CHAPTER 18. Antineoplastic Therapy
Lisa Schulmeister, MN, RN, APRN-BC, OCN®, FAAN∗
Cell Cycle, 351
Chemotherapy Classifications and Nomenclature, 352
Chemotherapeutic Agents, 353
Chemotherapy Dosing, 360
Chemotherapy Administration, 361
Symptom Management, 361
Safe Handling, 367
Future Directions in Oncology, 369
Summary, 369
Cancer is a chronic condition that consists of a large group of diseases characterized by abnormal cells. An estimated 1,444,920 new cases of cancer were diagnosed in the United States in 2007 and 559,650 deaths were caused by cancer. Cancer is the second most common cause of death in the United States, exceeded only by heart disease. Because of advances in cancer treatment, more than 10.5 million Americans with a history of cancer are alive and considered cancer survivors (American Cancer Society, 2007).
The term antineoplastic therapy refers to treatment intended to prevent, halt, or eradicate cancer. Antineoplastic therapies include surgery, radiotherapy, hyperthermia, cancer vaccines, gene therapy, immunotherapy, biotherapy, molecularly targeted therapies, hormonal therapy, and chemotherapy (Polovich, White, and Kelleher, 2005). Because chemotherapy is often administered as an infusion therapy, it is the focus of this chapter.
Chemotherapy may be given as the primary cancer treatment or administered adjuvantly, in conjunction with other treatments such as radiotherapy. Neoadjuvant chemotherapy is administered before another cancer treatment; for example, chemotherapy is sometimes given before surgery to reduce the size of a large cancer so it may be more easily removed. Induction chemotherapy, initial treatment with high doses of chemotherapy, is used to treat aggressive types of cancer, such as acute leukemia, with the goal of inducing remission of the disease. Postremission chemotherapy may consist of standard dose consolidation chemotherapy, intensification chemotherapy, or ablative chemotherapy (high-dose chemotherapy followed by stem cell transplantation). The goal of postremission chemotherapy is to prevent disease recurrence (Polovich et al, 2005).
For some types of cancer, chemotherapy is curative. In the 1950s only 10% of children responded to cancer treatment. Today, nearly 80% are cured (Singh, 2006). Similarly, the overall survival for all stages of testicular cancer, including metastatic disease, is 80%, and 99% of patients with early-stage testicular cancer are cured (Kopp et al, 2006). Chemotherapy also may be administered with the goal of controlling disease progression and extending survival. In some cases, such as patients with large tumors obstructing organs or causing pain, chemotherapy is given for palliation.
Although chemotherapy has historically been considered a cancer treatment, chemotherapy also has efficacy in treating noncancerous conditions, such as rheumatoid arthritis (Donahue et al, 2008), and has become an accepted alternative to surgical options for treatment of ectopic tubal pregnancy (Lipscomb, 2007).
Chemotherapy may be administered by the oral, subcutaneous, intravenous (IV), intraarterial, intrathecal, intraperitoneal, intrapleural, intravesicular, and intralesional routes. Some treatment protocols include chemotherapy administered via multiple routes; for instance, two chemotherapy agents are administered intravenously while a third is given intrathecally. Whenever the route of chemotherapy administration is unclear or not specified, clarification of the order should be sought in order to prevent a medication error (wrong route) from occurring (Polovich et al, 2005).
CELL CYCLE
Normal cells and cancer cells go through the same division cycle (or cell cycle) (Figure 18-1). The DNA in a parent cell replicates and divides, and two “daughter” cells are produced. The initial phase of the cell cycle is the resting phase, designated as G 0. A cell in this phase performs its designated function during this stage of the cell cycle; for example, renal cells filter the blood and gastric cells digest food. Enzymes needed for DNA synthesis are produced in this phase. Although G 0 is commonly referred to as the resting phase of the cell, it is sometimes called the postmitotic or presynthetic phase (Park and Lee, 2003 and Vermeulen et al., 2003).
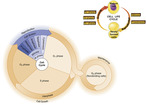 |
| FIGURE 18-1 Cell cycle. (From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.) |
When a cell is ready to divide, it enters into the next phase, called gap 1 and designated as G 1. Synthesis of the proteins for RNA occurs during the G 1 phase. Enzymes necessary for synthesis of DNA are activated in the S or synthesis phase. The length of time that the cancer cell is in this phase usually differs from that of normal cells. The second gap phase is designated as G 2. At this time, DNA synthesis stops and RNA synthesis and protein synthesis continue while the cell prepares for mitosis (Park and Lee, 2003 and Vermeulen et al., 2003).
Mitosis occurs during the final stage of the cell cycle, the M phase, and usually lasts 30 to 90 minutes. This phase is subdivided into four steps. In prophase the nuclear membrane is broken down and the chromosomes clump. In metaphase the chromosomes line up in the middle of the cell. During anaphase the chromosomes segregate into centrioles. In telephase, the final step, there is chromosome replication and cell division, which produces two daughter cells. These cells then go into the resting phase, G 0 (Park and Lee, 2003 and Vermeulen et al., 2003).
Although a few chemotherapy agents are given alone as single agents, most are administered in combination with other chemotherapy agents. Combining agents that act in different phases of the cell cycle increases the number of cells exposed to their cytotoxic effects and enhances cytotoxicity.
CHEMOTHERAPY CLASSIFICATIONS AND NOMENCLATURE
Chemotherapy agents can be classified by their effect on the cell cycle, pharmacological action or chemical structure, potential to cause tissue necrosis if extravasated, or emetogenic potential.
Cell cycle–specific chemotherapy kills cells that are in a specific phase and actively dividing. For example, a type of chemotherapy derived from vinca rosea plants and classified as plant alkaloids is cell cycle specific in the M phase. These chemotherapy agents kill only those cells in the M phase of the cell cycle. Cell cycle–nonspecific chemotherapy kills cells in any phase of the cell cycle, including G 0, the resting phase. An example of this type of chemotherapy is the anthracyclines, which are classified by their action as antitumor antibiotics. Vesicant chemotherapy has the potential to cause tissue necrosis when extravasated while nonvesicant chemotherapy does not alter tissue integrity when it infiltrates (Polovich et al, 2005). Chemotherapy agents have varying risks of causing nausea and vomiting, from minimal risk (<10% without antiemetics) to high risk (>90% without antiemetics), and may be classified by their emetogenic potential (Feeney et al, 2007). Each of these chemotherapy classifications is discussed in more detail in the following sections.
Chemotherapy treatment protocols often are assigned an acronym or protocol name and/or number. For instance, the breast cancer treatment acronym “CMF” refers to the multidrug combination of cyclophosphamide, methotrexate, and fluorouracil. Southwest Oncology Group (a national clinical research group) protocol SWOG-50337 is a clinical trial evaluating gemcitabine treatment for patients with recurrent bladder cancer. Most cancer clinical trials are funded and coordinated by the National Cancer Institute (NCI), and its website contains a database of trials that are open to patient enrollment (NCI, 2008). Some of the trials involve chemotherapy agents that are commercially available but are being studied in a new way while others are trials of investigational agents to determine their efficacy and toxicity.
CHEMOTHERAPEUTIC AGENTS
Chemotherapy agents have a very narrow therapeutic window; under- and overdosing of these agents can negatively impact the patient’s treatment outcome. Similarly, other errors in administration—such as wrong drug, wrong dose, wrong time, wrong route, or wrong patient—may cause patient harm. Chemotherapy orders must be reviewed and verified before administration by a trained clinician in chemotherapy administration to ensure that the correct chemotherapy drug and correct dose are administered to the right patient at the right time. Nurses and other health care providers should always compare a patient’s chemotherapy orders with the patient’s prescribed plan of care, treatment protocol, and other resources to ensure that the orders do not inadvertently contain errors or omissions (Schulmeister, 2006). Because chemotherapy doses are individualized and vary with the disease being treated, usual dosages are not stated in this chapter and the reader is referred to the manufacturer’s package insert for full prescribing information.
ANTIMETABOLITES
Antimetabolites are cell cycle–specific agents that act in the S phase and interfere with DNA and RNA synthesis by acting as “false” metabolites, which block the production of essential enzymes and prevent cell division. Their major toxicities are hematopoietic and gastrointestinal. Other common side effects are elevated liver function tests, photosensitivity, and alopecia (Wilkes and Barton-Burke, 2008).
Gemcitabine HCl
Gemcitabine (Gemzar), an antimetabolite, inhibits DNA synthesis and is cell cycle specific in the S phase. It is indicated for first-line treatment of pancreatic cancer (in combination with paclitaxel), unresectable non–small cell lung cancers (in combination with cisplatin), and women with metastatic breast cancer (in combination with paclitaxel) who have not responded to prior anthracycline chemotherapy. Gemcitabine (in combination with carboplatin) also is indicated for the treatment of patients with advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy. Myelosuppression is the dose-limiting toxicity, with a nadir of 7 to 14 days. Gemcitabine has a low (10% to 30% risk) emetic risk. Diarrhea, stomatitis, and constipation also occur. Other common side effects are fever, flulike symptoms, and mild to moderate rashes involving the trunk and extremities. Alopecia is minimal, involving about 15% of patients, and is reversible. Flulike symptoms occur in 20% of patients with the first treatment dose, and transient febrile episodes occur in 41% of patients (Thigpen, 2006 and Lilly 2007).
Methotrexate
Methotrexate (amethopterin, Mexate, Folex) is a folic acid antagonist that interferes with the synthesis of purine and thymidylate. It is cell cycle specific in the S phase, and it is used as a single agent or in combination with other drugs. Methotrexate is active against acute lymphocytic leukemia, lymphomas, sarcomas, mycosis fungoides, gestational trophoblastic carcinomas, and cancers of the breast, lung, head, and neck. Methotrexate can crystallize and precipitate in acidic solution and can cause renal damage in the presence of urine with a low pH. When high doses of methotrexate are given, the patient’s urine should be alkalinized both before and after methotrexate administration. Sodium bicarbonate is given orally or intravenously to neutralize the urine and keep the urinary pH greater than 7. Following high doses, leucovorin (folinic acid) is usually given as a rescue agent within 24 to 36 hours. The blood urea nitrogen (BUN), creatinine, and serum methotrexate levels determine the dosage and frequency of leucovorin. The purpose of the leucovorin is to reduce bone marrow toxicity. Methotrexate has a low (10% to 30%) emetic risk. Antiemetics should be given beforehand to control the dose-related nausea associated with this drug. Hydrating fluid is usually given before and after methotrexate dosing. The patient needs to be encouraged to increase oral fluids, and the fluid balance should be monitored. Stomatitis and esophagitis, which can impair eating and swallowing, can range from mild irritation and tenderness with sensitivity to spicy or acidic foods to full-blown, painful sores. With prolonged use of methotrexate, an elevation in liver function tests, hepatic fibrosis, and occasionally cirrhosis can occur. Pneumonitis may develop and is not always reversible. Methotrexate can cause photosensitivity, with the patient’s skin having an increased tendency to burn if exposed to bright, intense sunlight. It is recommended that the patient cover the exposed skin or use a sunscreen with a sun protection factor (SPF) of 15 or more. Radiation recall has been seen with this drug. Methotrexate interacts with many drugs, including 5-fluorouracil, asparaginase, and vincristine. The patient should be instructed to avoid the use of aspirin, nonsteroidal anti-inflammatory drugs, folic acid preparations, sulfa vaccines, and alcohol during treatment with methotrexate (Bedford 2005a and Wilkes and Barton-Burke, 2008).
5-Fluorouracil
Another antimetabolite, 5-fluorouracil (fluorouracil, Adrucil, 5-FU), inhibits the synthesis of DNA and RNA and is cell cycle specific for the S phase. It is indicated in the treatment of gastrointestinal (GI) malignancies, especially colorectal, pancreas, head, neck, breast, prostate, skin (administered topically), and liver (administered intraarterially) cancers. The dose-limiting toxicities are stomatitis and diarrhea. Toxicity may be delayed 1 to 3 weeks. 5-Fluorouracil has a low (10% to 30%) emetic risk. Rarely, GI ulceration can lead to hemorrhage. Myelosuppression is usually moderate, with a nadir of 7 to 14 days. Other side effects are anorexia, fatigue, alopecia, and photosensitivity. 5-Fluorouracil is an irritant, and a central venous access device is recommended for continuous infusions. The patient should be instructed to avoid exposure to direct sunlight, to wear long sleeves and pants outdoors, and to use a sunscreen with an SPF of 15 or higher. Hyperpigmentation of the nail beds and peripheral veins is common. Hand-foot syndrome may occur, involving painful, erythematous desquamation of palms and soles (Wilkes and Barton-Burke, 2008).
Floxuridine
Floxuridine (FUDR) interferes with cell replication and inhibits DNA synthesis. It is cell cycle specific for the S phase and is indicated in the treatment of liver, biliary, pancreatic, oral cavity, and breast cancers. Floxuridine is administered intraarterially into the hepatic artery, usually via continuous infusion for 14 to 21 days. When given intravenously, floxuridine is transformed to 5-fluorouracil; IV administration is being investigated. Dose-limiting toxicities are stomatitis and diarrhea. Myelosuppression is mild. Alopecia and dermatitis are common. Plantar-palmar syndrome may occur and is characterized by painful swelling and peeling of the hands and feet. Hyperpigmentation of the veins occurs with peripheral infusions (Bedford Labs, 2000).
Fludarabine
Fludarabine (Fludara, fludarabine phosphate) interferes with and inhibits DNA function. It is used to treat mycosis fungoides, low-grade lymphomas, and chronic B-cell lymphocytic leukemia that have not responded to standard treatment. Fludarabine has a minimal (<10%) emetic risk. Common side effects are anorexia, diarrhea, fever, chills, rash, myalgia, fatigue, weakness, GI bleeding, visual disturbances, and urinary tract infections. Cardiac problems such as arrhythmias, congestive heart failure, and myocardial infarction have occurred. Respiratory side effects include cough, dyspnea, pneumonia, and upper respiratory tract infections. The concurrent use of pentostatin is not recommended because of severe risk of pulmonary toxicity. Because myelosuppression can be severe, with a nadir of 7 to 14 days, fludarabine should not be administered with other severe myelosuppressants such as 5-fluorouracil or methotrexate. Confusion, coma, and death have been reported with very high dosages. Tumor lysis syndrome is common and usually begins with flank pain and hematuria. Most toxic effects are dose dependent and increase with advanced age, bone marrow impairment, and renal insufficiency (Bayer Pharmaceuticals, 2007).
Cytarabine
Cytarabine (ara-C, Cytosar-U, cytosine arabinoside) is a cell cycle–specific (S phase) antimetabolite that inhibits DNA synthesis. It is indicated in the treatment of acute nonlymphocytic leukemia, acute lymphocytic leukemia, acute myelogenous leukemia, chronic myelocytic leukemia, and meningeal leukemia (administered intrathecally). Myelosuppression is the dose-limiting toxicity, with a nadir of 7 to 14 days. Anemia with megaloblastic changes in the bone marrow is common. Cytarabine has a low (10% to 30%) emetic risk. Anorexia, diarrhea, and anal ulceration may occur. Alopecia is common, as are rashes, especially hand-foot syndrome (i.e., a rash on the palms and soles followed by blisters and desquamation). Tumor lysis syndrome, which is caused by rapid lysis of tumor cells, may occur when a patient with acute leukemia undergoes induction therapy or has a large tumor burden. Corticosteroid eyedrops are usually started before cytarabine administration to prevent conjunctivitis, which is often seen. Cytarabine is incompatible with 5-fluorouracil and heparin when given through the same administration set (Upjohn Pharmaceuticals, 2002).
Pentostatin
Pentostatin (Nipent) is an antimetabolite that interferes with DNA replication and disrupts RNA processing. It is cell cycle nonspecific and is indicated primarily in the treatment of refractory hairy cell leukemia and other leukemias unresponsive to therapy. Anorexia, nausea, vomiting, stomatitis, abdominal pain, diarrhea, headache, fatigue, rashes, chills, fever, pain, depression, and nervousness are common. Leukopenia and thrombocytopenia are severe with a granulocyte nadir of 15 days. Pulmonary complications such as pulmonary edema can be life-threatening. Infections and hypersensitivity reactions are common and severe. Myocardial infarctions, arrhythmias, heart failure, and death have occurred. Coma has been reported in more than half of patients (SuperGen, 1998).
Pemetrexed
Pemetrexed (Alimta) is an antimetabolite that inhibits key metabolic enzymatic steps that are needed for pyrimidine and purine synthesis. It is indicated in the treatment of malignant pleural mesothelioma (in combination with cisplatin) and non–small cell lung cancer. Bone marrow depression is the dose-limiting toxicity. Pemetrexed has a low (10% to 30%) emetic risk. Common side effects include anorexia, stomatitis, and diarrhea. A rash occurs in 22% of patients; oral dexamethasone is usually prescribed on the day before, day of, and day following pemetrexed treatment. The antitumor effect of pemetrexed is dependent upon the size of the cellular folate pools, so oral folic acid must be concurrently administered to increase the efficacy of pemetrexed and minimize its toxicity. Patients also receive vitamin B 12 injections every three treatment cycles, beginning 1 week before treatment and continuing throughout treatment every three treatment cycles thereafter (Lilly 2007 and Wilkes and Barton-Burke, 2008).
PLANT ALKALOIDS
The plant alkaloids consist of two groups: the vinca alkaloids, derived from the vinca rosea plant; and other plant alkaloids, such as etoposide, which is a derivative of mandrake (may apple plant). The plant alkaloids inhibit mitosis and are cell cycle specific for the M phase. Major toxicities of these agents affect the hematopoietic, integumentary, neurological, and reproductive systems. All of the vinca alkaloids have a minimal (<10%) emetic risk. The vinca alkaloids are for IV administration only and are usually fatal when inadvertently given intrathecally. All of the vinca alkaloids are vesicants (Feeney et al., 2007 and Wilkes and Barton-Burke, 2008).
Vinblastine
Vinblastine (Velban) is cell cycle specific for the M phase and blocks cellular division. It is used to treat Hodgkin’s disease (stages 3 and 4); lymphoma; acquired immunodeficiency syndrome (AIDS)-related Kaposi’s sarcoma; mycosis fungoides; histocytosis; testicular, bladder, renal cell, and non–small cell lung cancers; and choriocarcinoma. The major toxicity of this drug is dose-related bone marrow depression. Neurotoxicity may occur after several courses of treatment; symptoms include jaw pain, paresthesias, loss of deep tendon reflexes, and peripheral neuropathy. The patient should be monitored for footdrop and difficulty performing fine motor movements (e.g., fastening buttons). Acute bronchospasm and shortness of breath may occur, especially if the patient is also receiving mitomycin. Constipation is common, and the prophylactic use of stool softeners is recommended. Nausea, vomiting, and alopecia are mild (Bedford Labs, 2001).
Vincristine
Vincristine sulfate (Oncovin) is cell cycle specific for the M and S phases and blocks cell division during metaphase. It is indicated in the treatment of acute leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, rhabdomyosarcomas, neuroblastoma, Wilms’ tumor, multiple myeloma, and breast carcinoma. Vincristine has the same neurotoxicities as vinblastine, but those for vincristine are usually more severe and may be permanent. Fine motor movements, such as the ability to pick up and handle small objects, should be monitored. The patient’s gait should also be monitored for difficulty in walking, especially for a slapping gait, which indicates footdrop. Other side effects of vincristine are much the same as those for vinblastine, except myelosuppression is milder (Mayne Pharma USA, 2004 and Schulmeister, 2004).
Vinorelbine
Vinorelbine tartrate (Navelbine) is a semisynthetic vinca alkaloid that inhibits mitosis. It is used to treat Hodgkin’s disease and metastatic breast and ovarian cancers. It is also indicated in the treatment of nonresectable advanced non–small cell lung cancer as a single agent or in combination with cisplatin, and it is used in combination with cisplatin for stage III non–small cell lung cancer. Granulocytopenia is the dose-limiting toxicity and the risk of bone marrow suppression is increased when used in combination with cisplatin. Acute shortness of breath and severe bronchospasm have been noted to occur, especially when vinorelbine is administered with mitomycin. Mild to moderate nausea and vomiting, stomatitis, anorexia, and diarrhea are common. As with other vinca alkaloids, constipation is common, as is peripheral neuropathy. Transient elevations in liver enzymes, alopecia, and fatigue are common (Bedford Labs, 2005b).
Etoposide
Etoposide (VP-16, VePesid), another plant alkaloid, is indicated in the treatment of small cell lung, testicular, and ovarian cancers; relapsed Hodgkin’s and non-Hodgkin’s lymphoma; gestational trophoblastic tumors; and Ewing’s sarcoma. Etoposide is cell cycle specific, with activity in the G 2 and S phases and inhibition of DNA synthesis. Etoposide also inhibits topoisomerase II, an enzyme necessary for cell division. Myelosuppression is dose limiting. Patients experience mild to moderate nausea and vomiting, anorexia, and diarrhea. Alopecia is seen in about 66% of patients, with thinning of the hair in the remainder. Etoposide should be administered over 30 to 60 minutes to minimize the risk of hypotension and bronchospasm (wheezing). Anaphylactic reactions have been reported and are more common during the initial infusion. A metallic aftertaste may occur throughout the infusion of the drug and can sometimes be relieved by having the patient suck on hard candy. Radiation recall has also been reported. Etoposide is considered an irritant if it infiltrates from the vein (Bedford Labs, 2006).
ALKYLATING AGENTS
Most alkylating agents are cell cycle nonspecific. They interfere with DNA replication by crosslinking DNA strands, breaking DNA strands, and abnormally pairing base proteins. Major toxicities are hematopoietic, gastrointestinal, and reproductive (Wilkes and Barton-Burke, 2008).
Mechlorethamine HCl
The first nonhormonal chemotherapeutic agent, introduced in 1946, was mechlorethamine hydrochloride (nitrogen mustard, Mustargen). It is a cell cycle–specific alkylating drug. Its multiple mechanisms of action result in DNA miscoding, breakage, and failure of the cell to replicate. Although it was widely used in the treatment of Hodgkin’s disease, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, bronchogenic carcinoma, and polycythemia vera, its use has diminished in the past decade, largely because of the introduction of newer, more effective, and less toxic chemotherapy agents. Nitrogen mustard has a very short stability when reconstituted and rapidly undergoes chemical transformation and decomposition. Therefore nitrogen mustard is given by IV bolus within 15 minutes of preparation. If inadvertent contact with skin occurs, the area should be washed with copious amounts of water, followed by a rinse of 2.5% sodium thiosulfate solution. Nitrogen mustard is a vesicant and can cause severe tissue necrosis and sloughing in the event of an extravasation. Nitrogen mustard has a high (90%) emetic risk. Nausea and vomiting, which usually start within 30 minutes of administration, may last up to 36 hours. Anorexia and diarrhea are also common. The patient may note a metallic taste immediately after administration. Bone marrow suppression can be profound when nitrogen mustard is given with radiation therapy. Patients receiving this form of chemotherapy not uncommonly develop menstrual irregularities or impaired spermatogenesis (Merck and Co., 1999).
Dacarbazine
Dacarbazine (DTIC-Dome, imidazole, carboxamide) is cell cycle nonspecific and inhibits DNA, RNA, and protein synthesis. It possesses limited ability to cross the blood-brain barrier. Dacarbazine is active against Hodgkin’s disease, malignant melanoma, neuroblastoma, and soft tissue sarcomas. Patients may experience discomfort at the infusion site. Adequately diluting the drug and slowing the infusion may decrease the burning sensation and vasospasm. Slowing the infusion and applying ice above the injection site can also be helpful. Dacarbazine has a high (>90%) emetic risk. Myelosuppression, with a nadir of 7 to 14 days, and flulike symptoms are the most common side effects. The flulike syndrome can last for several days, with primary symptoms of fever and myalgias. Many patients complain of a metallic taste, which may interfere with nutritional intake. Skin reactions of pruritus, erythema, and photosensitivity have occurred. Exposure to the sun during the first 48 hours can cause facial flushing, paresthesia, and dizziness. Patients should avoid taking dong quai and St John’s wort while receiving dacarbazine treatment (these herbs increase photosensitization) (Merck Manuals Online Medical Library, and Wilkes and Barton-Burke, 2008).
Cyclophosphamide
Cyclophosphamide (Cytoxan, Neosar) is one of the most widely used alkylating agents. It is cell cycle nonspecific and causes cell death by crosslinking with DNA and interfering with RNA transcription. It is active in the treatment of lymphomas, leukemias, multiple myeloma, mycosis fungoides, neuroblastoma, retinoblastoma, sarcomas, and cancers of the breast, ovary, testes, lung, and bladder. Hemorrhagic cystitis is the most serious side effect; it can be severe and fatal. Vigorous hydration with 2 to 3 L of fluid a day is necessary before and after administration. The patient should be encouraged to void every 2 hours to lessen the time the drug dwells in the bladder. Mesna, a drug that prevents the formation of cyclophosphamide metabolites, is given with high-dose cyclophosphamide to lessen the incidence and severity of cystitis. Long-term administration of cyclophosphamide may cause fibrosis of the bladder. Leukopenia is the dose-limiting toxicity, with a nadir of 7 to 14 days. Thrombocytopenia and pulmonary fibrosis may occur with high dosages. Cardiotoxicity can occur with high dosages and in combination with doxorubicin. High-dose cyclophosphamide has a high (>90%) emetic risk while the risk is moderate (30% to 90%) with lower doses (Wilkes and Barton-Burke, 2008).
Ifosfamide
Ifosfamide (Ifex) is cell cycle nonspecific and inhibits DNA synthesis. It is indicated in the treatment of third-line germ-cell testicular cancer and is used to treat soft tissue sarcomas, non-Hodgkin’s lymphoma, and lung cancer. Myelosuppression is dose related and common side effects are alopecia, somnolence, and dose-dependent nausea and vomiting. Ifosfamide has a moderate (30% to 90%) emetic risk. Confusion has occurred, and coma and seizures have been reported. The most serious and dose-limiting side effect is hemorrhagic cystitis. Vigorous hydration is recommended before, during, and after administration. The patient should be instructed to void every 2 hours. Scheduled doses of mesna should always be administered with ifosfamide to prevent or lessen the severity of bladder toxicity (Wilkes and Barton-Burke, 2008).
Cisplatin
Cisplatin ( cis-diamminedichloroplatinum, platinum, Platinol) is a heavy metal that is cell cycle nonspecific and inhibits DNA synthesis. It is indicated in the treatment of testicular, ovarian, bladder, head, neck, non–small cell lung, small cell lung, esophageal, cervical, breast, gastric, thyroid, and neurological cancers, as well as in Hodgkin’s and non-Hodgkin’s lymphoma, sarcomas, melanoma, and advanced prostate carcinoma. Cisplatin has a high emetic risk (>90%). Antiemetics should be administered before the infusion because nausea and vomiting are usually severe, starting 1 to 4 hours after the infusion and lasting up to 5 days. Persistent anorexia and taste alterations often occur. Myelosuppression is dose dependent. Ototoxicity occurs in 31% of patients and is usually manifested by tinnitus and high-frequency hearing loss. Peripheral neuropathy occurs and has a cumulative effect. Although rare, anaphylactic reactions have occurred within minutes of the start of the infusion. Renal toxicity is common, severe, and dose dependent. Vigorous hydration is administered before and after treatment, along with mannitol or furosemide, to maintain urinary output. Renal function should be monitored during treatment. Amifostine (Ethyol) reduces the toxic effects of cisplatin and protects the renal cells without interfering with the action of cisplatin. The most common adverse effects of this cytoprotectant are transient hypertension, nausea, and vomiting, which can be severe. The patient should be well hydrated before administration, and should not be hypotensive or receiving antihypertensive therapy within 24 hours of administration. A baseline blood pressure should be taken, with blood pressure monitoring every 5 minutes. Amifostine is given rapidly (less than 15 minutes) with the patient in a supine position. Potassium and magnesium are usually added to the prehydration fluids to prevent hypokalemia and hypomagnesemia. Cisplatin reacts chemically with aluminum and forms a precipitate, so only stainless-steel needles should be used in preparation and administration (Hussain et al., 2003 and Kostova, 2006; Rybak, 2007; Yao et al, 2007).
Carboplatin
Carboplatin (Paraplatin), an analog of cisplatin, is cell cycle nonspecific. Its method of action is thought to be crosslinking of DNA strands. Carboplatin is indicated in the treatment of advanced ovarian cancer, endometrial carcinoma, non–small cell lung cancer, metastatic seminoma, recurrent brain tumors in children, relapsed and refractory acute leukemia, and head and neck cancers. Carboplatin has a moderate (30% to 90%) emetic risk. Mild anorexia and taste changes may occur. Myelosuppression is severe and dose related, with a nadir of 7 to 14 days. Hypersensitivity reactions are common after several courses of the drug have been administered (they are acquired rather than immediate reactions), and are characterized by flushing, rashes, dyspnea, hypotension, and tachycardia. Premedication with histamine-1 (H 1) and histamine-2 (H 2) blockers decreases the risk of developing carboplatin hypersensitivity. Epinephrine, corticosteroids, and antihistamines must be available. Cardiac failure, embolism, and cerebrovascular accident have been reported. Alopecia occurs in about 50% of patients. Needles or IV administration sets containing aluminum should not be used because aluminum reacts with carboplatin, causing precipitate formation and loss of potency. Carboplatin is contraindicated in patients with a history of allergies to cisplatin, platinum-containing compounds, or mannitol, as well as in those with severe bone marrow depression or bleeding. The patient should be instructed to avoid using products containing aspirin (Lenz, 2007, Navo et al., 2006, Winkeljohn and Polovich, 2006 and Lenz, 2007).
Oxaliplatin
Oxaliplatin (Eloxatin) is a third-generation platinum analog. It blocks DNA replication and transcription and is indicated for the adjuvant treatment of patients with stage III colon cancer and for first-line treatment of metastatic colon and rectal cancers (in combination with 5-fluorouracil and leucovorin). Oxaliplatin has a moderate (30% to 90%) emetic risk. Peripheral neuropathy is a dose-limiting toxicity. Two distinct neurotoxicity syndromes may occur: an acute syndrome that persists for less than 14 days and chronic persistent peripheral neuropathy. Acute neurotoxicity, caused by irritation of ion channels in the nerves, occurs in 56% of patients. It is often precipitated by exposure to cold and is characterized by decreased sensation, particularly in the hands and feet. Peripheral neuropathy affects 48% of patients and most often occurs when a cumulative dose of 800 mg/m 2 of oxaliplatin has been administered. Symptoms include decreased sensation (often in a stocking and glove distribution) and impairment of proprioception. Delayed hypersensitivity may occur after 10 to 12 courses of treatment. Symptoms range from rash to anaphylaxis. Common symptoms include dyspnea, hypotension, and urticaria. Desensitization protocols have been developed to reduce the risk of hypersensitivity (Kim and Erlichman, 2007, Sanofi-Aventis, 2006, Stordal et al., 2007 and Wilkes, 2007; Wilkes and Barton-Burke, 2008).
Thiotepa
Thiotepa (triethylenethiophosphoramide, TSPA, Thioplex) is cell cycle nonspecific and causes crosslinking of DNA. Thiotepa is indicated in the treatment of breast and ovarian cancer, Hodgkin’s disease, chronic granulocytic and lymphocytic leukemia, bronchogenic carcinoma, and superficial bladder cancer. Nausea and vomiting are dose dependent. Myelosuppression is the dose-limiting toxic effect. The patient should be instructed to avoid use of aspirin-containing products. Allergic reactions with hives, rash, and bronchospasm have been reported. The patient may experience pain at the infusion site, which may be relieved by diluting the drug, slowing the infusion, or placing an ice pack above the site (Wilkes and Barton-Burke, 2008).
Nitrosoureas
The nitrosoureas are lipid-soluble alkylating agents, most of which can cross the blood-brain barrier because of their lipid solubility. They interfere with DNA replication and repair and are cell cycle nonspecific. Their major toxicities are hematopoietic, gastrointestinal, and reproductive (Wilkes and Barton-Burke, 2008).
Carmustine
Carmustine (BiCNU, BCNU) is a nitrosourea that acts as an alkylating agent by interfering with DNA and RNA synthesis through alkylation. It is cell cycle nonspecific and is indicated in the treatment of brain tumors, Hodgkin’s disease, non-Hodgkin’s lymphoma, and malignant melanoma. Carmustine has a high emetic risk (>90%). Severe nausea and vomiting may occur 1 to 2 hours after infusion and last 6 to 8 hours. Stomatitis is common. Cumulative bone marrow suppression, which is delayed 4 to 6 weeks, is the major dose-limiting toxicity. Concomitant use of cimetidine is avoided because it may increase bone marrow toxicity. Pulmonary fibrosis has been reported with cumulative doses and may be progressive and fatal. Delayed onset has occurred from 9 days to 15 years after treatment. Nephrotoxicity progressing to renal failure and reversible hepatotoxicity have been reported. Carmustine is an irritant, causing intense pain at the infusion site. To decrease pain, the infusion can be slowed and ice placed above the infusion site. Contact of the drug with skin causes brown staining (Wilkes and Barton-Burke, 2008).
Streptozocin
Streptozocin (Zanosar, streptozotocin) is a cell cycle–nonspecific drug that inhibits DNA synthesis through crosslinking. It is indicated in the treatment of insulinomas, carcinoid tumors, non–small cell lung and colon cancers, hepatoma, and squamous cell carcinoma of the oral cavity. Renal toxicity, which is severe and often fatal, is dose related and cumulative. Proteinuria is an early sign of nephrotoxicity. Renal function should be evaluated before and after treatment. In patients with preexisting renal disease, the potential benefit of streptozocin must be weighed against the risk of further renal damage. The use of other nephrotoxic drugs, such as the aminoglycosides, should be avoided. Liver dysfunction and hypoglycemia caused by sudden insulin release have been reported. Myelosuppression is mild, with a nadir of 1 to 2 weeks. Severe nausea and vomiting is common, particularly with daily treatments. Streptozocin is an irritant and commonly causes pain and burning at the infusion site. Slowing the infusion, diluting the concentration of the drug, and applying ice above the site may decrease the symptoms (Wilkes and Barton-Burke, 2008).
ANTITUMOR ANTIBIOTICS
Although the antitumor antibiotics have some anti-infective qualities, their major action is cytotoxic. They interfere with nucleic acid synthesis and inhibit RNA synthesis by intercalation. They react with or bind to DNA and therefore inhibit DNA synthesis. Most are cell cycle nonspecific. Their major toxicities are cardiac, hematopoietic, gastrointestinal, and reproductive. All of the antibiotics except bleomycin are vesicants. The largest category of the antibiotics is the anthracyclines, including doxorubicin, daunorubicin, epirubicin, and idarubicin. In addition to myelosuppression, the anthracyclines can cause alopecia, nausea, vomiting, and stomatitis (Wilkes and Barton-Burke, 2008).
Doxorubicin HCl
Doxorubicin hydrochloride (Adriamycin, Rubex) inhibits DNA and RNA synthesis and is cell cycle nonspecific. It is indicated in the treatment of acute lymphoblastic and myeloblastic leukemia, soft tissue and bone sarcomas, neuroblastoma, Wilms’ tumor, Hodgkin’s and non-Hodgkin’s lymphoma, and breast, bladder, thyroid, lung, gastric, and ovarian cancers. Cardiotoxicity is the major dose-limiting toxicity, presenting as arrhythmias (which can be life-threatening), left ventricular heart failure, and irreversible cardiomyopathy. An electrocardiogram and cardiac ejection fraction (MUGA scan) should be obtained as a baseline before administering doxorubicin, and cardiac function is monitored periodically throughout the course of treatments. The recommended lifetime cumulative dose is 550 mg/m 2. Dexrazoxane is often given to those patients who have received a cumulative dose of 300 mg/m 2 and are continuing treatment. Dexrazoxane is specifically indicated for women with metastatic breast cancer who have reached the 300 mg/m 2 dose level and need to continue doxorubicin treatment. Myelosuppression is severe, with a nadir of 7 to 14 days. Doxorubicin has a moderate (30% to 90%) emetic risk; however, emetic risk increases when doxorubicin is given in conjunction with cyclophosphamide. Common side effects are stomatitis, photosensitivity, radiation recall, hyperpigmentation, and flare reaction. Complete alopecia occurs in 3 to 4 weeks. The patient should be informed that the urine turns red-orange for 1 to 2 days. Doxorubicin is physically incompatible with 5-fluorouracil and heparin when given through the same administration set. This drug causes very severe tissue damage and necrosis if extravasated (Cortes-Funes and Coronado, 2007, Hideg and Kalai, 2007 and Schulmeister, 2007a).
Daunorubicin HCl
Daunorubicin hydrochloride (daunomycin hydrochloride, Cerubidine) interferes with DNA synthesis and is cell cycle nonspecific. It is indicated for remission induction in acute nonlymphocytic and lymphocytic leukemia. Chronic cardiotoxicity is dose related and presents as congestive heart failure, with a mortality rate of 50%. A MUGA scan is indicated before treatment. Acute cardiotoxicity may occur within minutes of administration and presents as supraventricular arrhythmias. Preexisting cardiac disease or exposure to other anthracyclines or cardiotoxic drugs increases the risk for cardiotoxicity. The recommended cumulative lifetime dose is 500 to 600 mg/m 2. Bone marrow suppression occurs, with a nadir of 7 to 14 days. Daunorubicin has a moderate (30% to 90%) emetic risk. Common side effects are diarrhea, mucositis, hepatotoxicity, complete alopecia (3 to 4 weeks), radiation recall, flare reaction, and photosensitivity. Extravasation will cause severe soft tissue necrosis. Mixing daunorubicin hydrochloride with dexamethasone or heparin in the same administration set causes precipitation (Wilkes and Barton-Burke, 2008).
Epirubicin
Epirubicin (Ellence) is an anthracycline antitumor antibiotic analog. It inhibits nucleic acid and protein synthesis and causes cleavage of DNA by topoisomerase II. It is indicated in the treatment of breast cancer. Epirubicin has a moderate (30% to 90%) emetic risk. Common side effects are diarrhea, mucositis, and complete alopecia. Concurrent administration of cardiovascular drugs, such as calcium channel blockers, increases the risk of congestive heart failure. A MUGA scan is indicated before treatment, and cimetidine should not be given to patients receiving epirubicin; renal toxicity is increased. Tissue sensitization to radiotherapy may occur (radiation recall reaction). Soft tissue necrosis occurs if epirubicin extravasates. Secondary acute myelogenous leukemia (AML) has been reported in patients with breast cancer treated with anthracyclines, including epirubicin (Gluck, 2005, Pfizer, 2007 and Wilkes and Barton-Burke, 2008).
Idarubicin HCl
Idarubicin hydrochloride (Idamycin) is a synthetic anthracycline. It is cell cycle specific for the S phase and acts by inhibiting DNA synthesis. It is indicated in the treatment of acute myeloid leukemia, chronic myelogenous leukemia, and acute lymphocytic leukemia. Cardiotoxicity is the dose-limiting toxicity and can be fatal. Baseline cardiac function should be determined before the initial treatment and monitored throughout the course of treatment. Congestive heart failure, myocardial infarction, arrhythmias, electrocardiogram changes, and cardiomyopathy may occur. The maximum safe dose is unknown. Risk increases with preexisting cardiac conditions, radiation to the mediastinal area, and prior treatment with anthracyclines. Myelosuppression is severe and dose related, with a nadir of 7 to 14 days. Idarubicin has a moderate (30% to 90%) emetic risk. Other common side effects are cramps, diarrhea, and mucositis. Although rare, severe enterocolitis with perforation has occurred. Alopecia, generalized rash, urticaria, and bulbous erythrodermatous rash on the palms and soles are common. Radiation recall and flare reaction may occur. The patient should be informed that the urine may be red for 2 to 3 days. Idarubicin is a vesicant capable of severe tissue necrosis if extravasated. Idarubicin should not be mixed with heparin in an administrationset or central venous access device as the drug will precipitate (Wilkes and Barton-Burke, 2008).
Dactinomycin
Dactinomycin (actinomycin D, Cosmegen) is cell cycle nonspecific and inhibits DNA replication and RNA synthesis. It is indicated in the treatment of Wilms’ tumor, rhabdomyosarcoma, carcinoma of the testes and uterus, Ewing’s sarcoma, gestational choriocarcinoma, and melanoma. Nausea and vomiting are severe and usually occur within the first 1 to 2 hours after the start of treatment. Other common adverse reactions are anorexia, diarrhea, erythema, alopecia, and radiation recall. Myelosuppression may be severe, with a nadir of 7 to 14 days. Hepatotoxicity and anaphylaxis may occur. Dactinomycin is contraindicated in patients with existing or recent exposure to chicken pox or herpes zoster; life-threatening reactions may occur. Dactinomycin is a vesicant that can cause severe tissue necrosis if extravasated (Wilkes and Barton-Burke, 2008).
Mitoxantrone
Mitoxantrone (Novantrone) is cell cycle nonspecific and exerts its antitumor effect by interfering with DNA and RNA synthesis. It is indicated in combination therapy in acute and chronic leukemias, advanced or recurrent breast cancer, ovarian cancer, and advanced hormone-refractory prostate cancer pain. Myelosuppression is the dose-limiting toxicity, with a nadir of 7 to 14 days. Mitoxantrone has a low (10% to 30%) emetic risk. Common adverse effects are mucositis, diarrhea, abdominal pain, headache, fever, rash, dyspnea, and alopecia. Seizures, heart failure, arrhythmias, and a decrease in left ventricular ejection fraction have been reported. Cardiac toxicity may be more common in patients with a history of heart disease, radiation to the mediastinum, or prior anthracycline therapy. Hydration before and after administration is necessary to prevent uric acid nephropathy. Mitoxantrone turns the urine a blue-green color within 24 hours and may cause a bluish discoloration of the sclera. Mitoxantrone is an irritant with vesicant potential. If extravasation occurs, ulceration is rare unless a concentrated dose infiltrates. The skin in the involved area will turn blue. Mitoxantrone is incompatible in the same administration set with heparin (Wilkes and Barton-Burke, 2008).
Mitomycin
Mitomycin (mitomycin-C, Mutamycin) inhibits DNA and RNA synthesis and is cell cycle nonspecific. It is indicated in the treatment of adenocarcinoma of the stomach, pancreas, and colon and in advanced breast, non–small cell lung, ovarian, uterine, cervical, head, and neck cancers. Myelosuppression is delayed 4 to 8 weeks and is cumulative. Mitomycin has a low (10% to 30%) emetic risk. Anorexia, diarrhea, alopecia, purple bands on the nails, and pain at the infusion site are common. Interstitial pneumonitis may occur, with a nonproductive cough and fever as the presenting symptoms. When administered with vinca alkaloids, mitomycin may cause acute respiratory distress. Mitomycin is a vesicant, and extravasation should be avoided (Wilkes and Barton-Burke, 2008).
Bleomycin sulfate
Bleomycin sulfate (Blenoxane) is cell cycle specific in the G 2 phase and inhibits DNA synthesis. It is indicated in the treatment of testicular carcinoma, lymphoma, malignant pleural effusions, and squamous cell carcinoma of the head, neck, skin, cervix, vulva, and penis. Bleomycin has a minimal (<10%) emetic risk. The most significant toxicity of bleomycin is pulmonary. The earliest symptoms are dyspnea and rales, followed by pneumonitis and progressing to pulmonary fibrosis. This occurs in about 10% of patients and is fatal in 1%. Increased risk includes age older than 70 years, total cumulative dosage greater than 400 units, previous lung disease, history of radiation to the thoracic area, and concomitant use with other antineoplastics, especially methotrexate. Respiratory effort and lung sounds should be monitored frequently. Anorexia and stomatitis are common but mild. Myelosuppression, if it occurs, is mild, with a nadir of 10 days. Febrile reactions, which may be delayed for 3 to 6 hours, are very common, especially in patients with lymphoma. Premedicating with diphenhydramine and acetaminophen may lessen the fever and chills. Hyperpigmentation, photosensitivity, nail changes, erythema, rash, skin tenderness, and alopecia occur in nearly half the patients. A test dose of 1 to 2 units is recommended because anaphylaxis may occur, especially in patients with lymphoma. Bleomycin is the only antitumor antibiotic that is not a vesicant (Wilkes and Barton-Burke, 2008).
LIPOSOMAL ANTHRACYCLINES
Daunorubicin citrate liposome (DaunoXome) and doxorubicin hydrochloride liposome (Doxil) are two traditional drugs encapsulated with a polyethylene coating that allows the drug to evade detection by the immune system, thereby increasing the amount of drug reaching the tumor cell. The benefits of liposomal encapsulation are increased circulation time, decreased side effects, and the ability to penetrate altered vasculature. Toxicities are similar to those for daunorubicin and doxorubicin but are less severe. Liposomal anthracyclines do not discolor the urine, and alopecia, nausea, and vomiting are rare. Their most common side effects are myelosuppression, cardiac events, stomatitis, skin reactions (e.g., hand-foot syndrome), and hypersensitivity reactions. Mild to moderate cardiac events may occur, presenting as chest pain, palpitations, and tachycardia. Allergic reactions have been reported (7%) in the first 5 minutes,with presenting symptoms of back pain, facial flushing, chest tightness, headache, chills, or hypotension. Doxil and DaunoXome are approved for use in the treatment of advanced human immunodeficiency virus (HIV)-related Kaposi’s sarcoma. Doxil is also approved for the treatment of ovarian carcinoma and multiple myeloma. Doxil and DaunoXome should never be administered through an in-line filter because this will rupture the encapsulating coating. Liposomal anthracyclines are classed as irritants, not vesicants. Extravasations should be treated by applying ice. Although maximum lifetime dosage does not appear to be a factor, a baseline cardiac assessment including MUGA scan should be done before treatment. Neither Doxil nor DaunoXome should be mixed with 0.9% sodium chloride, bacteriostatic agents (benzyl alcohol), or any other solution (Batist, 2007, AuthorAnonymous, 2007, Petre and Dittmer,, Rahman et al., 2007, Samad et al., 2007, Udhrain et al., 2007 and Wilkes and Barton-Burke, 2008).
TAXANES
Docetaxel
Docetaxel (Taxotere) inhibits cancer cell growth by preventing cellular mitosis or division. It is indicated in the treatment of locally advanced or metastatic breast cancer, non–small cell lung cancer, prostate cancer, gastric adenocarcinoma, and head and neck cancer. Bone marrow suppression is the most common and dose-limiting toxicity. Docetaxel has a low (10% to 30%) emetic risk. Other common adverse reactions are stomatitis, diarrhea, weakness, fluid retention, alopecia (80%), and severe hypersensitivity reactions. All patients should be premedicated with corticosteroids before and during administration of docetaxel to reduce the incidence and severity of fluid retention and hypersensitivity reactions. Docetaxel is contraindicated in patients with a known allergy to polysorbate 80, a sorbitol ester used in drug manufacturing (Sanofi-Aventis, 2007 and Wilkes and Barton-Burke, 2008).
Paclitaxel
Paclitaxel (Taxol) is a natural product obtained by a semisynthetic process from the needles and bark of the Western yew tree. Paclitaxel is an antimicrotubule agent and induces abnormal arrays or “bundles” of microtubules throughout the cell cycle (Jordan and Kamath, 2007). It is indicated as first-line and subsequent therapy for the treatment of advanced ovarian carcinoma, as adjuvant treatment of node-positive breast cancer, and as treatment for breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. In combination with cisplatin, it is indicated for the first-line treatment of non–small cell lung cancer in patients who are not candidates for potentially curative surgery and/or radiation therapy. Paclitaxel is also indicated for the second-line treatment of AIDS-related Kaposi’s sarcoma. Bone marrow suppression, especially neutropenia (90%), is dose related and is the major dose-limiting toxicity. If paclitaxel and cisplatin or carboplatin are being administered in the same treatment, paclitaxel should be given first; studies have shown that myelosuppression is profound if it is given after cisplatin. Paclitaxel has a low (10% to 30%) emetic risk. Mucositis and diarrhea are common and occur more often with 24-hour infusions versus 3-hour infusions. Alopecia is very common, occurring in about 82% of patients. Hypersensitivity occurs in 10% of patients, and anaphylaxis characterized by dyspnea and hypotension requiring treatment, angioedema, and generalized urticaria has occurred in 2% to 4% of patients receiving paclitaxel. Fatal reactions have occurred in patients despite premedication. All patients should be pretreated with corticosteroids, diphenhydramine, and H 2 antagonists, and the paclitaxel should be infused over 3 hours via an infusion pump or controller. Patients who experience severe hypersensitivity reactions to paclitaxel should not be rechallenged with the drug. Peripheral neuropathy has been observed in 62% of patients, and the occurrence increases with cumulative doses. This is usually manifested by numbness, tingling, and pain in the hands and feet. There may be loss of deep tendon reflexes and fine motor skills. A baseline electrocardiogram with cardiac assessment should be performed before administering the first dose because of the potential cardiac side effects of the drug. Severe conduction abnormalities have been documented. If this occurs, the patient should receive cardiac monitoring with subsequent doses. Paclitaxel must be mixed in a glass or polyolefin container and infused via a paclitaxel-compatible administration set to prevent leaching of DEHP (diethylhexylphthalate) found in most polyvinylchloride IV administration sets. An in-line 0.2-micron filter must be used to remove particulate matter that forms in the solution. Paclitaxel is contraindicated in patients with a hypersensitivity to polyoxyethylated castor oil, which is a vehicle often used to transport drugs in solution (>Bristol-Myers, 2007, Kingston and Newman, 2007 and Marupudi et al., 2007).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


