedure should support their abdomen with a soft pillow if they need to cough. They should also avoid any heavy lifting or exertion as designated by their physician.
Role of Fibroblasts
Fibroblasts play a key role during the proliferation phase, appearing in large numbers within 3 days of injury and reaching peak levels on the 7th day. During this period, they undergo intense proliferative and synthetic activity. Fibroblasts synthesize and deposit extracellular proteins during wound healing, producing growth factors and angiogenic factors that regulate cell proliferation and angiogenesis.6
Granulation tissue is composed of many mesenchymal and nonmesenchymal cells with distinct phenotypes, inflammatory cells, and new capillaries embedded in a loose ECM composed of collagens, fibronectin, and proteoglycans.
Role of ECM Proteins
ECM consists of proteins and polysaccharides and their complexes produced by cells in the wound space. The two main classes of matrix proteins are fibrous proteins (collagens and elastin) and adhesive proteins (laminin and fibronectin). In addition, the ECM contains polysaccharides called proteoglycans and GAGs.
Collagen is the most abundant protein in animal tissue and accounts for 70% to 80% of the dry weight of the dermis.7 The collagen molecule consists of three identical polypeptide chains bound together in a triple helix. Made mainly by fibroblasts, at least 19 genetically distinct collagens have been identified. Collagen synthesis and degradation are finely balanced.2
Elastin is a protein that provides elasticity and resilience.8 It is composed of fibrous coils that stretch and return to their former shape, much like metallic coils. Because of these properties, elastin helps maintain tissue shape. Elastin represents only 2% to 4% of the human skin’s dry weight; it’s also in the lungs and blood vessels. It’s secreted into the extracellular space as a soluble precursor, tropoelastin, which binds with a microfibrillar protein to form an elastic fiber network.
Laminin and fibronectin are two fiber-forming molecules. Their function is to provide structural and metabolic support to other cells. Fibronectin is found in plasma and contains specific binding sites on its molecular wall for cells, collagens, fibrinogens, and proteoglycans. It plays a central role in tissue remodeling, acting as a mediator for physical interactions between cells and collagens involved in ECM deposition, thereby providing a preliminary matrix.
Proteoglycans consist of a central core protein combined with a number of GAG chains that may be one or several types. GAGs consist of long, unbranched chains of disaccharide units that can range in number.2 A highly complex group of molecules, proteoglycans are characterized by their many diverse structural and organizational functions in tissue. Forming a highly hydrated gel-like “ground substance,” they can contain up to 95% (w/w) carbohydrates. Originally, however, they were thought to contribute to tissue resilience due to their capacity to fill much of the extracellular space.
Angiogenesis
Angiogenesis is the formation of new vessels in the wound space and is an integral and essential part of wound healing.9 The vascular endothelial cell (VEC) plays a key role in angiogenesis and arises from the damaged end of vessels and capillaries. New vessels originate as capillaries, which sprout from existing small vessels at the wound edge. The endothelial cells from these vessels detach from the vascular wall, degrade and penetrate (invade) the provisional matrix in the wound, and form a knob-like or cone-shaped vascular bud or sprout. These sprouts extend in length until they encounter another capillary, to which they connect to form vascular loops and networks, allowing blood to circulate. This pattern of vascular growth is similar in skin, muscle, and intestinal wounds.
Epithelialization
Epithelial healing, or epithelialization, which begins a few hours after injury, is another important feature of healing. Marginal basal cells, which are normally firmly attached to the underlying dermis, change their cell adhesion property and start to lose their firm adhesion, migrating in a leapfrog or train fashion across the provisional matrix. Horizontal movement is stopped when cells meet. This is known as contact inhibition.
Wound Contraction
The final feature of the proliferation phase is wound contraction, which normally starts 5 days after injury. Wound contraction appears to be a dynamic process in which cells organize their surrounding connective tissue matrix, acting to reduce the healing time by reducing the amount of ECM that needs to be produced. The contractile activity of fibroblasts and myofibroblasts provides the force for this contraction. These cells may use integrins and other adhesion mechanisms to bind to the collagen network and alter its motility, bringing the fibrils and, subsequently, the wound edges closer.2 Such contraction may not be important in a sharply incised, small, and noninfected wound; however, it’s critical for wounds with large tissue loss.10
The myofibroblast theory suggests that the contraction force occurs when the movement of microfilament (actin) bundles (also termed stress fibers) contracts the myofibroblast in a muscle-like fashion. Because the myofibroblast displays many cell:cell and cell:matrix (fibronexus) contacts, the cellular contraction pulls collagen fibrils toward the body of the myofibroblast and holds them until they’re stabilized into position. This gathering of collagen fibers toward the myofibroblast cell “body” leads to the shrinkage of granulation tissue. The ECM of the wound is continuous with the undamaged wound margin, enabling the granulation tissue shrinkage to pull on the wound margin, leading to wound contraction. The myofibroblast theory further proposes that the coordinated contraction (cellular shortening) of many myofibroblasts, synchronized with the help of gap junctions, generates the force necessary for wound contraction.6
The traction theory proposes that fibroblasts bring about a closer approximation of matrix fibrils by exerting “traction forces” (analogous to the traction of wheels on tarmac) on ECM fibers to which they’re attached. This theory proposes that fibroblasts neither shorten in length nor act in a coordinated multicellular manner (as proposed by the myofibroblast theory); rather, a composite force, made up of traction forces of many individual fibroblasts, is responsible for matrix contraction. Such traction forces act as shearing forces tangential to the cell surface generated during cell elongation and spreading. According to the traction theory, the composite effect of many fibroblasts gathering collagen fibrils within the wound is thought to bring about wound contraction.11
 Patient Teaching
Patient Teaching
Teach your patient who has a wound left open to heal (secondary intention) that clinicians will be looking for indications that wound healing is progressing normally:
- Healthy pink tissue in the wound bed
- Signs of new tissue growth at the wound edges
- Decreasing wound size over time
Maturation Phase
The maturation phase normally starts 7 days after injury and may last for 1 year or more. The initial component in the deposited ECM is fibronectin, which forms a provisional fiber network. Other components include hyaluronic acid and proteoglycans. The network has two main roles: as a substratum for the migration and growth of cells and as a template for subsequent collagen deposition. Collagen deposition becomes the predominant constituent of the matrix and soon forms fibrillar bundles and provides stiffness and tensile strength to the wound.
Collagen deposition and remodeling contribute to the increased tensile strength of skin wounds. Within 3 weeks of injury, the tensile strength is restored to approximately 20% of normal, uninjured skin. As healing continues, the skin gradually reaches a maximum of 70% to 80% tensile strength. Different organs regain tensile strengths to differing degrees. The remodeling process involves the balance between the synthesis and degradation of collagen. A range of collagenases regulates the latter. This process is also characterized by a gradual reduction in cellularity and vascularity.3 Differentiation of fibroblasts into myofibroblasts with resultant apoptosis (programmed cell death) are also features of tissue remodeling.12
 Practice Point
Practice Point
A patient history should always include information about prior wounds. Healed wounds never achieve the same tensile strength as uninjured skin, thereby increasing the potential for reinjury.
 Patient Teaching
Patient Teaching
Remind the patient that the wounded area is never as strong as uninjured tissue so it is always vulnerable to damage. Simple measures such as keeping the scar tissue moisturized will help to optimize the condition of the tissue. Advise the patient to seek help if the scar begins to break down.
The scar is the final product of wound healing and is a relatively avascular and acellular mass of collagen that serves to restore tissue continuity and some degree of tensile strength and function. However, the strength of the scar remains less than that of normal tissue, even many years following injury, and it’s never fully restored (Box 5-1).
Box 5-1 Summary of Wound Healing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


