CHAPTER 19. Pain Management
Melanie H. Simpson, PhD, RN-BC, OCN®, CHPN∗
Pain Management, 372
Physiology of Pain, 373
Pain Management Basics, 375
Moderate Sedation/Analgesia (Conscious Sedation), 383
Summary, 387
PAIN MANAGEMENT
Pain is the most common reason patients seek health care and yet most health care professionals have very little current education in assessing, managing, and treating pain. According to the International Association for the Study of Pain (IASP), the definition of pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage described in terms of such damage.” Patient advocacy is at the forefront of pain management, whether it involves acute pain or chronic pain. Nurses in any setting are obligated to support the patient’s right to appropriate pain management (Brennan, Carr, and Cousins, 2007). To accomplish this, nurses must be armed with the knowledge of what is appropriate—of what constitutes undertreatment and overtreatment. When technologies are used, one must be able to assess their appropriateness and whether they fit into the patient’s lifestyle, frame of mind, and support system. The Joint Commission now requires that hospitals provide pain management across the continuum of care. This requirement demands that the pain management plan is outlined from admission to discharge and that patients are part of that planning process when possible. Nurses need to ensure that patients and their families are well educated regarding what is available for pain management intervention and what to expect from each intervention, including side effects and complications.
BARRIERS TO EFFECTIVE PAIN CONTROL
Many health care professionals have inadequate knowledge and skills regarding the pharmacology of pain medications, physiology of pain, assessment of pain, and pain management techniques. This lack of knowledge persists despite efforts to set standards of practice and to educate health care providers. The Agency for Health Care Policy and Research (now known as the Agency for Healthcare Research and Quality) developed and distributed pain management guidelines in the early and mid 1990s, and the American Pain Society (APS) continues to provide guidelines with the most recent publication in 2003 (APS, 2003). Yet pain control for both acute and chronic pain conditions continues to be inadequate.
A landmark study published in the Annals of Internal Medicine (Marks and Sacher, 1973) showed that pain is greatly undertreated. This study revealed that authorized prescribers underprescribe analgesic agents, nurses administer fewer analgesics than prescribed, patients request fewer analgesic medications than they need, and the as-needed regimen of administering opioid agents ensures that the patient will experience pain. More recent studies (Furstenberg et al., 1998 and Peretti-Watel et al., 2003) show very little has changed over the years in health care providers’ attitudes, knowledge, and behaviors in managing pain appropriately.
Studies have shown that we can educate health care professionals extensively and repeatedly about pain management; however, without institutional commitment for pain management implementation, it will not likely be done (Cromley and Banks, 2000 and Farber et al., 1998). A pain management champion is needed to start the process. Guidelines, policies, and standards must then be established to facilitate appropriate pain management. Continuous quality improvement must be in place as well to ensure continued assurance to these guidelines.
One of the most difficult barriers in pain management is the use of negative language. For example, the term “narcotic” is now pharmacologically obsolete, because it is more a historical term (Bond and Simpson, 2006), yet both patients and health care providers continue to use this word. The word narcotic carries a negative connotation; it is therefore difficult to educate patients on the benefits of the use of these types of medications in managing pain. The correct word or term is opioid. Opioids are natural, semisynthetic, and synthetic drugs that bind at opioid receptor sites and provide analgesia.
Another frequent misuse of a term is when the health care provider says or writes that a patient “complains of pain.” Patients are asked about their pain and then it is reported that the “patient is complaining of pain of eight out of ten,” instead of the “patient reports or states a pain level of eight out of ten.” Patients, for the most part, want to be “good” patients and not seen as complainers, so it is then difficult to get them to report the information needed for a thorough pain assessment if they feel they are perceived as complaining.
Attitudes and beliefs of health care professionals serve as additional barriers to effective pain control. Some professionals believe that pain is normal or expected so there is no sense of urgency when a patient reports severe pain. The concern for iatrogenic addiction (addiction inadvertently caused from valid medical use of opioids) from families and health care providers alike is completely out of proportion to the true incidence, which in psychologically intact patients is less than 1%. The daily media are filled with information about drug abuse and addiction that feeds the fears of health care providers and the public about potential drug addiction. These concerns can inhibit the use of opioids even when recent articles (Bloodworth, 2006, Guarino and Myers, 2007 and Kahan et al., 2006) show they can provide more effective pain control without a significant majority of the patients developing a problem.
Health care providers and lay people alike use the terms addiction, physical dependence, and tolerance interchangeably, yet they are all very different. This led to the publication of a consensus statement from the American Academy of Pain Management, the American Pain Society, and the American Society of Addiction Medicine (Savage et al, 2001) with the following definitions:
• Addiction is a primary, chronic, neurobiological disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
• Physical dependence is a state of adaptation that is manifested by a drug class–specific withdrawal syndrome following abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
• Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Health care providers are cautioned about using terms in patients’ charts or in conversations with patients and other health care providers that are negative or suggestive of misuse of pain medications as well. The use of words or phrases such as drug seeking, clock watcher, or addicted to their pain medications should be avoided. Very often the patient is in no way behaving inappropriately; it is simply that the treatment for the patient’s pain is not the right medication, dose, or dosing interval and needs to be addressed.
Laws and regulations were once designed to prohibit the use of opioids except in severely limited circumstances. Current regulations have tried to emphasize using opioids for treating pain in the appropriate patient population. Many organizations that address pain management issues, such as the American Pain Foundation, the American Pain Society, and the American Society of Pain Management Nursing, have been formed to organize efforts to overcome the lack of knowledge and unclear regulations. These organizations provide education for health care providers about pain management practices. Most have government relations’ committees that lobby legislators and regulatory authorities regarding current pain management issues.
DISCIPLINES INVOLVED
The discipline of pain management has transformed over the years. This is no longer a subspecialty of anesthesiology alone. Physicians from anesthesiology, physical medicine and rehabilitation, neurology, psychiatry, oncology, palliative care, internal medicine, and family medicine specialties have chosen to specialize in pain management as well. Some organizations also have nurses, pharmacists, psychologists, and ancillary professionals (e.g., physical and occupational therapists and social workers) who specialize in pain management. Teams of nurses responsible for pain management have been formed in some hospitals, hospices, and home health agencies. The nursing responsibilities of these teams range from actually administering the pain medications to educating nurses and other health care providers about proper dosing, delivery, and side effects. A pain team may also identify and solve problems associated with pain management.
A pain management medical director is usually a physician who specializes in pain management. The medical director serves as a resource to those involved in pain management and also acts as liaison and educator of other physicians. The medical director needs to understand and respect the nursing role in pain management, thereby enhancing the care that nurses give to patients in pain.
The rapidly developing discipline of pain management is in need of professional support and development. To provide this support and development with education and networking, societies and associations have been organized to advance the art of pain management for health care professionals. The American Society for Pain Management Nursing, founded in 1991, recognizes this need and responds by educating nurses and supporting nursing research in pain management. The society has established the role of the nurse in pain management, provides a professional publication, Pain Management Nursing, and offers national certification. As more information becomes available about pain pathways, new medications, and new routes of analgesia administration, nurses are invited to help implement advances in pain management. Continuing education regarding advances in pain management warrants high priority.
PHYSIOLOGY OF PAIN
Pain warns the individual that something is wrong, but once it serves that purpose, it should be relieved. Pain is harmful to the body if left untreated (Box 19-1). There are significant endocrine responses to pain—increased heart rate, vasoconstriction, and decreased gastrointestinal (GI) motility. Pain also causes muscle splinting, which can diminish pulmonary function and lead to atelectasis or pneumonia. These findings make it even more evident that health care professionals need to take pain control seriously to reduce morbidity and mortality, reduce the length of the hospital stay, and promote a more rapid general recovery (Bond and Simpson, 2006).
Box 19-1




SELECTED HARMFUL EFFECTS OF UNRELIEVED PAIN
| Area affected | Response to pain |
|---|---|
| Cardiovascular | Increased heart rate, increased cardiac output, increased peripheral vascular resistance, hypertension, deep vein thrombosis |
| Cognitive | Decreased cognitive function, mental confusion |
| Developmental | Increased behavioral and physiological responses to pain, irritability, higher somatization, addictive behavior, anxiety states |
| Endocrine | Increased adrenocorticotropic hormone, increased cortisol, increased antidiuretic hormone, increased epinephrine, increased norepinephrine, decreased insulin, decreased testosterone |
| Future pain | Debilitating chronic pain syndromes, phantom pain, postherpetic neuralgia, postmastectomy pain, post-thoracotomy pain |
| Gastrointestinal | Decreased gastric and bowel motility |
| Genitourinary | Decreased urinary output, urinary retention, fluid overload, hypokalemia |
| Immune | Decreased immune response |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycemia, glucose intolerance, insulin resistance, muscle protein catabolism |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, increased thoughts of suicide |
| Respiratory | Decreased flows and volumes, atelectasis, shunting, hypoxemia, decreased cough, sputum retention, infection |
Not all of these are body systems (future pain, developmental, quality of life).
Adapted from McCaffery M, Pasero C: Pain: clinical manual, ed 2, St Louis, 1999, Mosby.
PAIN NOCICEPTION
Matching clinical indicators with the appropriate pain intervention requires at least basic knowledge of the biochemical response to pain, the pain pathway, and opioid receptor sites. Melzak and Wall (1965) proposed the gate control theory of pain modulation. Current theory supports four processes involved to describe how pain becomes conscious or the nociception of pain. Those four basic processes in the nociception of pain are: (1) transduction, (2) transmission, (3) perception, and (4) modulation (McCaffery and Pasero, 1999) (Box 19-2).
Box 19-2
PROCESSES INVOLVED IN PAIN NOCICEPTION
| 1 | Transduction | Noxious stimulus causes cell damage → release of sensitizing substances → action potential begins pain signal |
| 2 | Transmission | Signal moves through afferent or peripheral nerve fibers to spinal cord → up spinothalamic tract to thalamus and cortex |
| 3 | Perception | Cortex interprets signal and pain becomes conscious |
| 4 | Modulation | Neurons from brain stem send signals down descending pathway → release of endogenous opioids, serotonin, and norepinephrine to inhibit or modulate pain signal |
The first process, transduction, begins in the periphery when the skin is cut or damaged. This trauma or pain stimulus causes a number of sensitizing substances to be released at the site of injury, including prostaglandins, bradykinin, serotonin, substance P, and histamine. In order for this pain stimulus to be changed to an impulse, an action potential must be generated. Changes in the neuronal membrane and the processing or abnormal processing of the sensitizing substances establish if the pain will be perceived as nociceptive or neuropathic (see Categories of Pain).
Transmission is the next process, which allows the impulse to be carried through the afferent or peripheral nerve fibers into the spinal cord. The pain impulse continues to be carried through the dorsal horn and ascends the spinothalamic tract to the brain—in particular, the thalamus. The pathway continues as the impulse travels to the cerebral cortex, which interprets the signal as pain at the site of the cut.
The third process is called the perception of pain, during which the cortex interprets the signal of pain and the pain becomes conscious.
The fourth process is modulation; during this process neuronal impulses from the brain stem move down the descending pathway and release substances that inhibit the pain message, such as endogenous opioids, serotonin, and norepinephrine. This is a very basic explanation of how pain occurs. It is important to understand the sequence of these four distinct processes involved to help understand how specific treatments interact within a process.
Many other factors influence the pain response. These factors include past experiences of pain; anxiety and anticipatory pain; emotional, physical, or sexual abuse; history of chemical dependency; and the patient’s support structures. This is why a comprehensive assessment is so important and experts agree that the patient’s self-report is the gold standard for measuring pain.
PAIN NERVE FIBERS
Specialized nerve endings in the skin and viscera send messages of noxious stimuli, such as mechanical, chemical, or thermal, to the brain. These specialized nerve endings, or receptors, send impulses along specific fiber types, all of which are peripheral nerves. Most fibers that transmit acute nociceptive pain information are identified as A-delta and C fibers (Sorkin, 2005). These fibers differ in their rate of impulse conduction and their diameter. The myelinated A-delta fibers are the largest and most rapid conductors, and they transmit well-localized, sharp pain (McCaffery and Pasero, 1999). Unmyelinated C fibers have the smallest diameter and are the slowest conductors, transmitting poorly localized, dull, and aching pain. A-delta fibers tend to conduct intense pain but are more receptive to local anesthetics and nonsteroidal anti-inflammatory drugs (NSAIDs). C fibers tend to conduct dull pain and are most responsive to opioids by any route.
Dermatomes constitute the segmental distribution of the spinal nerve sensations and are labeled according to their exit point on the spinal cord. Dermatome charts (Figure 19-1) are useful for tracking the nerves innervating the area of pain. With a nerve block or the intraspinal route of analgesia, medications can be delivered directly to those nerves that are the origin of an individual’s pain (Bonica, 1990).
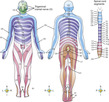 |
| FIGURE 19-1 Dermatome chart. (From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.) |
By understanding the dermatomal distribution of pain when working with epidural infusions of opioids and local anesthetics, the nurse can collaborate with the anesthesiologist to determine the appropriate dermatomal distribution of opioid and local anesthetic to the painful area. For example, when fentanyl, a lipid-soluble opioid, is used, the rate of infusion may need to be increased to widen the spread of analgesia (see Figure 19-1). The intraspinal route for medication delivery is addressed in Chapter 27.
PAIN MANAGEMENT BASICS
People are now more aware of pain and the significant problems associated with it than in any time in history. Therefore it is important that nurses and other health care professionals have up-to-date knowledge of pain including pathophysiology, pain assessment, and treatment strategies. Years ago, the International Association for the Study of Pain established three different categories of pain: acute, chronic, and cancer (Bonica, 1990). Current theory supports that the patient’s perception of pain is not different simply because the patient has a diagnosis of cancer, and therefore both cancer pain and noncancer pain should be treated equally.
CATEGORIES OF PAIN
Acute pain
Acute pain is caused by such occurrences as traumatic injury, a surgical procedure, or a medical disorder. With acute pain, the patient may show a clinical picture of tachycardia, hypertension, tachypnea, shallow respirations, agitation or restlessness, facial grimacing, or splinting. The incidence of acute pain in hospitalized patients is astounding. The challenges of acute pain become greater when individuals also suffer from chronic pain and have other co-morbidities, a history of substance abuse, or any communication barriers.
Chronic pain
Chronic pain is persistent, often lasting more than 6 months. However, some practitioners believe that pain that exists for a shorter duration than a 6-month period may qualify as chronic pain. An individual who has chronic pain may show the same clinical picture as the person suffering from acute pain, or the individual may not appear to be in pain at all. It is simply from the patient’s self-report that we know the severity of the patient’s pain. It is estimated that about 26% of Americans, or 76.5 million Americans, report that they have had a persistent pain problem with a duration of more than 24 hours (of note, this does not include acute pain) (National Center for Health Statistics [NCHS], 2006).
Nociceptive pain
Nociceptive pain is the result of complex interactions between the peripheral nerves and the central nervous system as mentioned earlier. Nociceptive pain is divided into two categories as well: somatic and visceral. Somatic pain is soft tissue or musculoskeletal pain. It is usually well localized and described as achy, throbbing, or tender. Visceral pain is often caused by the abnormal stretching or distention of the smooth muscle wall of the visceral muscles or mucosa (Silver and Mayer, 2007). Visceral pain is not well localized and is described as tight, pressure, cramping, stretching or distention. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are the treatment of choice for these types of pain.
Neuropathic pain
Neuropathic pain is a result of disrupted or injured nerves of the peripheral or central components of the nervous system (Strassman, 2003). This type of pain is often described as numbness, burning, tingling, radiating, shooting, or electric-like. A clue that the patient is describing neuropathic pain is when the pain moves (e.g., wraps around the waist, goes down the back of the leg). Opioids alone are usually not very helpful for neuropathic pain. Antidepressants and anticonvulsants are the treatment of choice.
ASSESSMENT OF PAIN
A comprehensive pain assessment is the key to effectively managing pain. Pain assessment requires an extensive knowledge of pain, its causes, and its management. Taking the time to ask the important questions gives the nurse the information needed to formulate an accurate treatment plan. Yet sometimes in clinical practice we know there are exceptions. A brief assessment of pain is performed when the patient is in absolute distress and delaying pain management would have harmful effects. A brief assessment includes the patient’s description and rating of the pain, its timing, and location; any associated symptoms; what lessens the pain or makes it worse; and what medications are being used. The brief assessment is beneficial for postoperative pain, trauma pain, and acute medical disorders. The goal of a brief assessment is to determine what pathways the pain is taking and to intervene with medications that affect those pathways directly.
A comprehensive assessment of pain is used to formulate a long-term plan for pain management. There are many components to a comprehensive pain assessment and many ways to document them (Figure 19-2). Regina Fink (2000) developed one such acronym, WILDA, as a way for health care providers to remember the primary components. WILDA stands for words, intensity, location, duration, and aggravating and alleviating factors.
 |
| FIGURE 19-2 Pain assessment form. |
The words the patient uses to describe the pain give the nurse information about what type of pain the patient may be experiencing. For example, when the patient uses words like numbness, tingling, or burning, the nurse can document that the patient has described a neuropathic pain syndrome. This should also give the nurse an idea of the appropriate intervention for that type of pain (i.e., the use of an antidepressant or anticonvulsant) (Backonja, 2002).
Knowing the intensity or pain rating is very important in selecting the treatment. In 1990 the World Health Organization (WHO) introduced a three-step ladder to use as a framework for the use of analgesics in cancer pain, and those in the field of pain are currently discussing the necessity of revising this concept (Figure 19-3) (IASP, 2005). While a very good guideline, some of the terms are outdated. The original intent for this ladder was to assist the health care provider in choosing which class of analgesic or adjuvant to use in relation to the intensity of the patient’s pain.
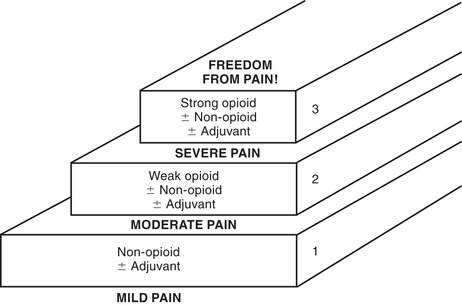 |
| FIGURE 19-3 Analgesic ladder. (1) Assess pain severity. (2) Begin treatment at proper step of ladder. (3) Move up ladder until pain controlled. (Adapted from World Health Organization: Cancer pain relief, Geneva, 1990, Author.) |
The WHO analgesic ladder uses a 0 to 10 scale for pain intensity. On that scale, 0 represents no pain, a pain intensity of 1 to 3 represents mild pain, 4 to 6 is moderate pain, and 7 to 10 is severe pain. Each level has suggestions for what class of analgesic or adjuvant medication to use for that patient’s level or intensity of pain.
For those patients who are unable to rate their pain with a number, there are many other scales that can be used. For example, with the verbal descriptor scale, patients are asked to describe their pain as mild, moderate, severe, very severe, or worst pain possible. With the faces scale, patients are asked to choose the facial expression that best represents how their pain makes them feel.
Assessing the location of pain is important because very often patients have more than one site of pain. It is essential that each location be fully assessed to determine if the patient has more than one type of pain. The duration of pain is assessed by asking, “Is the pain present more than 50% of the time or does it come and go?” If a patient has constant pain then the use of a long-acting pain medication or a basal or continuous rate of opioid infusion may be warranted. If the patient reports only episodic or transient pain, then the use of “as needed” medications is appropriate.
Aggravating and alleviating factors can be assessed by asking the patient, “What makes the pain better, and what makes the pain worse?” This information can help to determine the etiology of the pain. Most importantly, it can help decide how effective the treatments have been. In addition, other factors that need to be assessed include pain history, medical history, psychosocial issues, and any other accompanying symptoms, such as sleep, appetite, activity, concentration, mood, and relationships. Asking these questions will give the health care provider an indication of how disruptive the pain is for the patient’s quality of life, such as level of activity and the ability to sleep and interact with others. When the variables of the pain have been determined, then an appropriate treatment plan can be implemented.
NON-OPIOID, ADJUVANT, OR CO-ANALGESIC AGENTS
Non-opioid, adjuvant, or co-analgesic medications can be useful in pain control, either alone or in combination with opioids. They may have independent analgesic activity, enhance the effects of opioids, or counteract the side effects of opioids (APS, 2003). They are recommended on each level of the WHO analgesic ladder. These drugs include NSAIDs (e.g., ibuprofen, naproxen) and COX-2-selective NSAIDs (e.g., celecoxib), tricyclic antidepressants (e.g., amitriptyline, nortriptyline), anticonvulsants (e.g., gabapentin, pregabalin), alpha 2-adrenergic agonists (e.g., clonidine), and many others. For the purposes of this chapter, just a few are listed as examples. These medications need to be considered when evaluating patients with acute and chronic pain.
NSAIDs and COX-2s
NSAIDs and COX-2s target two types of cyclooxygenase (COX) enzymes in the process of relieving pain. Cyclooxygenase type 1 (COX-1) produces prostaglandins that are beneficial to renal and gastric function and are responsible for platelet aggregation. Cyclooxygenase type 2 (COX-2) produces prostaglandins related to the inflammatory process. NSAIDs that are nonselective inhibit both COX-1 and COX-2, which means patients taking these NSAIDs can be at risk for gastrointestinal effects or increased bleeding. COX-2-selective agents selectively inhibit COX-2. When this happens, inflammation is reduced. COX-2-selective agents provide the benefit of analgesia by blocking COX-2, without affecting COX-1, which benefits the kidneys and stomach lining.
NSAIDs and COX-2s, which can be given orally, intravenously, or rectally, inhibit the synthesis of prostaglandin by inhibiting cyclooxygenase. Because of this prostaglandin-inhibiting activity, studies have shown that adjuvant use of NSAIDs (Ballantyne et al., 2007 and McCaffery and Pasero, 1999) and COX-2s (Recart et al., 2003 and Rosenquist and Rosenberg, 2003) (Celebrex is currently the only one available) is opioid-sparing in controlling pain postoperatively. Careful consideration should be given to the patient before administering NSAIDs or COX-2s, especially in the elderly. The patient’s medical history and physical condition should be evaluated, and current medications need to be identified. For instance, if a patient has a history of renal disease or has an elevated creatinine level, the use of NSAIDs and COX-2s may be contraindicated. If the patient has a coagulopathy or is taking anticoagulants, the use of NSAIDs needs to be evaluated carefully. Because certain NSAIDs can be irritating to the stomach, patients with a history of peptic ulcer disease need to be evaluated carefully for this method of pain management because NSAIDs inhibit the production of the very prostaglandins that protect the stomach lining from gastric acids. As a result, the patient may need to receive gastrointestinal (GI) prophylaxis.
Tricyclic antidepressants
Tricyclic antidepressants are important medications for deafferentation of neuropathic pain (Iosifescu et al, 2003) caused by surgical trauma, radiation therapy, chemotherapy, or malignant nerve infiltration and have been used for many years. They are beneficial in pain control because they contribute to an increase in serotonin level in the descending pain pathway, resulting in a release of enkephalins (see Opioids section) in the spinal cord and a decrease in pain. Because of this function, tricyclic antidepressants are physiologically responsible for terminating nerve-transmitting activity. Side effects include hypotension, sedation, constipation, and dry mouth. A contraindication for tricyclic antidepressant therapy is coronary artery disease in patients with ventricular arrhythmias.
Anticonvulsants
Anticonvulsants are used to relieve lancinating neuropathic pain arising from peripheral nerves. They can be used to treat conditions such as trigeminal neuralgia and postherpetic neuralgia. Gabapentin has been a popular anticonvulsant over the years for the treatment of neuropathic pain (Wiffen et al, 2005) because it has a low side effect profile, has no metabolite, and rarely interferes with other medications. Recent studies (Montazeri et al., 2007 and Turan et al., 2006) have shown that the use of gabapentin preemptively and throughout the perioperative setting decreases the use of opioids postoperatively and improves patients’ satisfaction with their pain management dramatically. However, the gabapentin dose must be reduced in patients with renal insufficiency. Currently there are many other anticonvulsants as well now being used with good pain response (Backonja, 2002).
Alpha 2-adrenergic agonists
Clonidine has been approved by the U.S. Food and Drug Administration for the treatment of pain. Clonidine is effective against tactile allodynia or neuropathic pain (Wallace, 2006). It is available for epidural administration in a 100 mg/mL concentration. Hypotension, bradycardia, and sedation are possible side effects, but these are uncommon at low dosages.
OPIOIDS
Endogenous opioids
The body has its own protection against pain—endogenous opioids. These substances keep us pain-free through normal daily living. Endogenous opioids include endorphins and enkephalins. Endorphins, which are located in the brain, are mimicked by the systemic administration of opioids. Enkephalins, which are located in the spinal cord, are mimicked by the intraspinal administration of opioids. Activities that promote the release of endogenous opiates are physical exercise, deep relaxation, sexual activity, crying, and laughter. Situations that decrease the release of endogenous opioids are stress, chronic pain, chemical dependency, and depression.
Opioid receptors
Understanding opioid receptors helps us understand how opioids work to break the painful impulses. Opioid receptors are parts of cells that link with particular opioids to create analgesia and various side effects. There are three opioid receptors found within the dorsal horn of the spinal cord: mu, kappa, and delta. Of these, mu and kappa receptors are targeted by analgesics. Mu receptors are the most dense (Davis, Glare, and Hardy, 2005) in the spinal cord but are also present in the gut.
The most effective opioid receptor for producing superior analgesia is the mu receptor. Opioids that bind only at mu receptor sites are considered pure mu opioid agonists. Common examples of these include codeine, fentanyl, hydrocodone, hydromorphone, morphine, oxycodone, and oxymorphone. It has been discovered that there are subsets of the mu receptor: mu-1 and mu-2. The mu-1 receptor is responsible for analgesic effects and mu-2 for side effects. Efforts are now being made to find opioids that can combine only with mu-1. The kappa and delta receptors are much weaker than mu and may be less likely to produce physical dependency.
Agonist-antagonist
An agonist-antagonist can also reverse a mu receptor opioid (Goodman, Le Bourdonnec, and Dolle, 2007). Agonist-antagonist opioids combine at the kappa receptor site, thus producing lower quality analgesia than would a mu agonist. A commonly used agonist-antagonist that combines with the kappa opiate receptor site is nalbuphine (Nubain). Working with agonist-antagonist medications requires understanding when this kappa agonist is administered in relation to a mu agonist. When given alone, nalbuphine produces mild analgesia. Nalbuphine is given while a mu agonist is in the patient’s system; however, it acts to reverse the mu agonist’s analgesia and side effects. For example, a chronic back pain patient who is accustomed to taking sustained-release oral morphine (240 mg/day) is seen in the emergency department of the hospital for sudden severe onset of headache pain. An order is written to administer 10 mg of nalbuphine intravenously now for pain to supplement the oral morphine. The nalbuphine may be ordered because it has fewer side effects, but in this situation it will reverse the effects of the oral morphine, thereby producing severe pain and, most likely, symptoms of withdrawal.
Antagonist
The advantage of administering opioids for pain management is that their effects can always be reversed. Early intervention allows opioid side effects to be reversed before the situation becomes an emergency. Two types of medication can reverse a mu receptor opioid. A commonly used pure antagonist is naloxone (Narcan). This drug competitively inhibits opioids at the opioid receptor sites and thus reverses their side effects. However, naloxone (Narcan) should be given with great caution (see Box 19-3 for naloxone administration).
Box 19-3
GUIDELINES FOR USE OF NALOXONE (NARCAN) FOR RESPIRATORY DEPRESSION
1. Dilute naloxone 0.4 mg in 9 mL of 0.9% sodium chloride.
2. Give 0.5 mL slow IV push every 2 minutes until patient awakens.
Note: Respiratory depression is rare in patients who have been receiving chronic opioid therapy, but is a significant risk in opioid-naïve patients requiring high doses for acute pain. If naloxone must be used, careful titration is required to avoid the production of acute withdrawal, seizures, and severe pain. The duration of action of naloxone is shorter than that of most opioids, so repeated dosing may be necessary.
PARENTERAL OPIOIDS
Parenteral opioid administration is considered IV (intravenous), IM (intramuscular), and Sub-Q (subcutaneous) (see Chapter 26 for subcutaneous infusion). Parenteral opioids are available in a variety of forms including continuous infusions, intermittent doses, combinations of these, and patient-controlled analgesia. Opioids may be delivered through central or peripheral venous access. Selection of opioid depends on the type of pain reported by the patient and the availability of the nursing staff for administering it appropriately.
Continuous infusion
Continuous opioid infusion provides analgesia at a steady state. For example, if the patient reports constant pain, a continuous infusion of opioids may be indicated. Before the continuous infusion is initiated, it is best to administer small doses of the opioid around the clock for 24 hours or until the pain is controlled. A problem with this system is that accumulation may occur, causing the patient to feel oversedated and possibly develop respiratory depression. To ascertain the appropriate hourly dose, consideration needs to be given to the patient’s age, size, disease process, concurrent diseases, and opioid tolerance. Continuous infusions of opioids are appropriately used in trauma, postsurgical, and terminal care settings. The routes of continuous infusion include the intravenous, subcutaneous, and intraspinal (epidural or intrathecal) routes.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
