Classification systems, clinical vocabularies, and terminology
Marsha C. Steele
Key words
Administrative terminology
Administrative terminology
ASC-X12N
Backward mapping
Classification system
Clinical document ontology
Clinical interface terminology
Clinical terminology
Clinical Terms Version 3
Code
Code sets
ConceptID
Controlled clinical reference terminology
Controlled vocabulary
CPT
Crosswalk
Desiderata
Document naming nomenclature
DRGs
DSM-5
Electronic data interchange (EDI)
Electronic transaction standards
Federal medication terminologies
Forward mapping
General equivalency mappings GEMs
Granularity
HACs
HCPCS
Hierarchical relationships
HIPAA
ICD-10
ICD-10-CM
ICD-10-PCS
ICD-9
ICD-O
ICNP
IHTSDO
Interface terminology
Interoperability—basic, functional, semantic
IPPS
Location code
LOINC
Mapping
Metathesaurus
MS-DRGs
NDCs
NDF-RT
NIC
NOC
Nomenclature
Omaha System
Ontology
Patient Medical Record Initiative
PMRI
POAs
Point of care
ReadCodes
RELMA
RXCUI
RxNorm
Semantic network
Shared terminology
SNODENT
SNOMED CT
SNOMED RT
Specialist lexicon
Standardized terminology
Structured data
Structured output
Template
Terminology
Thesaurus
UMDNS
UMLS
Universal identifiers
Vocabulary
Abbreviations
AAFP—American Academy of Family Physicians
AAP—American Academy of Pediatrics
ACP—American College of Physicians
AHCPR—Agency for Health Care Policy and Research
AHIC—American Health Information Community
AHIMA—American Health Information Management Association
AHRQ—Agency for Healthcare Research and Quality
AMA—American Medical Association
ANA—American Nurses Association
AP-DRG—All Patient Diagnosis-Related Groups
AMA—American Medical Association
ASTM—American Society for Testing and Materials
CAP—College of American Pathologists
CCHIT—Certification Commission for Healthcare Information Technology
CDC—Centers for Disease Control and Prevention
CHCF—California Health Care Foundation
CHI—Consolidated Health Informatics
CINAHL—Cumulative Index of Nursing and Allied Health Literature
CIS—commercial information system
CMS—Centers for Medicare and Medicaid Services
CPRS—Computerized Patient Record System
CPT—Common Procedural Terminology
CT—Computed Tomography
DHHS—Department of Health and Human Services
DICOM—Digital Imaging and Communications in Medicine
DOD—Department of Defense
DRG—Diagnosis-Related Groups
DSM—Diagnostic and Statistical Manual of Mental Disorders
EHR—Electronic Health Record
EO—Executive Order
FDA—Food and Drug Administration
HCPCS—Healthcare Common Procedure Coding System
HIM—health information management
HIMSS—Health Information Management Systems Society
HIPAA—Health Insurance Portability and Accountability Act
HITPA—Health Information Technology Promotion Act
HITSP—Health Information Technology Standards Panel
HL7—Health Level 7
ICD-9—International Classification of Diseases, Ninth Revision
ICD-9-CM—International Classification of Diseases, Ninth Revision, Clinical Modification
ICD-10—International Classification of Diseases, Tenth Revision
ICD-10-PCS—International Classification of Diseases, Tenth Revision, Procedural Coding System
ICD-O—International Classification of Diseases for Oncology
IEEE—Institute of Electrical and Electronics Engineers
IOM—Institute of Medicine
ISO—International Organization for Standardization
JCAHO—Joint Commission on Accreditation of Healthcare Organizations (now The Joint Commission)
LOINC—Logical Observation Identifiers Names and Codes
MESH—Medical Subject Headings
NAHIT—National Alliance for Health Information Technology
NANDA—North American Nursing Diagnosis Association
NCHS—National Center for Health Statistics
NCPDP—National Council of Prescription Drug Programs
NCVHS—National Committee on Vital and Health Statistics
NDC—National Drug Codes
NDF-RT—National Drug File Reference Terminology
NEC—Not Elsewhere Classified
NIC—Nursing Interventions Classification
NLM—National Library of Medicine
NOC—Nursing Outcomes Classification
ONCHIT—Office of the National Coordinator of Health Information Technology
PBM—Pharmacy Benefits Management
PCS—Procedural Coding System
PMRI—Patient Medical Record Information
PNDS—Perioperative Nursing Data Set
RELMA—Regenstrief LOINC Mapping Assistant
SCD—Steering Committee of Databases to Support Clinical Nursing Practice
SDO—standards developing organization
SNODENT—Systematized Nomenclature of Dentistry
SNOMED CT—Systematized Nomenclature of Medicine, Clinical Terms
SNOMED RT—Systematized Nomenclature of Medicine, Reference Terminology
SNOP—Systematized Nomenclature of Pathology
UMLS—Unified Medical Language System
VA—Department of Veterans Administration
VAMC—Department of Veterans Affairs Medical Center
WHO—World Health Organization
Student Study Guide activities for this chapter are available on the Evolve Learning Resources site for this textbook. Please visit http://evolve.elsevier.com/Abdelhak.
When you see the Evolve logo,  go to the Evolve site and complete the corresponding activity, referenced by the page number in the text where the logo appears.
go to the Evolve site and complete the corresponding activity, referenced by the page number in the text where the logo appears.
The challenge of clinical communications and information exchange
In any health care environment, we rely on a variety of systems and applications to register a patient, identify the payer and the provider, capture the patient’s history and findings of the physical, communicate the orders and plan, track diagnostics and test results, record vital signs and inputs from monitoring devices, do the assessments, provide the treatment and care, document the response to care, and so on. You may recognize the system applications by the functional names of various processes such as the MPI (Master Patient/Person Index), ADT (admission, discharge, and transfer), order entry, results management, and communications systems, to name a few. The most common communication need is between the clinician and ancillary departments, and among other members of the health care team. The complexity of communications accelerates with the need to share these communications between systems in different health care settings, between clinical sources such as electronic health records (EHRs) and research databases, and with sharing patient information with other health care institutions or regional health information exchanges.1 Health care is a long way from having a true longitudinal patient record with the ability to track episodes of care across providers throughout the patient’s lifetime. Every aspect of health care is dependent on information. We must be able to create and exchange health information with ease and flexibility to enter information as demanded by clinicians, while at the same time managing its costs, maximizing its benefits, and protecting its security.
Interoperability and shared terminologies
Interoperability means the ability to communicate and exchange data accurately, effectively, securely, and consistently with different information technology systems, software applications, and networks in various settings, and exchange data such that clinical or operational purpose and meaning of the data are preserved and unaltered.”2
At the application level, interoperability means the capacity of an authenticated user to access and transmit or receive and exchange usable information with others. At the systems level, it means the ability of different information systems and software applications to communicate and exchange data accurately, effectively, and consistently. It includes the ability to use the information that has been exchanged. On an expanded level, it means the ability of health information systems to work together within and across organizational boundaries and disparate systems to other authorized entities in real time.3
The U.S. National Committee on Vital and Health Statistics describes three levels of interoperability:
2. Functional interoperability—defines the format of messages. This ensures messages between computers can be interpreted at the level of the data fields, so that the data can pass from a structured field in one system to a comparably structured field in another. Neither system, however, has understanding of the meaning of the data within the field(s).4 This is similar to e-mail in that the format is agreed on with To/From/Subject/Message. Like basic interoperability, technical connectivity is achieved and must follow a standard format, but the system is not trying to understand the meaning of the message.
3. Semantic interoperability—provides common interpretability, that is, information within the data fields can be used intelligently.5 It has the ability to allow information to be understood by shared systems. This level of interoperability is dependent on the degree of agreement of data terminology and its quality. An example of this is found at Methodist University Hospital in Memphis, Tennessee, where they use robots in their pharmacy. Because they use semantic interoperability, various fields mean the same thing across systems. This enables CPOE (computerized physician/provider order entry) information to be “understood” by the robots.
The system uses customized operating software, advanced robotics, and pharmacy system middleware to automate pharmacy orders. The robot is able to measure, compound, and fill medications orders. It can apply patient-specific bar-code labels, do compliance checks, and keep records. The robot can fill up to 600 doses per hour with precision, thereby increasing patient safety and reducing medication errors. The robot can also package daily doses for specific patients, remove expired drugs from inventory, and mix and fill intravenous syringes.6
The Health Level 7 (HL7) EHR Interoperability Work Group stated that “interoperability is not a quality or qualification, but rather a noun describing a relationship between systems.”1 Interoperability is not simply a successful transfer of information from one system to another in the correct format. To understand interoperability more fully, we must pursue the notion of semantics to ensure the preservation of the “meaning” of the message. This means that we must also employ shared terminologies and definitions. “In health care, semantic operability, or shared terminology, is as important as system interoperability, or shared functions, and must occur in order to achieve the maximum benefit to use the information that has been exchanged. Interoperability is one of the most critical concepts confronting the adoption and implementation of enhanced electronic information technologies into our national healthcare infrastructure.”7 Clinical data in health information systems need to be recorded at the appropriate level of detail. It must remain consistent over time and across boundaries and be able to be transmitted without any loss of meaning or accuracy. Without the use of semantic interoperability and shared terminologies, the potential for patient errors can increase, and data reliability and validity can be reduced.8
Putting terminologies in a framework
Structured versus unstructured text
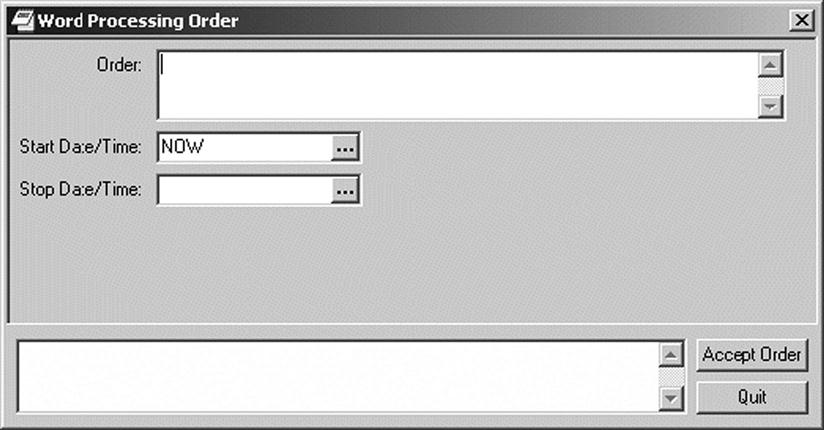
In Chapter 5, and Helbig build a contextual framework for understanding how terminologies and classifications are used in EHR systems. For EHR systems to produce predictable data, they require standardized terminologies to represent concepts and to communicate them effectively and in the manner intended. Clinicians document in the electronic health record in a variety of ways. They may use free text or “unstructured” data to enter information directly online or may continue to dictate their notes directly or with voice-activated dictation. Figure 6-1 illustrates an order form used to enter unstructured text directly on line.10
Clinicians often enter documentation using a combination of free text and structured data. Structured data entry allows users to draw from standard phrases or pick lists and pull-down menus to help guide the entry and ensure that complete information is included. These data “tools” make use of predefined text scripts, lists, and terminology.
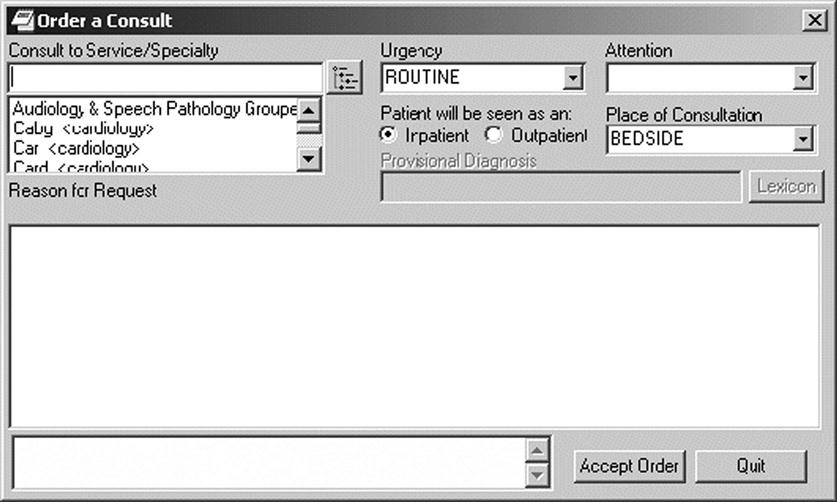
Figure 6-2 illustrates the ability to type free text or unstructured data to indicate the Reason for Request when ordering a consultation. Pick lists also appear on the consult order to prompt the use of standardized data to name and direct the order to the appropriate service or specialty. Other structured data needed for the consult order include selections to indicate urgency, inpatient or outpatient service, and the location where the consultation will take place.9
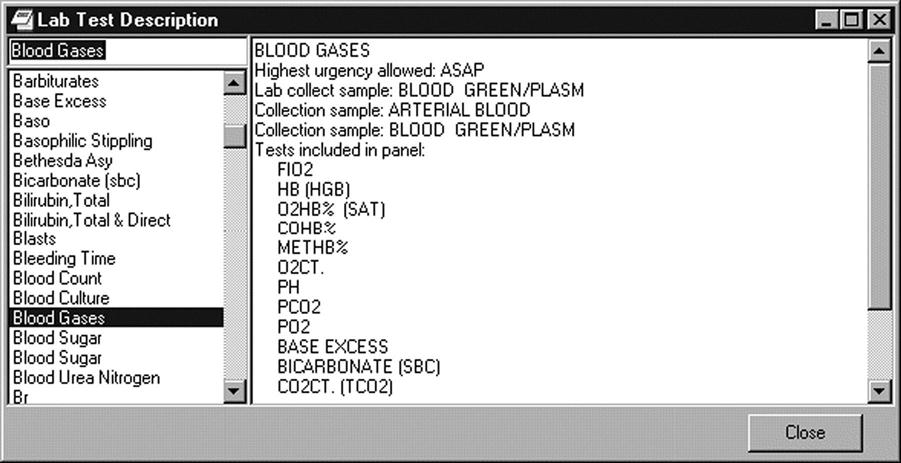
In addition to documenting in the patient record, standardized text can be used to look up information, as shown in Figure 6-3. By selecting a lab test from the pick list on the left, the description of the test is displayed on the right and indicates the tests that are included in the lab panel.
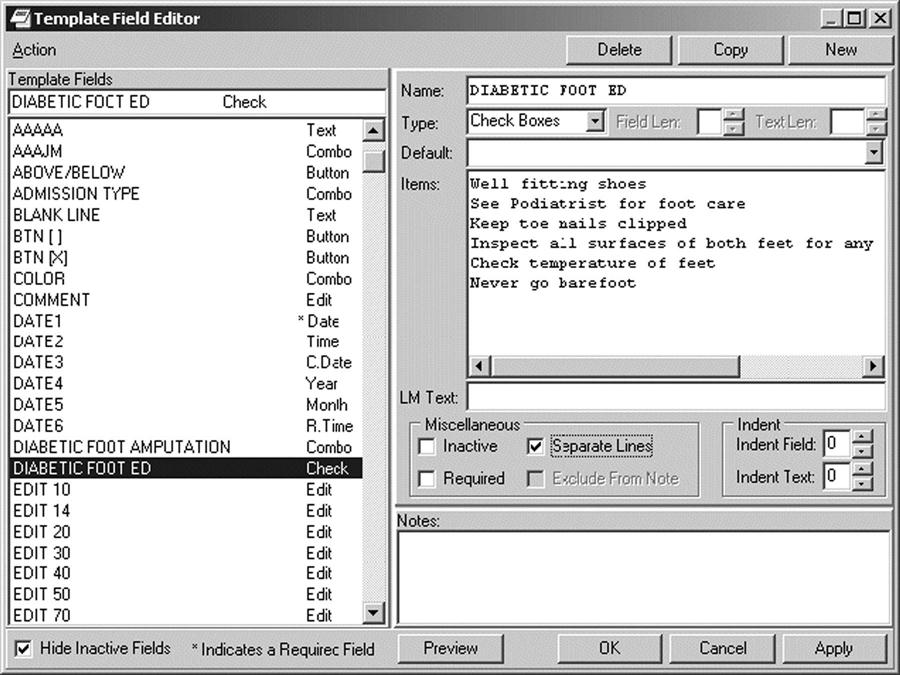
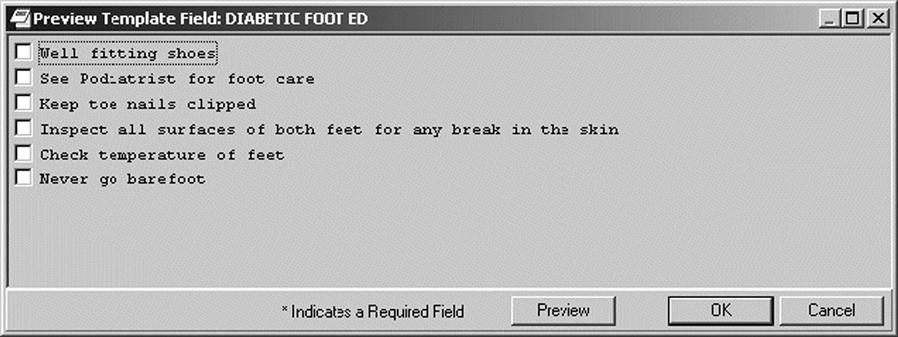
In some settings, templates are used to guide entries and facilitate data capture.10 A template is constructed like an electronic form, and guides the user to enter specific content. The template has a combination of drop-down lists and areas for entering free text that are visible to the person documenting the note. The template becomes transparent to the reader as the system will pull the structured and free text into a note that reads like a narrative report. You can select desired items from the pick lists and preview how they will read in the note. In designing the template, the service requesting it must reach consensus and decide which phrases and content are the most clinically appropriate and reflect the most natural expression of the provider. The order of the content should also follow the natural work-flow process used by the clinician. Figure 6-4 shows patient instructions for diabetic foot care created via a template.
Standardized terminology
Regardless of the tools employed, standardized terminologies are needed to represent concepts and to communicate them accurately. The terms and concepts used to create clinical documentation for symptoms, diagnoses, procedures, test findings, health status, problem lists, and plans in the EHR need to be interpreted and understood by persons authorized to access that information in that same way that the author wanted it to be represented, exchanged, and interpreted. Uniform definitions are necessary to convey accurate and useful information consistently. This is especially important when information is shared and exchanged. In health care, semantic interoperability, or shared terminology, is as important as systems interoperability, or shared functions, and must occur to achieve maximum benefit to use the information.1
The adoption of a single common medical language to meet all information needs is not practical. Although there are many excellent and robust clinical terminologies available, each terminology differs in purpose, scope, and granularity. Terminologies used to enter and capture clinical documentation in the health record must also be interoperable with subsystems, such as the lab or pharmacy. Standardized terminology and structured clinical data enable computer processing and are a prerequisite for interoperability, sharing, and exchanging health care information.11 A balance must be sought to find terminologies that offer the level of detail and ease of use that clinicians need to provide and document care, achieve semantic interoperability, and work with the messaging and exchange infrastructure within the EHR and across systems. A standard electronic health record and a national health information infrastructure require the use of standardized medical language and terminologies to transmit clinical data across diverse information systems.12
Ehealth: standardized terminology
The World Health Organization’s report eHealth: Standardized Terminology underscores the importance of a terminology that ensures that information exchanged between different users will be faithfully understood and reliably used. Content standards, independent of technical standards, must reflect the most advanced scientific understanding of terms and concepts and adhere to the best available knowledge-representation principles.13 Today’s health care environment is experiencing an explosion of knowledge that requires standardization to collect, store, archive, retrieve, process, analyze, and exchange vast amounts of health data. Agreed upon definitions and the usage of terms and concepts underpin the reliability and accuracy of the health information that we collect, whether it is about a single patient or about many patients aggregated into statistics and reports. Standardized terminology is the key to integration and to managing the complexities of moving from data to knowledge for the purposes of medical knowledge and improving patient care.
The ability to move data to knowledge has significant implications. An individual provider can document the condition and care provided to one patient. These problems and treatments are then coded. This data can then be aggregated and grouped with similar conditions and treatments. After the data are grouped, we can look at the similarities and differences in the problems, as well as the successes and failures in the treatment, to enable us to learn something. If this grouping never happens, it is up to individual care providers to learn from their own patient base—and the immediate group with whom they share information—likely a less effective and slower process. Knowledge is the collective information, understanding, and experience that gives individuals the power to learn from this collective experience and make informed decisions to improve care.
Basic understanding of terms
Advocates believe that strong terminologies or coding schemes are essential components of health information systems and that the fastest way to establish and secure consistent data from health care organizations is through standard terminologies. There continues to be national and international interest in identifying “ideal” terminologies to secure accurate and efficient data capture from the care delivery experience to accurately represent clinical concepts.
Health information management (HIM) professionals play an important role in helping health care organizations achieve the benefits of health information technology. David Brailer, MD, PhD, the first national coordinator for health information technology, noted that “HIM professionals know what to do with records, how they flow through the systems, who uses them, who has access to them, and how to release information appropriately.” With expertise in health information, data standards, coding, documentation, EHR functionality, and business processes in a variety of health care settings, health information managers serve as advocates and subject matter experts. They help to resolve a wide range of health information technology issues from data standardization to privacy. To be effective in this evolving role, HIM professionals must understand the uses and limitations of different health care terminologies and be able to assist in the selection of appropriate terminologies for use with EHR systems.
Vocabulary
A vocabulary typically means all the terms that are recognized for communication within a domain; it is a collection of words or phrases.
A controlled vocabulary means that the terms within the vocabulary are carefully selected for their inclusion; terms outside that boundary are not included. You already use controlled vocabularies in everyday activities. The Yellow Pages makes use of a controlled vocabulary. If you look up “doctors,” you will be redirected to “See Physicians & Surgeons,” using the preferred terminology rather than the vernacular. In a controlled vocabulary, a list of approved terms have been enumerated explicitly. Two rules are generally followed: (1) if the same term is commonly used to mean different concepts in different contexts, then its name is explicitly qualified to resolve the ambiguity; (2) if multiple terms are used to mean the same thing, one of the terms is identified as the preferred term, and the others are listed as synonyms.14 The Dental Informatics Online Community provides these examples: white spots or tooth decay will be mapped to the preferred term “DENTAL CARIES.” Similarly, oral cancer or mouth tumor will be mapped to the preferred term “MOUTH NEOPLASMS.”15 The most standard controlled medical vocabularies used for coding patient information are ICD-9-CM, SNOMED, LOINC, UMLS, and READ (see the list of abbreviations at the beginning of this chapter for the full name of these vocabularies). They are discussed in further detail in this chapter.

Terminology
A terminology generally means a set of terms representing the system of concepts of a particular subject or field. When used in health care, a terminology is a set of terms that describe health concepts. In contrast to a vocabulary, a terminology includes a prescribed set of terms authorized for a specific use. An example would be CPT (Common Procedural Terminology) used for reporting diagnostic and therapeutic procedures and other services performed by physicians.16 CPT is intended for use for administrative and reimbursement purposes. Generally, procedures are lumped into categories.
The CPT codes in Table 6-1 demonstrate concepts to distinguish between the donor and the recipient, the segment of the liver used, whether the specimen came from a living or cadaver donor, or is from the same species in liver transplantation.17
Table 6-1
CPT CODES FOR LIVER TRANSPLANTATION
| Donor Hepatectomy | Recipient Liver Transplant |
| 47133 Donor hepatectomy from cadaver donor | 47135 Liver allotransplantation; partial or whole; from cadaver or living donor, any age |
| 47140 Donor hepatectomy from living donor; left segment only | 47136 Liver allotransplantation; heterotopic, partial or whole; from cadaver or living donor, any age |
| 47141 Donor hepatectomy from living donor; total left lobectomy 47142 Donor hepatectomy from living donor; right total lobectomy |
A clinical terminology is used to represent the information in a health record, whether it is in paper or electronic form. It is composed of standardized terms, concepts, and their synonyms. Clinical terminologies are used to record patient findings, circumstances, events, and interventions with sufficient detail to support clinical care, decision support, outcomes research, and quality improvement. They are used for many tasks, from simple archiving and retrieval of medical information to complex clinical decision support and data sharing between systems. Clinical terminologies can be efficiently mapped to broader classifications for administrative, regulatory, oversight, and fiscal requirements.18 Clinical terminologies provide a way to input clinical data into a health record and support the transmission of patient data across information systems.19
Terminologies have been developed to represent diseases, procedures, interventions, medications, devices, anatomy, functional status, demographics, and other types of information. Classifications, code sets, vocabularies, and nomenclatures all come under the umbrella of terminology.
Classification system
A classification system is a scheme for grouping similar things in a logical way on the basis of common characteristics. It is a system that is clinically descriptive and arranges or organizes like or related entities.20 Items are grouped according to type or class within an organized system. Libraries organize their collections using the Dewy Decimal System. It, too, is a classification system. Like classifications we use in health care, it must be updated to include new topics and discoveries. The Dewey Decimal System maps to the Library of Congress Subject Headings (LCSH) and to Medical Subject Headings (MeSH) to organize medical literature. Classification systems vary in their level of detail; some offer very general groupings, whereas others allow more detailed groupings and subgroupings, enabling the user to capture a greater degree of specificity (a level of detail), or granularity, of data. As an example, ICD-9-CM contains 17,000 codes, whereas ICD-10-CM and ICD-10-PCS code sets have more than 155,000 codes. This improved granularity greatly assists in identifying medical conditions and procedures with high precision. There is only one code for angiography in ICD-9. In contrast, ICD-10 provides 1,170 coded descriptions that can identify the location of the blockage and the device used for the procedure. You can move from a highly granular code set to a more generalized one without disrupting the integrity of the codes; however, if you move from a generalized code set to a more specific one, it is difficult to achieve the level of granularity required. As with paint, you can put two specific colors together in one bucket, but it is almost impossible to separate them back into the original colors they once were.
When used in health care, a classification system groups together similar diseases and procedures and organizes related entities for easy retrieval. It groups clinical conditions and procedures into manageable, predetermined categories for external reporting purposes, including reimbursement for health care services and statistical data analysis, such as epidemiological analysis or trending of disease incidence. Because the main purpose of a classification is statistical analysis and reporting, this type of system is intentionally limited to a relatively small number of mutually exclusive categories. To support statistical reporting needs, classifications use a complex system of conventions, instructional notes, and reporting rules. However, they are not as comprehensive as terminologies because classifications are not intended to represent the complete clinical content of the health record. They are not designed for primary clinical documentation care at the point of care.13
Essentially, terminologies and nomenclatures are more granular than classifications, because a classification categorizes and aggregates clinical concepts rather than supports detailed descriptions of distinct clinical concepts. A classification provides the ability to aggregate the terms in a reference terminology for administrative purposes. Because a classification aggregates or classifies clinical terms, it is also considered a type of clinical terminology and may be referred to as an “administrative,” “aggregate,” or “reporting” terminology.13 Some examples of classification systems are included in Box 6-1.
Nomenclature
A nomenclature is a naming convention or systematic listing of names that have been assigned according to preestablished rules.
An example of a nomenclature would be Universal Medical Device Nomenclature System (UMDNS). It is a proprietary, standardized, and controlled international nomenclature and coding system for medical devices that has been adopted by more than 90 countries and more than 5,000 institutions. Table 6-2 shows a section of UMDNS codes and the device term.21 This nomenclature is discussed later in the chapter.
Table 6-2
UNIVERSAL MEDICAL DEVICE NOMENCLATURE SYSTEM CODES
| UMDNS CODE | UMDNS TERM English |
| 16421 | Trocars, Suprapubic |
| 16422 | Cannulae, Nasal |
| 16424 | Capsules, Dental Amalgam |
| 16425 | Sponge Carriers, Endoscopic |
| 16426 | Carvers, Dental Amalgam |
| 16427 | Carvers, Dental Wax |
| 16428 | Tissue Cassettes |
| 16429 | Catheters, Cholangiography |
| 16430 | Catheters, Anesthetic Conduction |
| 16431 | Catheters, Intrauterine |
| 16432 | Catheters, Nasopharyngeal |
| 16433 | Catheters, Peritoneal |
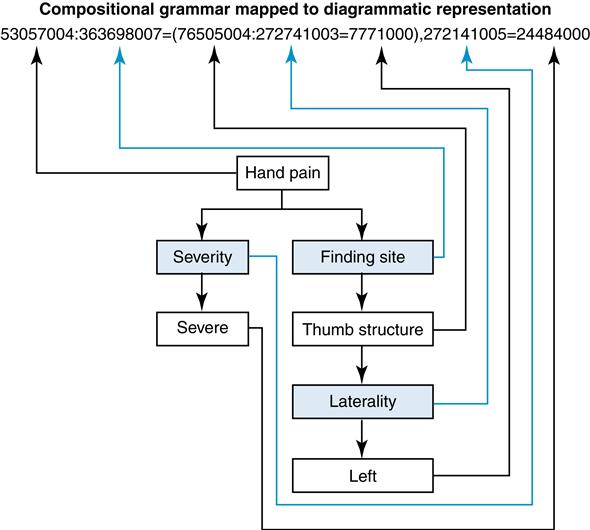
There is often overlap between the terms nomenclature and vocabulary. For example, the Systematized Nomenclature of Medicine, Clinical Terms, known as SNOMED CT, is both a vocabulary and a nomenclature. There is a naming structure in SNOMED that maps relationships. It contains more than 311,000 active concepts and approximately 1,360,000 links or semantic relationships between SNOMED CT concepts.22 Let’s look at a patient that has severe thumb pain because of an infection under his nail (Table 6-3).
Table 6-3
SNOMED-CT NAMING STRUCTURE MAPPING TO RELATIONSHIPS
| Type of Relationship | Manifestation |
| “IS” a relationship between concepts | Pain in the hand Pain + hand |
| Finding site relationship | |
| Laterality of site relationship | |
| Causative relationship | |
| Associated morphology relationship | |
| Severity relationship | 6. Severe pain |
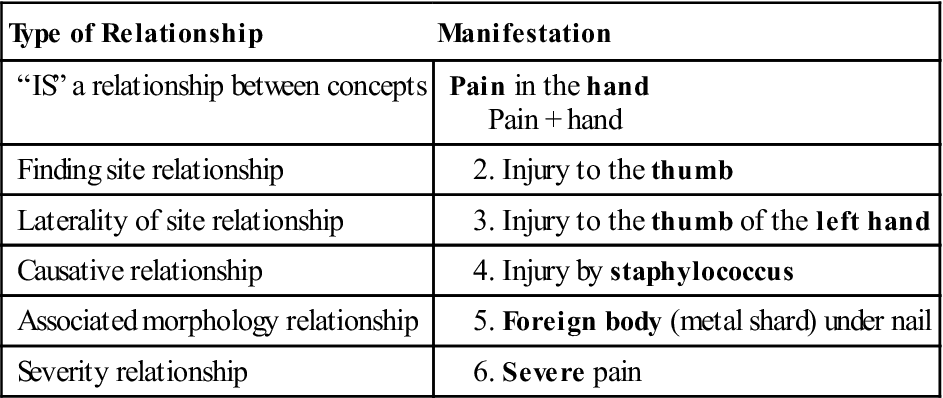
Compare the table to the diagrammatic representation (Figure 6-5) offered by the International Health Terminology Standards Development Organization (IHTSDO).23
Codes
A code is a unique identifier assigned to a specific term, description, or concept. A list of codes and the terms or descriptions with which they are associated is known as a code set. In some code sets, the codes serve as a kind of shorthand—or a way of conveying meaning with a minimum of complexity. For example, in the fourth edition of the Current Procedural Terminology (CPT), developed by the American Medical Association (AMA), codes between 80048 and 89356 are all related to pathology and laboratory.
In HIM, coding commonly refers to the selection of alphanumeric codes to represent diseases, procedures, and supplies used in the delivery of health care and the assessment of the quality of care. As an example, CPT Category II codes are often used to code services that support performance measures and approved clinical treatment protocols. Statistical analysis may be based on formulae such as these:
Administrative coding may be done manually by using code books and other reference sources or with the assistance of electronic tools.24
However, when used in the broader context of EHRs, coding (or encoding) refers to the assignment of unique identifiers to the terms within a particular clinical terminology. Rather than being assigned manually, codes are assigned according to some predetermined algorithm, usually without any manual intervention. In a well-designed terminology, no meaning can be discerned from a code itself; it is simply a unique identifier that represents a more detailed description, concept, or term. The codes themselves remain unknown to most users. When a user selects a term to use, the associated code is stored electronically. These codes are used to transmit health data from one electronic system to another for analysis, aggregation, or use at the point of care.25
As an example, location codes enable systems to identify the source from which data originate. A facility may have 15 different locations or “location codes.” These code numbers do not have meaning but make the data easier to handle. The users do not have to enter the codes and are unaware that that a unique identifier is being used.
Codes provide a more concise and efficient means to store and transmit detailed information than free text does. Reliance on free text information limits our ability to aggregate and compare information from different sources and presents challenges to the designers of advanced EHR tools. With encoded data, information can be displayed in a form that human beings can read and understand and store in a form that computers can exchange and manipulate. Clinical terminologies that use codes to represent detailed concepts provide a way to combine the expressiveness and flexibility of free text information with the clarity and computability of encoded information.26
Classifications and terminologies are used with code sets to define and classify individual health terms. They serve as a way to relate terms to one another so that they are easily and consistently understood by users. Classifications arrange related terms for easy retrieval, whereas vocabularies are sets of specialized terms that facilitate precise communication by eliminating ambiguity. A coding classification system groups related terms or conditions together into classes. For this reason, classification systems have less granularity and specificity than nomenclatures. Nomenclatures use precise naming conventions to represent a term or condition in more detail. A coding system with more specificity can be more readily converted to one that groups or classifies, whereas once grouped, the detail is lost, and it is more difficult to revert back with accuracy.
As an example, ICD-9 has only one code for angioplasty, a procedure for widening a narrowed or obstructed artery; ICD-10 offers 1,170 codes with a granularity that pinpoints the location of the blockage and the device used for each procedure. Similarly, ICD-9 does not have codes to distinguish laterality; ICD-10 has codes that allow the identification of the left or right side. These are just some of the differences between these coding systems. When comparing the 17,000 codes in ICD-9 to the over 155,000 codes in ICD-10, it is easy to understand the concept of granularity and data loss if reverting back to ICD-9 from ICD-10. Important classifications include ICD-9-CM, ICD-10, ICD-10-CM/PCS, and ICF. Important terminologies include LOINC and SNOMED. There are subtle differences among vocabularies, nomenclatures, terminologies, and classifications.
Administrative terminologies
Administrative terminologies are classification and coding systems that are primarily designed to support the administrative, financial, and regulatory functions associated with patient care. They serve as the primary communication tool between those providing services and those paying for those services. They are also used by providers, payers, researchers, governmental agencies, and others to create secondary statistical reports as well as facilitate the activities that follow:
• Measuring the quality, safety, and efficacy of care
• Managing care and disease processes
• Tracking public health and risks
• Providing data to consumers regarding costs and outcomes of treatment options
• Payment system design and processing of claims for reimbursement
• Research, epidemiological studies, and clinical trials
• Designing health care delivery systems and monitoring resource utilization
• Identifying fraudulent practices
Clinical terminologies
Clinical terminologies are those designed to represent the information in the patient record, whether in paper or electronic form. When clinical terminologies are used, much of the ambiguity inherent in natural language can be eliminated because the definitions, concepts, and relationships that represent the concepts have been predetermined. Human readers can easily interpret narrative information and automatically add context that helps them interpret that information. In reviewing another clinician’s progress note about a patient, for example, a physician might recall a previous conversation that was not documented in the record, think about practices at the other hospital where the patient had been treated, or even consider who wrote the note to capture subtleties that a computer might miss.
In contrast, computers are quite literal in their interpretation of data. A classic example of this is illustrated by the sentence, “Baby swallows fly.” This sentence could be interpreted in different ways, depending on whether the verb in the sentence is “swallows” or “fly” and on whether “baby” is a noun or an adjective.
Health data can also be ambiguous. The word “cold,” for example, can refer to many different things, including temperature, mood, illness, and influenza. For example, a patient might report to her physician that she has a cold or that she feels cold all the time. If the letters “COLD” were transmitted from one provider to another as part of the patient’s medical record, the recipient might wonder whether the patient had chronic obstructive lung disease unless additional information were provided to clarify the use of the term. When clinical terminologies are used, much of the ambiguity inherent in natural language can be eliminated.
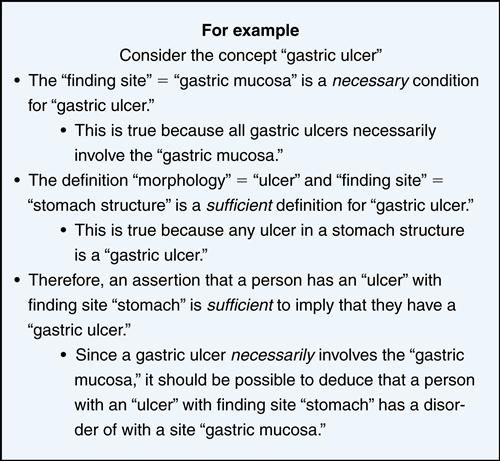
A controlled medical terminology or controlled clinical reference terminology is a coded vocabulary of medical concepts and expressions used in health care. It is concept-based and allows for the complex organization and aggregation of clinical information. The term controlled refers to managing the content to ensure that it remains structurally sound, biomedically accurate, and consistent with current practice. It facilitates the use of standardized terminology and the collection of structured data when creating electronic health records. An example of a controlled reference terminology is SNOMED-CT. It allows a variety of practitioners to use multiple terms to express the same concept. The system inserts an interpretive layer of semantics between the term entered by the user and the underlying database to better represent the original intention of the terms of the user. This helps others to understand the entry made by the author in the way it should be interpreted14 (Figure 6-6).
Clinical terminologies
Desirable characteristics of clinical terminologies
In 1998, a medical informaticist named James Cimino, MD, identified 12 desiderata, or desirable characteristics, that would make clinical terminologies more useful.28 These desiderata have since gained broad acceptance and are now considered the fundamental principles for the development and integration of clinical terminologies. The 12 characteristics are as follows:
1. Content
The usefulness of a given terminology is largely determined by its content. There must be a formal method for adding content when gaps in content coverage are discovered.
2. Concept orientation
Each terminology should have a concept orientation—that is, each term should correspond to a single concept and that concept should have a single, coherent meaning. A given concept may be interpreted differently when used in different contexts; for example, a condition recorded under the personal history of a patient may have different implications than the same condition recorded under family history.
3. Concept permanence
Concept permanence means that once a concept has been created within a terminology, its meaning cannot change and it cannot be deleted. The preferred name of the concept can change, or the concept may be deemed inactive, but its meaning must remain fixed as long as the terminology is in use. Concept permanence helps prevent the users from misinterpreting historical data.
4. Nonsemantic concept identifier
There must be a nonsemantic concept identifier associated with each term. This means that an identifier does not carry any meaning in itself and does not indicate the hierarchical position of the term it represents.
5. Polyhierarchy
Many terminologies are arranged hierarchically. A hierarchical structure can help users locate concepts, group related concepts, and add context. It is not necessary to have a single hierarchy; in fact, polyhierarchy is necessary to support different users and uses of the same terminology. (Example: SNOMED accomplishes polyhierarchy through the use of “is-a” relationships.)
6. Formal definitions
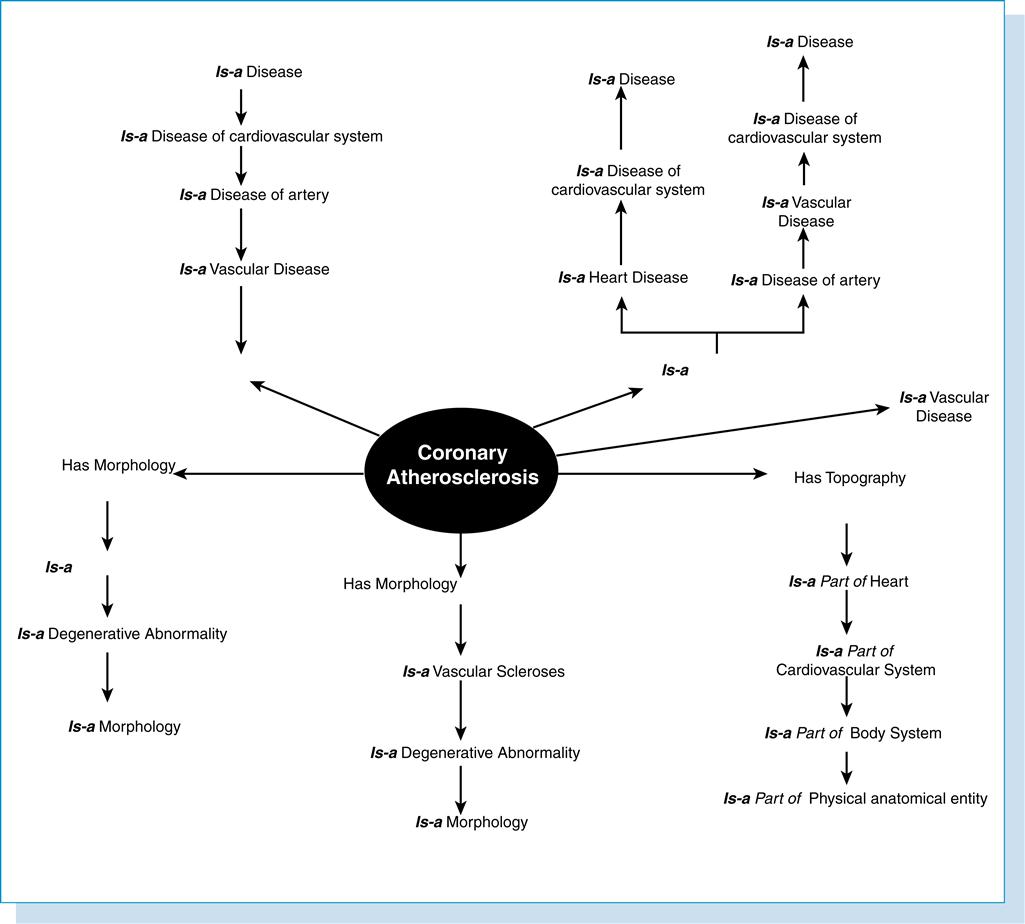
Formal definitions are those that follow a formal structure, that is, definitions that are represented in a form that can be manipulated by a computer, as opposed to narrative text definitions intended for human readers. These definitions can be expressed through the use of “is-a” relationships connecting concepts in a vocabulary.
In Figure 6-7, the concept “Coronary Atherosclerosis” can be defined with an “is-a” link to the concept “Arteriosclerotic Vascular Disease” and a “site” relationship to “coronary artery.”
7. Reject “not elsewhere classified”
Not Elsewhere Classified (NEC) terms are used to collect concepts that are not covered elsewhere in a terminology. Because NEC terms are defined by exclusion, their meaning can change every time a new concept is added to the terminology.
8. Multiple granularities
Granularity refers to the degree of specificity and refinement of a term. Different users are likely to require different levels of granularity. Terminologies that include multiple granularities of the same content can support many more users that those with a single level of granularity.
9. Multiple consistent views
Multiple consistent views are required to support the different views of the vocabulary depending on the utility. Cimino states that polyhierarchical terminologies with multiple levels of granularity must not permit inconsistent views of a single concept.28
10. Context representation
A well-designed terminology must allow for context representation. It should include formal, explicit information about how concepts are to be used.28 This means that there is a “grammar” that can be used to manipulate data and that there is also context-specific information about what is “sensible to say.” For example, it would be sensible to see the word rupture used in relation to appendicitis but not to the term eyebrow. The ability to represent context is essential to avoid the misinterpretation of health data.29
11. Graceful evolution
The structure and content of a terminology must change over time to reflect scientific advances, administrative changes, and other relevant factors. There must be a formal method for adding content to the terminology so that does not become obsolete as the field of medicine evolves, and all changes should be described and documented in detail.
12. Recognized redundancy
Redundancy is a characteristic of language. Redundancy occurs within a terminology when there is more than one term with the same meaning. This is desirable as long as the redundancy is recognized. Recognized redundancy means that terms with the same meaning are treated as synonyms and are represented by the same identifying code. Ideally, these redundant entries map to the same concept; for example, Myocardial Infarction and Heart Attack are synonyms and should be treated as though they represent the same concept.
On the basis of experience in implementing a clinical terminology for Kaiser Permanente, Campbell identified six additional desirable characteristics.30,31
1. Copyrighted and licensed
The terminology should be licensed, copyrighted, and maintained by a single organization to prevent local modifications that might make the data incomparable across disparate sites, systems, or organizations.
2. Commercial information system vendor-neutral
The design of the terminology should not give any advantage to a particular commercial information system (CIS) vendor. This will ensure that the terminology is available nationally (or internationally), prevent limits on its use for profit reasons, and protect the terminology from events that adversely affect a vendor. ICD-9, CPT, and SNOMED are all vendor-neutral.
3. Scientifically valid
The terminology should be understandable, reproducible, useful, and reflective of the current understanding of science.
4. Well-maintained
There must be a central authority responsible for keeping the terminology current and for providing rapid response to new terms. ICD is updated on a regular basis. The ICD-9-CM Coordination and Maintenance Committee reviews proposals to update ICD-9-CM; changes are effective October 1 each year.
5. Self-sustaining
The terminology should be supported by public or endowment funding or by license fees to cover development and support costs.
6. Scalable infrastructure and process control
Tools and processes used to maintain the terminology should be scalable to the number of users so that timely maintenance is not jeopardized.
Table 6-4 from “Interface Terminologies: Facilitating Direct Entry of Clinical Data into “Electronic Health Record Systems” demonstrates desired attributes for a controlled medical terminology.32
Table 6-4
SOME DESIRED ATTRIBUTES FOR A CONTROLLED MEDICAL TERMINOLOGY, AS REPRESENTED IN CIMINO,81 CHUTE ET AL.,81 AND THE INTERNATIONAL ORGANIZATION FOR STANDARDIZATION TECHNICAL SPECIFICATIONS FOR TERMINOLOGIES81
| Terminology Attribute | Cimino | Chute et al. | ISO |
| Statement of purpose, scope, and comprehensiveness | x | ||
| Complete coverage of domain-specific content | x | x | x |
| Use of concepts rather than terms, phrases, and words (concept orientation) | x | x | |
| Concepts do not change with time, view, or use (concept consistency*) | x | x | |
| Concepts must evolve with change in knowledge | x | x | x |
| Concepts identified through nonsense identifiers (context-free identifier) | x | x | x |
| Representation if concept context consistently from multiple hierarchies | x | x | x |
| Concepts have single explicit formal definitions | x | x | x |
| Support for multiple levels of concept detail | x | x | x |
| Methods, or absence of, to identify duplication, ambiguity, and synonymy | x | x | x |
| Synonyms uniquely identified and appropriately mapped to relevant concepts | x | x | x |
| Support for compositionality to create concepts at multiple levels of detail | x | x | x |
| Language independence | x | ||
| Integration with other terminologies | x | x | |
| Mapping to administrative terminologies | x | x |
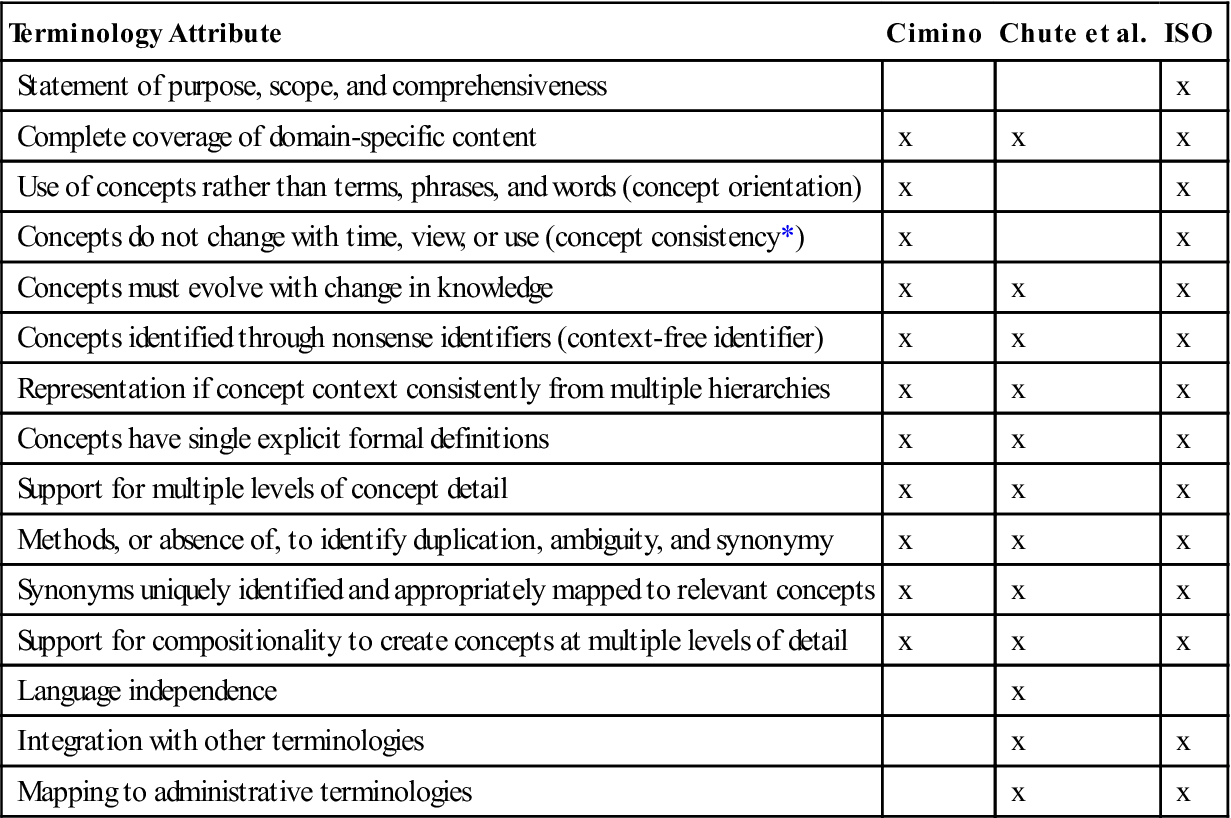
*Includes the concepts “multiple consistent views” and “concept permanence.”
Interface terminologies
Interface or application terminologies are designed to support efficient structured clinical documentation into EHR system interfaces and computerized note capture tools. They help users view categorical data by providing common colloquial terms as synonyms and enable natural language text generation. They are used to present preselected subsets or pick lists to end users to facilitate data entry and standardization when documenting in the electronic health record. The terms in the pick lists are generally selected from a reference terminology. Figure 6-8 Allergy or Adverse Reaction Template demonstrates the use of preselected subsets to facilitate data entry.10
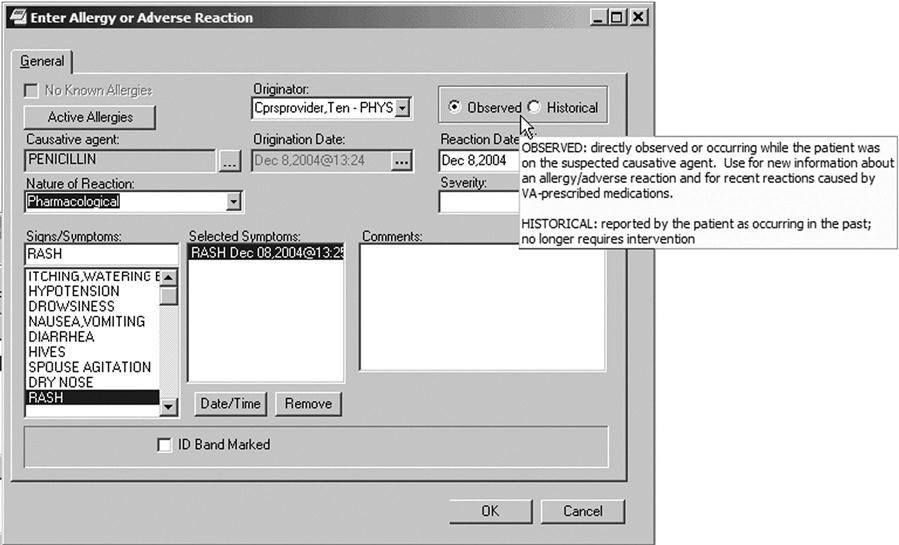
A clinical interface terminology is a systematic collection of health care–related phrases that supports clinician entry of patient-related information into computer programs. Interface terminologies also facilitate display of computer-stored patient information to clinician users as simple human-readable text. Thus, interface terminologies “provide the interface” between the clinician’s own conceptualizations of patient descriptors and the more structured, coded internal data elements used by specific clinical computer programs. Interface terminologies allow users to interact easily with concepts through common colloquial terms and synonyms.
An example of how interface terminologies can support provider entry of patient information into computer programs and display it can be found in the illustration of a reminder dialog. At the time of the patient encounter, the provider needs to store actions for follow-up. These follow-up reminders can be customized to the condition of the patient and the specialty of the physician, who then uses a check box to select the actions for follow-up that the patient needs. Each check can open more detail if the icon is selected. Future follow-up actions can be set to send reminder messages in the future, such as, to get a flu shot each fall or tetanus shot every 10 years. When the follow-up activity has taken place and the patient has received the services, the system facilitates entering a description of what has occurred (Figure 6-9).
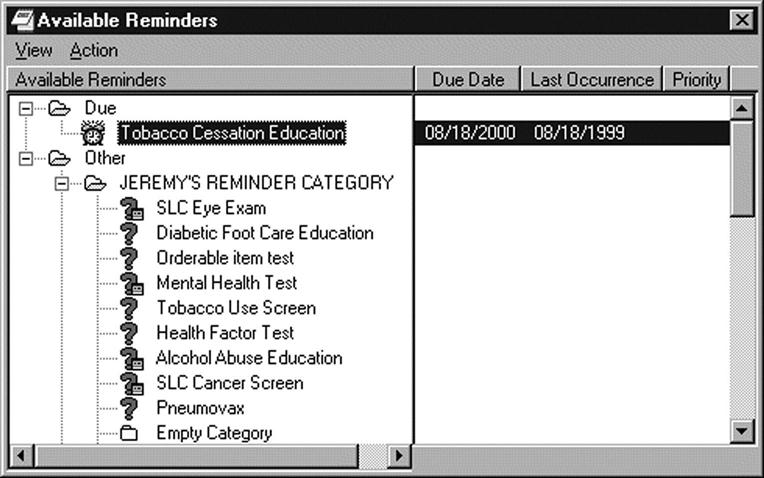
Electronic health record systems depend on interface terminologies for successful implementation in clinical settings because such terminologies provide the translation from the individual clinician’s own natural language expressions into the more structured representations required by application programs. This enables unlocking clinical data from narrative reports. Box 6-2 demonstrates the use of an interface to capture information from unstructured natural language to a structured representation.
Interface terminologies have three classes depending on the intended use or task:
1. Interface—supports a user-friendly structured data entry interface
2. Processing—optimizes natural language processing
3. Reference—enables storage, retrieval, and analysis of clinical data34
Clinical interface terminologies have been used for problem list entry, clinical documentation in EHRs, text generation, CPOE with decision support, and diagnostic expert programs.
Mapping
Data mapping is the process of creating data element mappings between semantic and representational terms residing in two distinct data models. Data mapping is used as a first step in data integration which involves combining terms residing in different sources and to provide users with a unified view of these data. Semantic mapping is analogous to an auto-connect feature that looks up a term and its synonyms. It is a way of graphically representing concepts by constructing a semantic map and identifying ideas and how they fit together. The aim is to explain precisely a subset of real information to improve communication with precision. For example, if the source system lists “LAST NAME” and the destination system lists “SIR NAME,” the mapping will recognize that these data elements are synonyms. There are limitations, however, in that mapping is only able to find exact matches. Metadata must be used to indicate that these two distinct terms are indeed synonyms.
In the future, tools based on semantic web languages will make data mapping a more automatic process for improved retrieval and navigation of health information by using search engine methodology to infer synonyms and semantic relationships.35

General equivalency mappings (GEMS)
The Centers for Medicare and Medicaid Services (CMS) and the Centers for Disease Control and Prevention (CDC) created the national version of the General Equivalence Mappings (GEMs) to ensure that consistency in national data is maintained. CMS and CDC will maintain the GEMs for at least 3 years beyond October 1, 2013, which is the compliance date for implementation of ICD-10-CM/PCS. The GEMs are a comprehensive translation dictionary that can be used to convert, accurately and effectively, any ICD-9-CM-based application or data to ICD-10-CM/PCS, including data for tracking quality, recording morbidity/mortality, and calculating reimbursement. The GEMs can be used to convert large applications with a high degree of complexity and still preserve the essential logic of the application.
The GEMs are a bidirectional tool that can be used to convert applications and data from ICD-9-CM to ICD-10-CM/PCS and vice versa. Mapping from ICD-10-CM and PCS codes back to ICD-9-CM codes is referred to as backward mapping. Backward mapping is used to map a newer code set to an older code set. Mapping from ICD-9-CM codes to ICD-10-CM and PCS codes is referred to as forward mapping. Forward mapping maps from an older code set to a newer code set.
Caution should be used in using GEMS, because they are not a simple one-to-one crosswalk. ICD-10 is much more specific than ICD-9 and contains many more codes (Box 6-3).