Chapter 5 Acid-Base Balance
Basic concepts
2 How does a change in 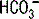 or in PCO2 affect pH?
or in PCO2 affect pH?
A change in 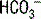 of 10 mEq/L up or down causes pH to increase or decrease by 0.15 unit, respectively. Acutely, a change in PCO2 of 10 mm Hg up or down causes pH to decrease or increase by 0.08 unit, respectively. A chronic change in PCO2 levels alters blood pH by 0.03 unit per 10 mm Hg of PCO2 (due to renal compensation; see Case 5-2 for details).
of 10 mEq/L up or down causes pH to increase or decrease by 0.15 unit, respectively. Acutely, a change in PCO2 of 10 mm Hg up or down causes pH to decrease or increase by 0.08 unit, respectively. A chronic change in PCO2 levels alters blood pH by 0.03 unit per 10 mm Hg of PCO2 (due to renal compensation; see Case 5-2 for details).
The first step in interpretation is to determine the primary disorder. Figure 5-1 can be used for this purpose. Normal values for [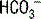 ] and PCO2 are 22 to 28 mEq/L and 35 to 45 mm Hg, respectively.
] and PCO2 are 22 to 28 mEq/L and 35 to 45 mm Hg, respectively.
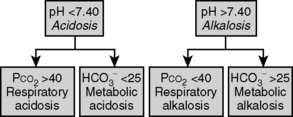
Figure 5-1 Determining the primary acid-base abnormality.
(From Piccini JP, Nilsson KR: The Osler Medical Handbook, 2nd ed. Baltimore, Johns Hopkins University, 2006.)
3 What else is considered in the differential diagnosis for non–anion gap metabolic acidosis?
An acidosis resulting from such a failure by the kidney to excrete 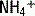 is called a renal tubular acidosis (RTA). See Table 5-1 for a description of the three types of renal tubular acidoses.
is called a renal tubular acidosis (RTA). See Table 5-1 for a description of the three types of renal tubular acidoses.
A negative UAG in the setting of a non-AG acidosis most often indicates diarrhea as the nonrenal cause. However, other causes include ureteral-colonic fistulas, exogenous acid ingestion or administration (consider parenteral nutrition in a hospitalized patient), posthypocapnic acidosis, early renal failure, and “dilutional acidosis.” In the case of ureteral-colonic fistulas, urine rich in Cl− enters the colon, where the Cl− is reabsorbed in exchange for 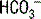 , resulting in
, resulting in 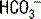 loss in a mechanism similar to diarrhea. Posthypocapnic acidosis is a result of persisting renal compensation to a chronic respiratory alkalosis (see Case 5-2, question 2). Acidosis in early renal failure can result from loss of the ability to generate ammonia; late renal failure, in contrast, generally results in a mixed AG/non-AG acidosis (see next question). Dilutional acidosis, which is commonly seen in hospitalized patients, is a result of excess normal saline administration; normal saline represents a large Cl− load (specifically, 154 mEq/L of Cl−), which results in increased bicarbonate excretion to maintain electrical neutrality.
loss in a mechanism similar to diarrhea. Posthypocapnic acidosis is a result of persisting renal compensation to a chronic respiratory alkalosis (see Case 5-2, question 2). Acidosis in early renal failure can result from loss of the ability to generate ammonia; late renal failure, in contrast, generally results in a mixed AG/non-AG acidosis (see next question). Dilutional acidosis, which is commonly seen in hospitalized patients, is a result of excess normal saline administration; normal saline represents a large Cl− load (specifically, 154 mEq/L of Cl−), which results in increased bicarbonate excretion to maintain electrical neutrality.
4 Is there appropriate compensation or is there a mixed disorder in this patient?
An appropriate compensation for metabolic acidosis is to decrease PCO2, that is, excrete volatile acid, through hyperventilation. Such respiratory compensation is virtually immediate. Using the equation in Table 5-2, the expected change in PCO2 = 1.2 × (25 – 7) = 21.6. The actual change in this case is (40 – 19) = 21, reflecting appropriate compensation.
Table 5-2 Respiratory Compensation for Metabolic Acid-Base Disturbances
| Condition | Primary Change | Expected Compensation |
|---|---|---|
| Metabolic acidosis | Decreased 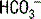 | Decreased 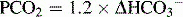 |
| Metabolic alkalosis | Increased 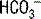 | Increased 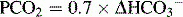 |
In looking at Table 5-2, it is apparent that the lungs, in responding to metabolic acid-base disturbances, can more readily excrete CO2 than retain it. This response is both rather intuitive (severe hypercapnia can be quite dangerous, rapidly leading to mental status changes and coma) and useful in remembering that the degree of respiratory compensation for metabolic acidosis is greater than the compensation. Note: Compensation during any acid-base disturbance is never complete.
5 What would it mean if, in the same patient, appropriate compensation was not present and PCO2 was instead 30 mm Hg?
Summary Box: Acid-Base Disturbances, Metabolic Acidosis
 First determine the primary disturbance using pH, PCO2, and
First determine the primary disturbance using pH, PCO2, and 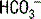 .
.
 Then determine if the degree of compensation is appropriate or if there is a mixed disorder.
Then determine if the degree of compensation is appropriate or if there is a mixed disorder.
 It is “easier” for the body to blow off CO2 than to retain it, that is, greater compensation for metabolic acidoses than for metabolic alkaloses.
It is “easier” for the body to blow off CO2 than to retain it, that is, greater compensation for metabolic acidoses than for metabolic alkaloses.
 For a metabolic acidosis, first determine the anion gap (AG).
For a metabolic acidosis, first determine the anion gap (AG).

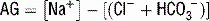 ) = unmeasured anions − unmeasured cations; normal anion gap is ~10.
) = unmeasured anions − unmeasured cations; normal anion gap is ~10.
 If a non-AG metabolic acidosis exists, search for clues in the history (e.g., diarrhea, rapid infusion of normal saline) and determine the urine anion gap (UAG).
If a non-AG metabolic acidosis exists, search for clues in the history (e.g., diarrhea, rapid infusion of normal saline) and determine the urine anion gap (UAG).
 UAG = (UNa + UK) − UCl = unmeasured anions − unmeasured cations.
UAG = (UNa + UK) − UCl = unmeasured anions − unmeasured cations.
 If the UAG is negative, the kidneys are working normally (i.e., are excreting NH4Cl).
If the UAG is negative, the kidneys are working normally (i.e., are excreting NH4Cl).
 If the UAG is positive, renal tubular acidosis (RTA) is likely; look at urine pH, serum pH, serum potassium, and the fractional excretion of
If the UAG is positive, renal tubular acidosis (RTA) is likely; look at urine pH, serum pH, serum potassium, and the fractional excretion of 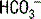 after a bicarbonate load to determine which type.
after a bicarbonate load to determine which type.
1 What is the primary acid-base disorder?
Following Figure 5-1, we see that the primary disorder is a respiratory alkalosis.
3 Is there appropriate compensation or is this a mixed disorder?
Because the full effect of renal compensation for respiratory disturbances is not immediate, for acid-base disturbances with a respiratory cause, one must first determine if the disturbance is acute or chronic. In this patient, the respiratory alkalosis is acute. The appropriate compensation for any respiratory alkalosis is to decrease serum 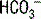 through increased renal excretion. Using the equation in Table 5-3, the appropriate acute change in
through increased renal excretion. Using the equation in Table 5-3, the appropriate acute change in 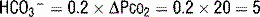 . The actual change in this case is 25 – 20 = 5; therefore, there is appropriate acute compensation.
. The actual change in this case is 25 – 20 = 5; therefore, there is appropriate acute compensation.
Table 5-3 Renal Compensation for Respiratory Acid-Base Disturbances
| Condition | Primary Change | Expected Compensation |
|---|---|---|
| Acute respiratory acidosis | Increased PCO2 | Increased 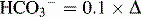 PCO2 PCO2 |
| Chronic respiratory acidosis | Increased PCO2 | Increased 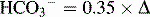 PCO2 PCO2 |
| Acute respiratory alkalosis | Decreased PCO2 | Decreased 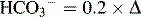 PCO2 PCO2 |
| Chronic respiratory alkalosis | Decreased PCO2 | Decreased 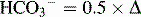 PCO2 PCO2 |
It is not likely that the USMLE will ask you to calculate the degree of renal or pulmonary compensation for an acute or chronic acid-base disorder using the formulas listed in Tables 5-2 and 5-3. However, these are important formulas to know for your clinical years, particularly because they can help you spot mixed disorders.
4 Does the degree of compensation allow you to draw any conclusions as to the duration of the condition?
If you are attempting to remember the preceding numbers, it is useful to realize that the kidneys more easily and more rapidly excrete 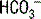 than retain
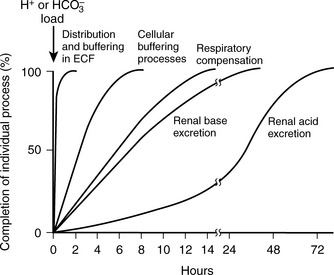
than retain 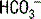 when responding to respiratory acid-base disturbances. Thus, renal compensation for respiratory alkaloses is both more complete and more rapid than the compensation for respiratory acidoses (see Table 5-3 and Fig. 5-2).
when responding to respiratory acid-base disturbances. Thus, renal compensation for respiratory alkaloses is both more complete and more rapid than the compensation for respiratory acidoses (see Table 5-3 and Fig. 5-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


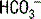 ] can be expressed as follows:
] can be expressed as follows: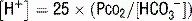
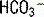 ]: 7 mEq/L
]: 7 mEq/L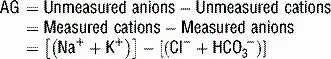
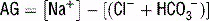 . In this patient, AG is 13 mEq/L (normal range = 8-16 mEq/L). In the normal state, these unmeasured anions consist primarily of albumin and phosphate.
. In this patient, AG is 13 mEq/L (normal range = 8-16 mEq/L). In the normal state, these unmeasured anions consist primarily of albumin and phosphate.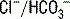 exchange process in the colon. Diarrhea shortens intestinal transit time, limiting the opportunity for colonic
exchange process in the colon. Diarrhea shortens intestinal transit time, limiting the opportunity for colonic 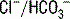 exchange and thereby increasing the concentration of bicarbonate in the stool. This loss of bicarbonate is thus electrically balanced by an increase in serum Cl− concentration. As mentioned in the preceding note, the AG remains unchanged. A similar process of Cl− retention occurs in all non-AG acidoses. This is why a non-AG metabolic acidosis can alternatively be described as a hyperchloremic metabolic acidosis.
exchange and thereby increasing the concentration of bicarbonate in the stool. This loss of bicarbonate is thus electrically balanced by an increase in serum Cl− concentration. As mentioned in the preceding note, the AG remains unchanged. A similar process of Cl− retention occurs in all non-AG acidoses. This is why a non-AG metabolic acidosis can alternatively be described as a hyperchloremic metabolic acidosis.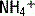 . This
. This 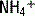 is generally paired with Cl− to maintain electrical neutrality. As such, the urine excretion of Cl− can be measured to determine renal
is generally paired with Cl− to maintain electrical neutrality. As such, the urine excretion of Cl− can be measured to determine renal 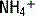 excretion. This calculation is specifically done using the equation for the urine anion gap (UAG):
excretion. This calculation is specifically done using the equation for the urine anion gap (UAG):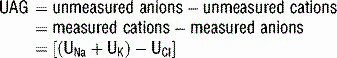
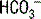 is excluded from this equation because, due to the acidity of urine, its concentration is typically negligible. A negative UAG signifies an appropriate renal response since
is excluded from this equation because, due to the acidity of urine, its concentration is typically negligible. A negative UAG signifies an appropriate renal response since 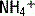 makes up a large portion of the unmeasured cations in the equation, and, likewise, urine Cl− concentration will be increased due to pairing with
makes up a large portion of the unmeasured cations in the equation, and, likewise, urine Cl− concentration will be increased due to pairing with 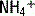 . A failure to excrete ammonium during an acidemia will give a positive UAG.
. A failure to excrete ammonium during an acidemia will give a positive UAG.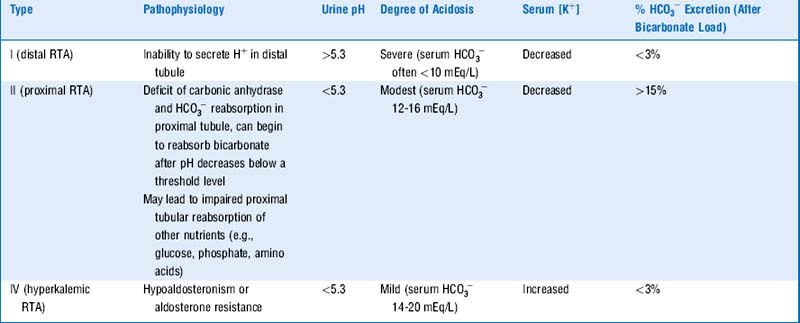
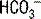 would have been 0.5 × ΔP
would have been 0.5 × ΔP