Barbara Lauritsen Christensen
Care of the patient with a blood or lymphatic disorder
Objectives
1. Describe the components of blood.
2. Differentiate between the functions of erythrocytes, leukocytes, and thrombocytes.
3. Discuss factors necessary for the formation of erythrocytes.
4. Define the white blood cell differential.
5. Describe the blood clotting process.
6. List the basic blood groups.
10. List six signs and symptoms associated with hypovolemic shock.
15. Discuss the primary goal of nursing interventions for the patient with lymphedema.
Key terms
disseminated intravascular coagulation (DIC) (d -S
-S M-
M- -n
-n t-
t- d, p. 289)
d, p. 289)
erythrocytosis ( -r
-r th-r
th-r -s
-s -T
-T -s
-s s, p. 279)
s, p. 279)
erythropoiesis ( -r
-r th-r
th-r -p
-p –
– -s
-s s, p. 265)
s, p. 265)
hemarthrosis (h -m
-m r-THR
r-THR -s
-s s, p. 287)
s, p. 287)
hemophilia A (h -m
-m -F
-F L-
L- –
– , p. 287)
, p. 287)
heterozygous (h t-
t- r-
r- -Z
-Z -g
-g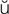 s, p. 277)
s, p. 277)
homozygous (h -m
-m -Z
-Z -g
-g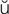 s, p. 276)
s, p. 276)
idiopathic (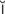 d-
d-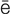 –
– -P
-P TH-
TH- k, p. 273)
k, p. 273)
leukopenia (l-k -P
-P -n
-n –
– , p. 281)
, p. 281)
lymphangitis (l m-f
m-f n-G
n-G -t
-t s, p. 292)
s, p. 292)
lymphedema (l m-f
m-f -D
-D -m
-m , p. 293)
, p. 293)
multiple myeloma (M L-t
L-t -p
-p l m
l m –
– -L
-L -m
-m , p. 291)
, p. 291)
myeloproliferative (m –
– -l
-l -pr
-pr -L
-L F-
F- r-
r- -t
-t v, p. 279)
v, p. 279)
pancytopenic (p n-s
n-s -t
-t -P
-P N-
N- c, p. 273)
c, p. 273)
pernicious (p r-N
r-N SH-
SH- s, p. 272)
s, p. 272)
Reed-Sternberg cells (r d-ST
d-ST RN-b
RN-b rg, p. 294)
rg, p. 294)
thrombocytopenia (thr m-b
m-b -s
-s t-
t- -P
-P -n
-n –
– , p. 286)
, p. 286)
Anatomy and physiology of the hematologic and lymphatic systems
Transportation and protection are two of the body’s most important functions. Without transportation and protection for the cells, the body’s homeostasis would be threatened. The systems that provide these vital services for the body are the circulatory and lymphatic systems. This chapter discusses the primary transportation fluid—blood—and presents an overview of the lymphatic system. Blood not only performs vital transportation services, but also provides much of the protection necessary to withstand foreign invaders. The lymphatic system helps maintain fluid balance, and lymphoid tissues help protect the internal environment.
Characteristics of blood
In ancient times, blood was referred to as the “river of life” or “fluid of life.” Some people believed it had magical properties. All knew it was necessary to maintain life.
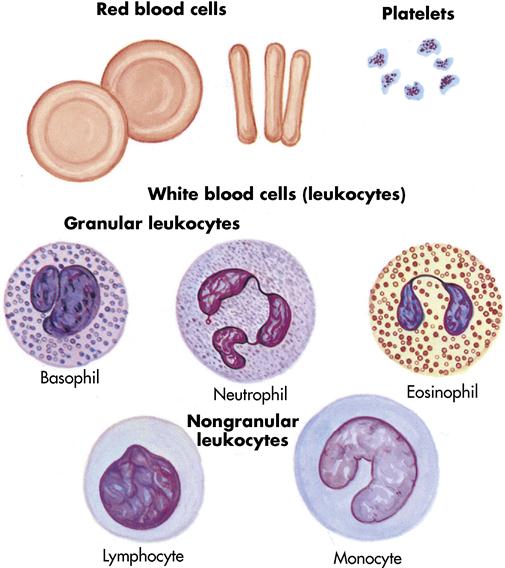
Blood is a viscous (thick), red fluid that contains red blood cells (RBCs), white blood cells (WBCs), and platelets, which are suspended in a light yellow fluid called plasma. Plasma constitutes 55% of the blood’s volume; the remaining 45% is composed of the blood cells and platelets (Figure 7-1). Blood is slightly alkaline, with a pH range of 7.35 to 7.45. It has a sodium chloride concentration of 0.9%. The average adult blood volume is 5 to 6 L (101⁄2 to 121⁄2 pints).
The blood performs three critical functions. First, it transports oxygen and nutrition to the cells and waste products away from the cells, and it transports hormones from endocrine glands to tissues and cells. Second, it regulates the acid-base balance (pH) with buffers, helps regulate body temperature because of its water content, and controls the water content of its cells as a result of dissolved sodium ions. Third, it protects the body against infection with special cells and prevents blood loss with special clotting mechanisms.
The following sections discuss individual components of the blood.
Red blood cells
Erythrocytes (RBCs) give blood its rich color. In men, RBCs average approximately 5.5 million/mm3 of blood; in women, they average approximately 4.8 million/mm3 (Table 7-1). A mature RBC contains cytoplasm and the red pigment hemoglobin, a compound in the blood that carries oxygen from the lungs to the cells and carbon dioxide away from the cells to the lungs. Erythrocytes are classified according to size, shape, and color. Hemoglobin content is expressed as normochromic or hypochromic anemia, whereas RBC size is usually expressed as macrocytic, microcytic, or normocytic. The normal hemoglobin level is 14 to 18 g/dL for men and 12 to 16 g/dL for women. The average life span of an RBC is 120 days. An erythrocyte is the major cellular element of the circulating blood; its principal function is to transport oxygen and carbon dioxide. Erythrocytes are continuously produced in the red bone marrow, principally in the vertebrae, ribs, sternum, and proximal ends of the humerus and femur.
Table 7-1
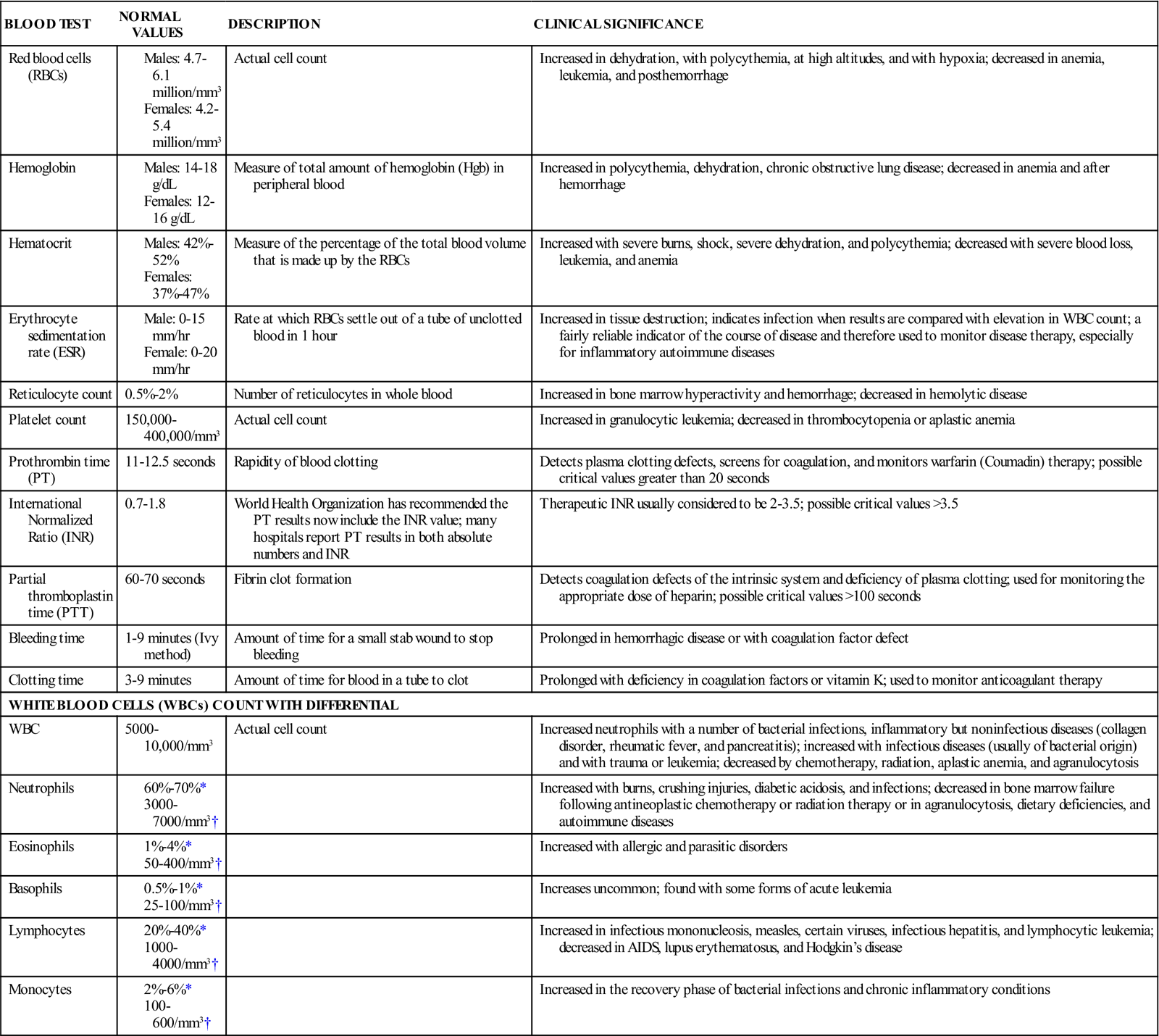
AIDS, Acquired immunodeficiency syndrome.
*Relative values: expressed as percentage of total WBC.
Erythropoiesis (the process of RBC production) depends on several factors, among them healthy conditions of the bone marrow; dietary substances such as iron and copper, plus essential amino acids; and certain vitamins, especially vitamin B12, folic acid, riboflavin (vitamin B2), and pyridoxine (vitamin B6). When the amount of oxygen delivered to the tissues by RBCs is decreased, it triggers the release of an enzyme, the renal erythropoietic factor, in the kidneys. Erythropoietin is carried to the bone marrow, where it initiates the development of mature RBCs. The increased number of RBCs allows more oxygen to be delivered to the tissues, and as a result shuts off the signal to increase RBC production.
A common laboratory test called the hematocrit (a measure of the packed cell volume of RBCs, expressed as a percentage of the total blood volume) can tell a great deal about the volume of RBCs in a blood sample. Normally about 42% to 52% of the blood volume in men and 37% to 47% in women consists of RBCs.
If hemoglobin falls below the normal level, as it does in anemia, an unhealthy chain reaction begins: less hemoglobin means less oxygen transported to cells, a slower breakdown and use of nutrients by cells, less energy produced by cells, and decreased cellular function. Understanding the relationship between hemoglobin and energy makes it clear why an anemic person complains of feeling “tired all the time.”
White blood cells
Unlike erythrocytes, leukocytes (WBCs) have nuclei, are colorless, and live from a few days to several years. They are primarily involved in body defenses, such as destruction of bacteria and viruses. They number 5000 to 10,000/mm3 of blood. Some WBCs can actually leave the bloodstream and move through tissue spaces to fight foreign invaders, such as bacteria. WBCs have two broad categories: granulocytes and nongranulocytes. The three types of granulocytes are neutrophils, eosinophils, and basophils. The nongranulocytes include lymphocytes and monocytes. A differential white blood cell count is an examination in which the different kinds of WBCs are counted and reported as percentages of the total examined. They also may be reported as absolute (actual number) (see Figure 7-1).
Because leukocytes respond predictably to symptoms of infection and recovery, they are a reliable gauge of the state of the body’s defenses. That is why the differential WBC is such a common blood test. Although the differential WBC cannot, by itself, be used to diagnose a disease or to discriminate between a bacterial and viral infection, it reveals activity that points to occult (hidden) infection or that signals the intensity of chemotherapy.
The granulocytes develop from the red bone marrow and contain granules in their cytoplasm. The granules are demonstrated when the cells are stained with Wright’s stain (a chemical solution). Neutrophils (granular circulating leukocytes essential for phagocytosis—the process by which bacteria, cellular debris, and solid particles are destroyed and removed) ingest bacteria and dispose of dead tissue. Neutrophils are the primary phagocytic cells involved in acute inflammatory response. A mature neutrophil is called a segmental neutrophil, or “seg,” because the nucleus is segmented into two to five lobes connected by strands. They also release lysozyme, an enzyme that destroys certain bacteria. The normal value of neutrophils is 60% to 70%.
Mature neutrophils have a short life span (approximately 7 hours), after which they die, along with the bacteria and debris they have engulfed. Bone marrow thus needs to manufacture neutrophils constantly; normally it stores approximately a 6-day supply. Because neutrophils respond in proportion to the severity of the infection, an overwhelming infection may deplete marrow reserves. When this happens, the marrow releases polymorphonuclear leukocytes (“polys”) that are in the final stages of development. These immature neutrophils are called bands. When the band count exceeds 8% of the total number of polys, the marrow has used up its reserve. In the differential white count, an increase in the number of band neutrophils is called bandemia. Bandemia is seen in patients with serious bacterial infections. The presence of excess bands in the peripheral blood was traditionally called “a shift to the left.” This term originated when laboratory reports were handwritten with the immature neutrophils recorded on the left side of paper. This term is still used in some areas (McCarron, 2004).
Eosinophils are WBCs that play a role in allergic reactions and are effective against certain parasitic worms. Normal values of eosinophils are 1% to 4%.
Basophils are WBCs that are essential to the nonspecific immune response to inflammation because they release histamine (vasodilator) during tissue damage or invasion. They have cytoplasmic granules that contain heparin, serotonin, and histamine. If a basophil is stimulated by an antigen or by tissue injury, it releases substances within the granules. This is part of the response seen in allergic and inflammatory reactions. Normal values of basophils are 0.5% to 1%.
Monocytes are WBCs that function like neutrophils; they circulate in the bloodstream and move into tissue, where they engulf foreign antigens and cell debris. Monocytes are the second type of WBC to arrive at the scene of an injury. They are useful in removing dead bacteria and cells in the recovery stage of acute bacterial infections. Normal values of monocytes are 2% to 6%.
Lymphocytes are WBCs that form antibody, a special protein that combats foreign invaders, or antigens. They set up the antigen-antibody process, which protects the body. Lymphocytes have two groups: B cells and T cells. B cells search out, identify, and bind with specific antigens. T cells, when exposed to an antigen, divide rapidly and produce large numbers of new T cells that are sensitized to that antigen. T cells work together with the B cells to destroy the foreign antigen. Normal values of lymphocytes are 20% to 40%.
Thrombocytes (platelets)
Thrombocytes, or platelets, are the smallest cells in the blood. They are circular cell fragments that do not contain nuclei. They have a life span of 5 to 9 days and number 150,000 to 400,000/mm3 of blood (see Figure 7-1). They are produced in the red bone marrow and have a role in the process of hemostasis (the prevention of blood loss). They assist in forming clots, which seal off a break in the continuity of the walls of the blood vessels (Figure 7-2).
Hemostasis
Hemostasis is a body process that arrests the flow of blood and prevents hemorrhage. Three actions take place: (1) vessel spasm, (2) platelet plug formation, and (3) clot formation. When a vessel has a tear or rupture, the smooth muscle in the walls of the vessel causes it to contract. Platelets rush in and attempt to seal the area, which is effective in small vessel tears. The third process, clot formation, is more detailed and occurs in larger injuries. This process can be summarized as follows (see Figure 7-2):
Blood types (groups)
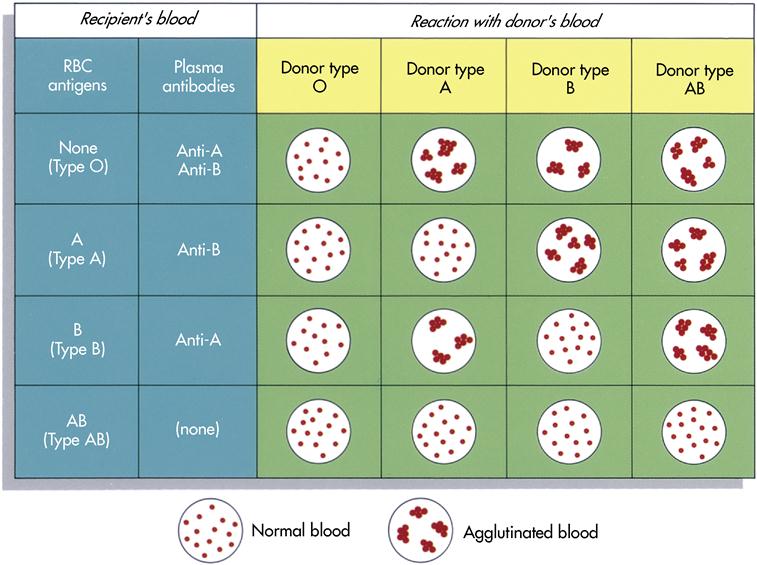
A person’s blood group or type is genetically determined and is inherited from his or her parents. Blood types are determined by the presence or absence of specific antigens on the outer surface of the RBCs. In certain types of blood, the antigens on the RBCs are accompanied by antibodies found in the blood plasma. In the ABO system, every person’s blood is one of the following types: type A, type B, type AB, or type O.
Forty-one percent of Americans have type A blood. The letter A stands for a certain type of antigen in the plasma membrane of the RBCs at birth. A person who is born with type A antigen does not form antibodies to react with it. In other words, this person’s blood plasma contains no anti-A antibodies; it does, however, contain anti-B antibodies. For some unknown reason, these antibodies are present naturally in type A blood plasma. The body did not form them in response to the presence of B antigen. In summary, then, in type A blood the RBCs contain type A antigen and the plasma contains anti-B antibodies.
Correspondingly, in type B blood, the RBCs contain type B antigen and the plasma contains anti-A antibodies. In type AB, as its name indicates, the RBCs contain both type A and B antigens, and the plasma contains neither anti-A nor anti-B antibodies. The opposite is true of type O blood: its RBCs contain neither type A nor type B antigens, and the plasma contains both anti-A and anti-B antibodies.
Harmful effects or even death can result from a blood transfusion if antibodies in the recipient’s plasma react to the donor’s blood and the RBCs become agglutinated. If the donor’s blood is type O, and therefore its RBCs do not contain any A or B antigen, the blood cannot be clumped by anti-A or anti-B antibodies. For this reason type O blood is known as universal donor blood; it can be used in an emergency as donor blood, no matter what the recipient’s blood type. Similarly, blood type AB has been called the universal recipient blood because it contains neither anti-A nor anti-B antibodies in its plasma. Therefore it does not clump any donor’s RBCs containing A or B antigens. In a normal clinical setting, however, all blood intended for transfusion is typed and crossmatched carefully to the blood of the recipient for a variety of factors. Figure 7-3 shows the results of combinations of donor and recipient blood.
Two types of reactions can occur: agglutination and hemolyzation. In agglutination the donor cells clump together because of the antibodies; this occludes arteries and can result in death. In hemolyzation the antibodies cause the RBCs of the recipient to rupture and release their cell contents; this can also lead to death.
Rh factor
Rh factor is located on the surface of the RBCs. People who have Rh factor are said to be Rh positive; people who do not have Rh factor are said to be Rh negative. Eighty-five percent of humans have Rh factor; 15% do not. Normally, human plasma does not contain Rh antibodies; these develop in response to an individual’s receiving the wrong type of blood (i.e., if an Rh-negative person receives Rh-positive blood). Within approximately 2 weeks, Rh antibodies are produced and remain in the blood. If the Rh-negative person then receives more Rh-positive blood, a severe reaction occurs because the Rh-positive antibodies react with the donor blood. The antibodies hemolyze the donor RBCs, causing them to rupture and lose their contents.
Rh incompatibility is seen most commonly in pregnancy. Fortunately, this incompatibility can be prevented. The mother’s blood is tested for antibodies, and if they are present, she can receive an intramuscular dose of Rho(D) immune globulin (RhoGAM)—a desensitization drug. This enables her to carry the next infant without the potential complications associated with Rh incompatibility.
Lymphatic system
The lymphatic system is a subdivision of the cardiovascular system. It consists of lymphatic vessels, the lymph fluid, and the lymph tissue. The system has three basic functions: (1) maintenance of fluid balance, (2) production of lymphocytes, and (3) absorption and transportation of lipids from the intestine to the bloodstream.
Lymph and lymph vessels
The constancy of the fluid around each body cell can be maintained only if numerous homeostatic mechanisms function together in a controlled and integrated response to changing conditions. The circulatory system plays a key role in bringing many needed substances to cells and then removing the waste products that accumulate as a result of metabolism. This exchange of substances between blood and tissue fluid occurs in capillary beds. Many other substances that cannot enter or return through the capillary walls, including excess fluid and protein molecules, are returned to the blood as lymph.
Lymph is a specialized fluid formed in the tissue spaces and transported by way of lymphatic vessels to eventually reenter the circulatory system. In addition to lymph and the lymphatic vessels, the lymphatic system includes lymph nodes and lymphatic organs such as the thymus and the spleen (Figure 7-4).
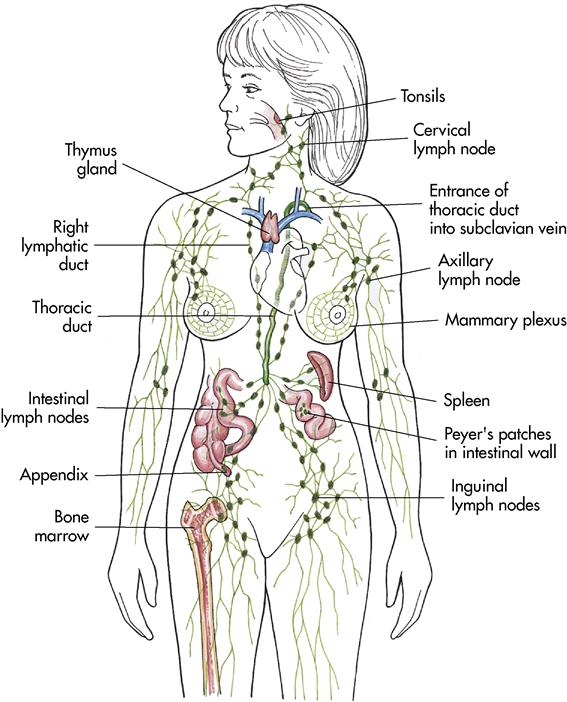
Lymphatic tissue
Lymph nodes
Lymph nodes (glands) have two functions: (1) to filter impurities from the lymph (much like an oil filter in a car) and (2) to produce lymphocytes (WBCs). The body contains 500 to 600 lymph nodes. They are small bean-shaped structures, usually appearing in groups. They range from 0.04 to 1 inch (1 to 25 mm) in length. Lymph nodes are most numerous in the axilla, the groin, the abdomen, the thorax, and the cervical regions (see Figure 7-4). The structure of the lymph nodes makes it possible for them to perform two functions: defense and WBC production.
Tonsils
The tonsils are masses of lymphoid tissue embedded in the mucous membrane of the oral cavity and the pharynx. The tonsils protect the body against invasion of foreign substances by producing lymphocytes and antibodies. They also trap bacteria and may become enlarged. The tonsils are larger in children and begin to atrophy (shrink) at about age 7.
Spleen
The spleen is a soft, roughly ovoid, highly vascularized organ located in the left upper quadrant of the abdominal cavity, just below the diaphragm (see Figure 7-4). The spleen is 5 to 6 inches (12.7 to 15.2 cm) long and 2 to 3 inches (5 to 7.6 cm) wide. It contains lymphatic nodules.
The spleen stores 1 pint of blood, which can be released during emergencies, such as hemorrhage, in less than 60 seconds. This large amount of blood gives the spleen its deep purple color. The main functions of the spleen are (1) to serve as a reservoir for blood; (2) to form lymphocytes, monocytes, and plasma cells; (3) to destroy worn-out RBCs; (4) to remove bacteria by phagocytosis (engulfing and digesting); and (5) to produce RBCs before birth (the spleen is believed to produce RBCs after birth only in cases of extreme hemolytic anemia).
Thymus
The thymus is located in the upper thorax posterior to the sternum and between the lungs in the mediastinum (see Figure 7-4). The thymus gland functions in utero (before birth) and a few months after birth to develop the immune system. The thymus is responsible for the development of T lymphocytes in the cell-mediated immune response before they migrate to the lymph nodes and the spleen. At puberty the thymus gland atrophies; it is eventually replaced by fat and connective tissue.
Laboratory and diagnostic tests
Complete blood count
The complete blood count (CBC) is an important part of routine screening and hospital admission. It involves several tests, each of which assesses the three major cells formed in the bone marrow. The CBC detects many disorders of the hematological system and provides data for diagnosing and evaluating disorders in other body systems. A CBC includes red and white cell counts, hematocrit and hemoglobin levels, erythrocyte indexes, differential white cell count, and examination of the peripheral blood cells (see Table 7-1). Prepare the patient by explaining that a blood sample will be taken from the hand or arm and evaluated for indicators of infection or anemia in the body.
Erythrocyte indexes
Erythrocyte indexes are measurements of the size and hemoglobin content of RBCs. This measurement provides information about the average volume or size of a single RBC (mean corpuscular volume [MCV]). Mean corpuscular hemoglobin (MCH) is a measure of the average amount (weight) of hemoglobin within an RBC. Mean corpuscular hemoglobin concentration (MCHC) is a measure of the average concentration or the percentage of hemoglobin within an RBC.
Peripheral smear
A peripheral smear along with the differential WBC count allows examination of the size, shape, and structure of individual RBCs and platelets. This information is useful in differentiating various forms of anemias and blood dyscrasias. All three hematological cell lines (RBCs, WBCs, platelets) can be examined. When adequately prepared and examined microscopically by an experienced technologist, a smear of peripheral blood is the most informative of all hematological tests.
Schilling test and megaloblastic anemia profile
The Schilling test is a laboratory blood test for diagnosing pernicious anemia. The test measures the absorption of radioactive vitamin B12, before and after parenteral injection of the intrinsic factor, by examination of the urinary excretion of vitamin B12. Normal findings are excretion of 8% to 40% of radioactive vitamin B12 within 24 hours. The Schilling test for pernicious anemia is being replaced by a serum test called megaloblastic anemia profile, which measures vitamin B12, methylmalonic acid, and homocysteine levels.
Gastric analysis
Gastric analysis is an older test for determining pernicious anemia. In pernicious anemia the gastric secretions are minimal and the pH remains elevated, even after injection of histamine.
Radiologic studies
Radiologic studies for the hematological system involve primarily the use of computed tomography (CT) or magnetic resonance imaging (MRI) for evaluating the spleen, the liver, and the lymph nodes. In the past, lymphangiography with contrast dye was a common procedure for evaluating deep lymph nodes. CT is now the preferred method (Lewis et al., 2007).
Bone marrow aspiration or biopsy
When the diagnosis is not clearly established by peripheral blood smears or further information is needed, bone marrow aspiration or biopsy helps establish the diagnosis and assess treatment response. The most common site for this procedure is the posterior iliac crest. The sternum can also be used, but generally only for aspiration. Normal bone marrow is soft and semifluid and can be removed by aspiration through a needle. Bone marrow aspiration is most commonly performed in people with marked anemia, neutropenia (decreased number of WBCs), acute leukemia, and thrombocytopenia (decreased number of platelets). Cell types, numbers, and maturation are examined. Although complications of bone marrow aspiration are minimal, there is a possibility of penetrating the bone and underlying structures. This hazard is greatest in an aspiration procedure involving the sternum.
Disorders of the hematologic and lymphatic systems
The hematologic and lymphatic systems include the blood and the organs of blood production—the bone marrow and lymphatic tissue. Disorders of blood production, bone marrow, or lymphatic tissues affect virtually all body systems. Disturbances in this delicate balance can produce life-threatening signs and symptoms, severe pain, and incapacitation.
Disorders associated with erythrocytes
Anemia
Anemia is a disorder characterized by levels of RBCs, hemoglobin, and hematocrit that are below normal range. In hemolytic anemia, increased RBC destruction also occurs. In persons with anemia, insufficient amounts of oxygen are delivered to tissues and cells.
Etiology and pathophysiology
Anemia can be caused by many factors, including blood loss (hemorrhage), impaired production of RBCs (bone marrow depression), increased destruction of RBCs (hemolysis), or nutritional deficiencies (long-term iron deficiency). Hemorrhage or blood loss accounts for temporary anemia, whereas nutritional deficit can cause long-term iron deficiency anemia. Marrow failure is linked to a disease process, toxic exposure, tumor, or unknown causes. A decrease in RBC production or increased destruction results in a lower number of circulating RBCs. Bone marrow hematopoietic function is unable to produce the needed quantity.
Loss of the oxygen-carrying element in the blood results in a supply/demand imbalance in vital organs. Peripheral circulation compensates by shunting blood to vital organs, thus causing hypoxia in other areas of the body. Rapid hematopoietic effort causes blood cell irregularities (immature RBCs) and inability to produce RBCs, with a resultant decrease in the RBC count.
Clinical manifestations
Most adults do not experience symptoms until the hemoglobin level is less than 8 g/dL. Older adults, however, may show symptoms with a hemoglobin concentration of less than 10 g/dL. Although each type of anemia has specific signs and symptoms, the decreased oxygen-carrying capacity leads to signs and symptoms that are common in all anemias. These include anorexia, cardiac dilation, disorientation, dizziness, dyspepsia, dyspnea, exertional dyspnea, fatigue, headache, insomnia, pallor (mucous membranes and skin), palpitations, shortness of breath, systolic murmur, tachycardia, and vertigo.
Assessment
Subjective data commonly include expressions of weakness, dyspnea, fatigue, and vertigo. Anorexia and dyspepsia may accompany headache and insomnia, but the patient generally does not link these complaints to the condition unless questioned. In older adult patients with impaired cardiopulmonary reserves, be alert to complaints of chest pain, dyspnea on exertion, palpitations, and dizziness.
Collection of objective data includes observing signs of bleeding or shock (hypovolemic anemia). Laboratory values will show a low RBC count and hematocrit and hemoglobin levels. Skin and mucous membranes are pale, and cardiac symptoms are related to anemia. With long-term anemia, the patient may have ulcerations of the extremities.
Diagnostic tests
Blood studies show RBC count and hemoglobin and hematocrit levels to be below normal. Serum iron, total iron-binding capacity, and serum ferritin levels are below normal. Reticulocyte count is increased because of immaturity of RBCs. A bone marrow study shows a deviation from normal findings. Peripheral blood smears enable identification of abnormalities of shape and color of cells. A megaloblastic anemia profile reveals decreased levels of vitamin B12.
Medical management
Intervention depends on the cause. Correction of the disease process may correct or lessen the anemic condition. Transfusion is appropriate for blood loss; iron and vitamin B12 are replaced if these are deficient. Treatment is often specific to the particular anemia (see Cultural Considerations box).
Nursing interventions and patient teaching
Nursing diagnoses and interventions for the patient with anemia include but are not limited to the following:
Tailor patient education to the individual conditions and needs.
Hypovolemic anemia (blood loss anemia)
Etiology and pathophysiology
Secondary anemia is when deficiencies in RBCs and other components are caused by an abnormally low circulating blood volume from hemorrhage. Blood loss of 1000 mL or more in an adult can be severe. Such a loss is usually related to internal or external hemorrhage caused by a surgical procedure, gastrointestinal (GI) bleeding, menorrhagia, trauma, or severe burns.
Loss of blood decreases the amount of circulating fluid and hemoglobin and thus decreases the amount of oxygen carried to the body tissues. The tissues must have oxygen to survive. The average adult has an approximate total blood volume of 6000 mL (6 L [12 pints]) and can tolerate a loss of up to 500 mL. If the loss approaches 1000 mL, acute complications, such as hypovolemic shock, may occur. The rapidity of blood loss is related to the severity and number of signs and symptoms. The sudden reduction in the total blood volume can lead to hypovolemic shock. RBC count and the hematocrit level drop to half the normal range.
Clinical manifestations
Signs and symptoms include restlessness; a subtle rise in respiratory rate; weakness; stupor; irritability; and pale, cool, moist skin. Excessive blood loss results in shock. Shock occurs when there is a deprivation of oxygen and nutrients to organs. Hemorrhagic blood loss results in a decrease in blood volume. In shock, vasoconstriction occurs in blood vessels to noncritical organs such as skin, muscles, and intestines. This decreases the blood flow to these organs and shunts blood to the vital organs such as the heart and the brain.
The amount of blood loss affects the heart rate and the blood pressure. In the early stages of shock, when 750 to 1000 mL of blood has been lost, the heart rate is less than 100 bpm with a normal blood pressure. As the blood loss increases to 1000 to 1500 mL, the pulse increases to more than 100 bpm and the patient has orthostatic blood pressure. When the blood loss is 1500 to 2600 mL (about 30% to 40% of the blood volume), the systolic blood pressure decreases to less than 90 mm Hg and the pulse increases to more than 120 bpm. With a blood loss of 1500 to 2000 mL, irreversible end-organ damage can result (Beattie, 2007a).
The patient’s clinical signs and symptoms are more important than the laboratory values. Be alert to the patient’s expression of pain. Internal hemorrhage may cause pain because of tissue distention, organ displacement, and nerve compression. Pain may be localized or referred. Decreased RBC, hemoglobin, and hematocrit levels may not be evident until several days after severe blood loss has occurred. The severity of the patient’s signs and symptoms correlates with the severity of the blood loss.
Assessment
Subjective data commonly include complaints of thirst, weakness, irritability, and restlessness.
Objective data include decreased blood pressure; rapid, weak, thready pulse; and rapid respirations. Cold, clammy skin with pallor is noted. Oliguria is often evident. Mental disorientation and physical collapse with prostration can occur.
Diagnostic tests
When blood loss is sudden, plasma volume has not yet had a chance to increase, the loss of RBCs is not reflected in laboratory data, and values may seem normal or high for 2 to 3 days. However, once the plasma is replaced, the RBC mass is less concentrated. RBC, hemoglobin, and hematocrit levels are severely decreased, often to half the normal values.
Medical management
In the case of massive hemorrhage, measures are taken to stop the blood loss and treat for shock and lost volume. Severe hemorrhaging often results in the need for mechanical ventilation. Oxygen therapy restores oxygen that is less available because of decreased hemoglobin in the blood. To replace fluid volume, intravenous (IV) saline is used. In severe fluid volume depletion, a bolus of 2 L of normal saline is given. If hypotension continues or if the hemoglobin is below 6 g/dL, packed RBCs are usually given. It is now recommended to keep the hemoglobin over 7 g/dL. Often platelets, fresh frozen plasma (FFP), or cryoprecipitate is included in the treatment to control hemorrhage (Beattie, 2007a).
Monitor the hemoglobin level to note the effectiveness of the treatment. Be aware that one unit of packed RBCs should increase the hemoglobin by 1 g/dL (Beattie, 2007a). The patient may also need supplemental iron because the availability of iron affects the marrow production of erythrocytes. Oral or parenteral iron preparations are often administered.
Nursing interventions and patient teaching
Monitor blood and fluid restoration and identify blood loss sites to control the bleeding. Keep patients flat and warm. Take vital signs at frequent intervals. Take precautions to prevent injury to a restless or disoriented patient. Measure intake and output (I&O), with careful monitoring of urinary output for oliguria caused by decreased renal perfusion. The decrease in urinary output correlates to the amount of blood lost. If a patient has a blood loss of 1000 to 1500 mL, the urinary output is 20 to 30 mL/hr; with a blood loss of 1500 to 2000 mL, the urinary output is less than 20 mL/hr; and a blood loss of 2000 mL or more would result in anuria (very low urinary output) (Beattie, 2007a).
If hemorrhage is caused by a chronic problem, teach the patient to monitor bleeding amounts and associated factors and to report to the physician immediately for treatment.
Prognosis
Without treatment, death will result. With aggressive treatment, the prognosis is favorable.
Pernicious anemia
Etiology and pathophysiology
A pernicious disease is one that is capable of causing great injury, destruction, or death. Without treatment, pernicious anemia would be fatal. This type of anemia is the result of a metabolic defect: the absence of a glycoprotein intrinsic factor secreted by the gastric mucosa. Intrinsic secretion fails because of gastric mucosal atrophy. Pernicious anemia is an autoimmune disease in which antibodies in the parietal walls of the stomach prevent the production of the intrinsic factor (Lewis et al., 2007). It is a progressive, megaloblastic, macrocytic anemia primarily affecting older adults. The intrinsic factor is essential for absorption of vitamin B12 (cyanocobalamin).
The intrinsic factor is not available to combine with vitamin B12, preventing transport of this necessary vitamin to the ileum (vitamin B12 is normally absorbed in the distal ileum). Deficiency of the vitamin affects growth and maturity of all body cells, including RBCs in the marrow. The erythrocyte membrane becomes fragile and ruptures easily. This vitamin is related to nerve myelination; its absence leads to progressive demyelination and degeneration of nerves and white matter.
Clinical manifestations
Extreme weakness is noted with dyspnea, fever, and hypoxia. As the condition progresses, weight loss is apparent, as is slight icterus (jaundice) with pallor. The skin color may appear a pale lemon-yellow because of the excessive destruction of the RBCs, which causes the bile pigments to increase in the blood serum. The patient experiences edema of the legs, intermittent constipation, and diarrhea.
Assessment
Subjective data include the patient’s complaints of palpitations, nausea, flatulence, and indigestion. The tongue is sore and burning. Weakness and difficulty swallowing (dysphagia) may occur. Neurologic symptoms include tingling of the hands and feet and loss of the sense of body position (impaired proprioception).
Collection of objective data includes observation of a smooth and erythematous tongue, with infection about the teeth and gums. Cerebral signs include mental disorientation, personality changes, and behavior problems. Severe neurologic impairments can result, including partial or total paralysis from destruction of the nerve fibers of the spinal cord.
Diagnostic tests
The Schilling test shows malabsorption of vitamin B12. This test is being replaced by the serum megaloblastic anemia profile, which reveals decreased serum levels of vitamin B12, serum methylmalonic acid, and homocysteine. Bone marrow aspiration reveals abnormal RBC development.
The erythrocytes appear large (macrocytic) and have abnormal shapes; serum cyanocobalamin (B12) levels are reduced. A gastric analysis may be done to determine the cause of the vitamin B12 deficiency. Pernicious anemia is caused by an absence of intrinsic factor, from either gastric mucosal atrophy or autoimmune destruction of parietal cells of the stomach. This results in a decrease of hydrochloric acid secretion by the stomach. An acidic environment in the stomach is required for the secretion of intrinsic factor.
Medical management
Oral vitamin B12 is ineffective if there is an absence of the intrinsic factor in the stomach or a malabsorption problem in the ileum (Lewis et al., 2007). Cyanocobalamin injections, folic acid supplement, and iron replacement are ordered. If the anemia is severe, the patient may be transfused with packed RBCs. The standard treatment includes initiating vitamin B12 replacement therapy; without it, these individuals will die in 1 to 3 years. Treatment is 1,000 units of vitamin B12 administered intramuscularly daily for 2 weeks, then weekly until the hematocrit is normal, and finally monthly for life. An intranasal form of cyanocobalamin (Nascobal) is self-administered once weekly. The patient’s blood values should return to normal within 2 months of B12 therapy. A CBC is necessary every 3 to 6 months to monitor the long-term success of treatment.
Nursing interventions and patient teaching
The nursing interventions depend to some extent on the stage of the disease. A symptomatic approach is appropriate. When the patient is confined to the hospital, check vital signs every 4 hours. Perform special mouth care several times daily. The diet should be high in protein, vitamins, and minerals. Anemic patients are especially sensitive to cold, so additional lightweight, warm blankets may be needed. Interventions should conserve energy and prevent injury.
Nursing diagnoses and interventions for the patient with pernicious anemia include but are not limited to the following:
| Nursing Diagnoses | Nursing Interventions |

To control the disease, the patient must understand the disease process and the importance of lifetime therapy of vitamin B12. Discuss the importance of a diet high in vitamin B12. Adjusting activities when signs and symptoms are present may lessen the patient’s stress. The need for assistance with ADLs and for frequent rest periods should be impressed on the patient and significant people involved in the care.
Prognosis
This condition, if untreated, can be considered terminal in 1 to 3 years. With treatment the patient may be asymptomatic. Because the potential for gastric carcinoma is increased in pernicious anemia, the patient should have frequent and careful evaluation for this problem.
Aplastic anemia
Etiology and pathophysiology
Aplastic anemia, or aplasia (a hematological term for a failure of the normal process of cell generation and development), has two etiologic classifications: congenital and acquired. Approximately 30% of aplastic anemias that appear in childhood are inherited, caused by chromosomal alterations. Acquired aplastic anemia is directly related to exposure to viral invasion, medications, chemicals (e.g., benzene, insecticides, arsenic, alcohol), radiation, or chemotherapy, in which the hematopoietic tissue is replaced by fatty marrow, causing a defect in RBC production. The causes of 70% of acquired cases of aplastic anemia are idiopathic (cause unknown). Aplastic anemia is probably an immune-mediated disease.
Depression of erythrocyte production results in lowered hemoglobin and RBCs. Leukopenia and thrombocytopenia may develop. People with aplastic anemia are usually pancytopenic; that is, all three major blood elements (red cells, white cells, and platelets) from the bone marrow are reduced or absent. The incidence of aplastic anemia is low, affecting approximately 4 of every 1 million people.
Clinical manifestations
The signs and symptoms of aplastic anemia may have an acute onset or develop slowly over several weeks to months. With suppression of all three major blood elements, the patient may have signs and symptoms related to each. For example, suppression of WBCs may result in infection, suppression of RBCs may lead to anemia, or suppression of thrombocytes may cause petechiae (Lewis et al., 2007). Repeated infections with high fevers may occur, along with fatigue, weakness, general malaise, dyspnea, and palpitations. Mortality is high from complications of infection and hemorrhage. Bleeding tendencies are reported: petechiae, ecchymoses, bleeding gums, epistaxis, and GI and genitourinary system bleeding.
Assessment
Subjective data include a history of exposure to chemicals such as insecticides and drugs in addition to a family history of aplastic anemia. Ask the patient about the ability to carry out ADLs without fatigue.
Collection of objective data includes monitoring the patient for pallor, signs of infection, and bleeding tendencies. Also, dyspnea and tachycardia may be noted.
Diagnostic tests
A bone marrow study (aspiration biopsy) shows hypoplastic or aplastic fatty deposits, a decrease in cellular elements with increased yellow marrow (fat content), and depressed hematopoietic activity. The diagnostic findings are especially important because the marrow is hypocellular, with increased yellow marrow, a finding termed dry tap. Peripheral blood smears show that blood cells may be normocytic and normochromic.
Medical management
The cause of aplastic anemia must be identified promptly and removed or discontinued. Bone marrow suppression is expected with certain antineoplastic medications or radiation therapy, and laboratory values should be monitored frequently to maintain control.
Avoid blood transfusions, if possible, to prevent iron overloading and the development of antibodies to tissue antigens. Platelet transfusions that are human lymphocyte antigen (HLA) matched are used to treat serious bleeding in a thrombocytopenic patient. Blood transfusions are used cautiously to minimize the risk of rejection for a bone marrow transplant candidate.
A splenectomy may be required in patients with hypersplenism that is destroying normal platelets. Steroids and androgens are sometimes used to stimulate the bone marrow. Immunosuppressive therapy with antithymocyte globulin and cyclosporine or high-dose cyclophosphamide (Cytoxan) has become important for patients who are not candidates for bone marrow transplantation or hematopoietic stem cell transplant (SCT). Bone marrow transplantation or hematopoietic SCT is the treatment of choice in patients younger than the age of 45 who have a compatible donor. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is used as biologic response modifier treatment for aplastic anemia.
Bone marrow transplant.
A bone marrow transplant is indicated in certain cases such as immunodeficient states, cancer, leukemia, and recurrent aplastic anemia. A matched donor and recipient are essential to avoid rejection or complications. Specimens from twins, siblings, or self (autologous) while in remission are preferred.
After emotional and physical preparation of the patient, perform blood studies to set baselines and assess the patient’s status. Establish a pathogen-free environment, with the immunocompromised patient placed on reverse isolation (neutropenic precautions). Monitor for fever or infection. The medication therapy used in this preparation may include immunosuppressants, antibiotics, and antianxiety agents.
Bone marrow transplants are used increasingly in hematological malignancies after large doses of chemotherapy or radiation therapy. A limited amount of chemotherapy or radiation can ordinarily be administered because of its toxicity to the bone marrow. When bone marrow is transplanted after these therapeutic modes, much larger therapeutic doses are possible.
Bone marrow is obtained by multiple marrow aspirations under general or spinal anesthesia, usually yielding 500 to 800 mL of marrow. The marrow is cryopreserved (frozen) until it is used. Shortly after chemotherapy (with or without radiation therapy) is completed, the patient receives the donated marrow through an IV catheter. This infusion of marrow is called the rescue process. The marrow travels through the bloodstream to the bone marrow, where it begins to manufacture new leukocytes, erythrocytes, and thrombocytes. The infused marrow repopulates the patient’s marrow after several weeks. The patient runs a great risk of toxicity, including infections, marrow rejection, and graft-versus-host disease. Medications supporting graft acceptance include cyclosporine (immunosuppressant) and chemotherapy (to prevent graft-versus-host complications).
Splenectomy.
Surgical excision of the spleen may be performed to treat blood dyscrasias with splenomegaly, to treat trauma to the spleen, or to remove a diseased spleen. Preoperative assessment includes cardiovascular observation, respiratory function determination, and GI evaluation. Postoperatively, compare these observations with the patient’s baseline evaluations, and observe the patient for infection or inflammation. Potential complications include infection, hemorrhage, shock, and paralytic ileus. Maintain parenteral therapy. Use nasogastric (NG) suction if a paralytic ileus develops. Address the patient’s postoperative pain. Also maintain movement and use positioning to prevent infection or postoperative pneumonia.
Nursing interventions and patient teaching
Proper observation and care after bone marrow study are essential. Patients with aplastic anemia are highly susceptible to infection; thus nursing interventions should be directed toward prevention. Adhere to strict aseptic techniques for dressing changes and IV site care. To prevent impaired skin and mucous membranes, avoid intramuscular injections and rectal medications or rectal temperatures. Use protective devices, such as an air mattress. In the presence of thrombocytopenia, observe carefully for any signs of bleeding and prevent even the slightest trauma. Monitor the patient’s urine and stool for occult or gross blood.
Nursing diagnoses and interventions for the patient with aplastic anemia include but are not limited to the following:
| Nursing Diagnoses | Nursing Interventions |
| Activity intolerance, related to inadequate tissue oxygenation | |
| Risk for infection, related to increased susceptibility |
Everyone with aplastic anemia needs to know how to protect themselves from excessive bleeding. Help the patient maintain a balance between rest and activity. Discuss with the patient how to avoid infection, especially of the respiratory or urinary tract (see Safety Alert box).

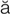 -N
-N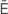 -m
-m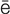 –
–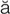 ,
, 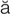 -PL
-PL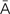 -zh
-zh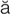 ,
, 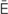 -m
-m –
– ,
,