CHAPTER 16. Biologic Therapy
Cora Vizcarra, MBA, RN, CRNI®
Immune System Review, 299
Disorders of the Immune System, 301
Biologic Therapy for Treatment of Autoimmune Disorders, 302
Immunoglobulin Therapy for Immune Deficiency, 307
Nursing Considerations and Patient Management, 310
Summary, 314
Biologic therapy includes the use of agents derived from biological sources or of agents that affect biological responses. Primarily, these are products derived from the mammalian genome and represent the new “medicine cabinet” because of the modern genetic engineering techniques used in the creation of these agents (Oldham, 2003). Traditionally, biologic therapies were used to modify the body’s immune responses. Although modulation of the immune response remains a main focus, the term “biotherapy” has replaced “immunotherapy” because the scope of the field has widened. Other names for biologic therapies include biologic agents, biologics, biologicals, and biological response modifier (BRM) therapy (Medicinenet, 2008). Biologics include a diverse range of products such as bacterial and viral vaccines; tissues; human blood and plasma and their derivatives; and certain products produced by biotechnology. These cells and biological molecules are extraordinarily specific in their interactions (Rieger, 2001). Because of this specificity, biotechnology’s tools and techniques are precise and are tailored to operate in known, predictable ways. Biologic agents differ from most drugs as they are not small chemical compounds but are proteins structurally similar to autologous proteins. Biologic agents are not metabolized like drugs but are processed like other proteins in the body; thus adverse reactions might be different from those elicited by drugs (Pichler, 2006). The mechanisms of action of biologic agents involve the individual’s own biological responses (Oldham, 2003). In this chapter, the discussion on biologic therapy will include immunoglobulin therapy and only biologic agents used in the treatment of select autoimmune disorders.
IMMUNE SYSTEM REVIEW
To understand how biologic agents interact with the immune system, it is important to recall how our immune system functions. There are two types of immunity—innate and adaptive immunity (Figure 16-1).
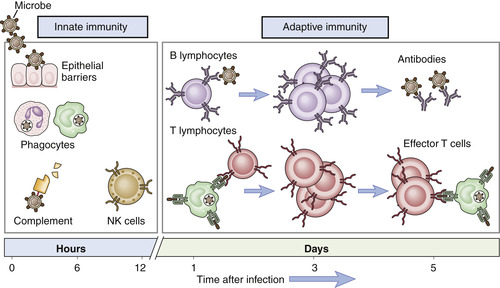 |
| FIGURE 16-1 Innate and adaptive immunity. (From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.) |
Innate immunity, also called nonspecific immunity, is the first line of defense against antigens, which are foreign substances that induce an immune response (Rieger, 2001). Innate immunity is present before exposure to an antigen and it is not enhanced by subsequent exposures. The first line of defense for the innate immune system includes mechanical barriers such as the intact skin and mucous membranes (Rieger, 2001). If these defenses fail, the innate immune system has other components as a second line of defense, including complement, phagocytes, and natural killer (NK) cells.
Adaptive immunity, also known as specific or acquired immunity, is characterized by specific recognition of foreign organisms and a memory response (Rieger, 2001). This allows the immune system to increase its ability to respond and defend the body with successive exposures to infectious organisms. There are two major branches of the adaptive immune responses—humoral immunity and cell-mediated immunity—depending on the components of the immune system involved in the response to the antigen.
Humoral immunity involves the production of antibody molecules in response to an antigen and is mediated by B lymphocytes. B lymphocytes, also called B cells, are specialized cells of the immune system and develop from the stem cells in the bone marrow. When matured, B lymphocytes can be found in the bone marrow, lymph nodes, spleen, some areas of the intestine, and, to a lesser extent, in the bloodstream. When B lymphocytes are stimulated by an antigen, they respond by maturing into another type of cell called plasma cells, the cells responsible for producing antibodies (Thibodeau and Patton, 2007).
Antibodies or immunoglobulins, produced by the plasma cells, interact with specific antigens to protect the host from potentially harmful substances. Each antibody consists of two identical heavy chains and two identical light chains, shaped to form a Y. The basic antibody structure is shown in Figure 16-2.
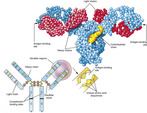 |
| FIGURE 16-2 Basic antibody structure. (From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.) |
The tips of the Y’s arms are called the variable region since these sections vary greatly from one antibody to another. These unique contours in the antigen binding site allow the antibody to recognize a matching antigen. The stem of the Y is called the constant region. This area is identical in all antibodies of the same class. It is this section that links the antibody to other participants in the immune defenses.
There are five classes of antibodies or immunoglobulins: immunoglobulin gamma (IgG), immunoglobulin alpha (IgA), immunoglobulin delta (IgD), immunoglobulin epsilon (IgE), and immunoglobulin mu (IgM) (Thibodeau and Patton, 2007). The major immunoglobulin in the blood is IgG; it is produced in large quantities that exist for more than 1 month. IgG can enter into tissue spaces and works efficiently to coat microorganisms, speeding their destruction by other cells of the immune system. It is the only class of immunoglobulins that crosses the placenta and passes immunity from the mother to the newborn. IgA is shaped as a doublet guarding the entrance to the body; IgA is found in body fluids such as tears, saliva, and secretions of the respiratory and gastrointestinal tracts. IgD remains attached to B cells and plays a key role in initiating early B-cell response. IgE is normally present in only trace amounts in the blood and is responsible for the symptoms of allergy. IgM is a star-shaped cluster that remains in the bloodstream, where it is very effective in killing bacteria (Figure 16-3) (Thibodeau and Patton, 2007).
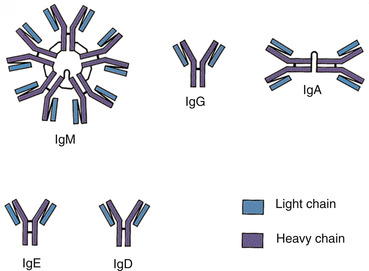 |
| FIGURE 16-3 Classes of antibodies. (From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.) |
Cell-mediated immunity, on the other hand, involves the production of cytotoxic T lymphocytes, activated macrophages, activated NK cells, and cytokines in response to an antigen, and is mediated by T lymphocytes (Rieger, 2001). T lymphocytes, also called T cells, do not produce antibodies; instead, they directly attack foreign antigens such as viruses, fungi, or transplanted tissues and act as regulators of the immune system. Mature T lymphocytes leave the thymus and populate other organs of the immune system, such as the spleen, lymph nodes, bone marrow, and blood. T lymphocytes have molecules on their surfaces that are similar to antibodies and recognize antigens. T lymphocytes vary in types and functions. The killer or cytotoxic T lymphocytes perform the actual destruction of the invading microorganism, and also respond to foreign tissues in the body, such as a transplanted kidney. The killer T lymphocytes directly bind to their target and kill it. The helper T lymphocytes assist B lymphocytes in producing antibodies and assist the killer T lymphocytes in attacking foreign substances. The regulatory T lymphocytes suppress or turn off other T lymphocytes and act as a thermostat to the lymphocyte system. Natural killer (NK) cells come from the bone marrow and are present in low numbers in the bloodstream and in tissues. NK cells play an important role in killing cells infected with viruses and are believed to play a role in preventing cancer (Thibodeau and Patton, 2007).
Macrophages are large white blood cells found in many organs, including the lungs, kidneys, brain, and liver. Macrophages act like scavengers, removing debris and worn out cells from the body, and they also secrete monokines, a powerful chemical signal vital to the immune response. Cytokines are diverse and potent chemical messengers secreted by cells of the immune system. They are the chief communication signals of T cells, and encourage cell growth, promote cell activation, direct cellular traffic, and destroy target cells. Cytokines include interleukins, growth factors, and interferons. Interleukins serve as messengers between leukocytes and are also known as either lymphokines or monokines. When cytokines attract specific cell types to an area, they are called chemokines, and are released at the site of injury or infection where they attract other immune cells to the area to help repair damage and defend against infection. Interferons are naturally occurring cytokines that boost the immune system’s ability to recognize cancer as a foreign invader (Thibodeau and Patton, 2007).
The major difference between humoral and cell-mediated immunity is that in cell-mediated immunity the immune response does not involve antibodies as it does in humoral immunity, but rather involves the activation of macrophages and NK cells, the production of antigen-specific cytotoxic lymphocytes, and the release of various cytokines in response to the antigen.
DISORDERS OF THE IMMUNE SYSTEM
The disorders of the immune system can be divided into two general categories: (1) excessive immune responses and (2) deficient immune responses. The category of excessive immune responses includes disorders in which the immune system is overfunctioning or hyperfunctioning. Examples include autoimmunity and hypersensitivity disorders. The category of deficient immune responses includes disorders in which the immune response is ineffective because of congenital, genetic, or acquired dysfunction. Examples include severe combined immunodeficiency (SCID) syndrome, DiGeorge syndrome, selective IgA deficiency, and the secondary immunodeficiencies associated with white blood cell malignancies. Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is a primary acquired immunodeficiency disorder (Copstead-Kirkhorn and Banasik, 2005).
In this chapter, the discussion will focus on disorders of the immune system treated with biologic agents and immune deficiency disorders treated with immunoglobulin.
AUTOIMMUNE DISORDERS
One of the most remarkable features of the immune system is its ability to distinguish between the self antigens, which are part of the host, and foreign antigens, which may present a threat to the host (Rieger, 2001). The immune system does not normally respond to self antigens. This immunological unresponsiveness to self antigens is called tolerance, and is important in understanding autoimmune disorders. The failure of this tolerance, because of the interaction of a wrong environment with the wrong genes, results in autoimmune disease (Mackay, 2000). Unfortunately, when a self antigen becomes immunogenic, it cannot be eliminated and the resulting inflammation becomes persistent and destructive. This is evident in autoimmune disorders such as rheumatoid arthritis, Crohn’s disease, and psoriatic arthritis, just to name a few. Examples of autoimmune diseases by main target organs are listed in Box 16-1.
Box 16-1



 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

AUTOIMMUNE DISEASE BY MAIN TARGET ORGANS
Nervous system
• Multiple sclerosis
• Myasthenia gravis
• Guillain-Barré syndrome
• Autoimmune uveitis
GI system
• Crohn’s disease
• Ulcerative colitis
• Primary biliary cirrhosis
• Autoimmune hepatitis
Blood/Blood vessels
• Autoimmune hemolytic anemia
• Pernicious anemia
• Autoimmune thrombocytopenia
• Temporal arteritis
• Antiphospholipid syndrome
• Vasculitides (Wegener’s granulomatosis)
• Behçet’s syndrome
Skin
• Psoriasis
• Dermatitis herpetiformis
• Pemphigus vulgaris
• Vitiligo
Endocrine glands
• Type 1 diabetes
• Grave’s disease
• Hashimoto’s thyroiditis
• Autoimmune oophoritis/orchitis
• Autoimmune adrenal gland disease
Musculoskeletal system
• Rheumatoid arthritis
• Systemic lupus erythematosus
• Scleroderma
• Polymyositis
• Ankylosing spondylitis
• Sjögren’s syndrome
Data from Understanding Autoimmune Disease (website). Accessed 3/1/08 from http://www.nutritionadvisor.com/autoimmune-diseases.htm.
IMMUNE DEFICIENCY DISORDERS
When either part of the immune system is absent or its function is hampered, the result is an immune deficiency disease. An immune deficiency disease may be caused by an intrinsic (inborn) defect in the cells of the immune system, termed primary immune deficiency disease, or caused by an extrinsic environmental factor or agent, known as secondary immune deficiency disease. Primary immunodeficiency diseases are a group of disorders caused by basic defects in immune function that are intrinsic to, or inherent in, the cells and tissues of the immune system. There are more than 150 primary immunodeficiency diseases, ranging from common to rare, affecting a single cell or protein or more than one component of the immune system (Immune Deficiency Foundation, 2007). Secondary immune deficiencies are conditions that impair immune function as a result of other processes. A number of physical, psychosocial, nutritional, environmental, and pharmacological factors can singly or in combination lead to the development of secondary immunodeficiency disorders (Copstead-Kirkhorn and Banasik, 2005). A list of primary and secondary immune deficiencies that may benefit from immunoglobulin therapy is presented in Box 16-2.
Box 16-2
PRIMARY AND SECONDARY IMMUNE DEFICIENCY TREATED WITH IMMUNOGLOBULIN THERAPY
| Primary Immune Deficiency | Secondary Immune Deficiency |
|---|---|
| Agammaglobulinemia (X-linked and autosomal form) | Chronic lymphocytic leukemia (CLL) with antibody deficiency and recurrent infection |
| Hypogammaglobulinemia with impaired specific antibody production: • Common variable immunodeficiency • Hyper IgM syndromes • Transient hypogammaglobulinemia | Pediatric HIV infection |
| Normogammaglobulinemia with selective antibody deficiency: • Wiskott-Aldrich syndrome • Specific polysaccharide antibody deficiency and/or “lacunar” antibody deficiencies | Hypogammaglobulinemia and/or specific antibody deficiency caused by chemotherapy and/or monoclonal antibody treatment |
From The Immune Deficiency Foundation Nursing Advisory Committee: IDF guide for nurses on immune globulin therapy for primary immunodeficiency diseases, ed 2, Bethesda, Maryland, 2007, Immune Deficiency Foundation. Accessed 3/1/08 from http://www.primaryimmune.org/publications/book_nurse/Nurses_Guide.pdf.
BIOLOGIC THERAPY FOR TREATMENT OF AUTOIMMUNE DISORDERS
There are a vast range of treatments available (Vizcarra, 2003) for traditional management of autoimmune disorders such as the following: rheumatoid arthritis, inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis, inflammatory skin conditions such as psoriasis, transplant-related diseases such as graft rejection, and neurological disorders such as multiple sclerosis. The traditional symptom-based treatment includes the use of anti-inflammatory drugs, corticosteroids, immunosuppressants, and even surgery, resulting in limited control and effectiveness often accompanied by safety and tolerability issues. It was this limitation and ineffective treatment partnered with the greater understanding of the underlying mechanisms of autoimmune disorders that facilitated the development of new biologic agents and revolutionized treatment (Olsen and Stein, 2004).
There are several biologic agents currently approved in the United States for the treatment of autoimmune disorders. Table 16-1 lists all types of biologic agents with approved indications related to the treatment of autoimmune disorders in the United States.
| Name (Generic/Trade) | Mechanism of action | Approved indications | Recommended dosage (Initial) | Recommended dosage (Subsequent) | Comments |
|---|---|---|---|---|---|
| Abatacept/Orencia | Selective co-stimulation modulator; inhibits T-cell activation by binding to CD80 and CD86, thereby blocking interaction with CD28 | Adult rheumatoid arthritis | Adult: <60 kg: 500 mg 60-100kg: 750 mg >100 kg: 1000 mg | Following initial dose, give at 2 and 4 weeks, then every 4 weeks | Administer as a 30-min infusion. Prepare vials using only silicone-free disposable syringe. Use sterile, nonpyrogenic, low protein binding filter. |
| Juvenile idiopathic arthritis (pediatric patients 6 years of age and older) | Pediatric: <75 kg: 10 mg/kg ≥75 kg: follow adult dosing regimen, not to exceed maximum dose of 1000 mg | Following initial dose, give at 2 and 4 weeks, then every 4 weeks | Administer as a 30-min infusion. Prepare vials using only silicone-free disposable syringe. Use sterile, nonpyrogenic, low protein binding filter. | ||
| Adalimumab/Humira | Binds specifically to TNFα and blocks its interaction with p55 and p75 cell-surface receptors Lyses surface TNF-expressing cells in vitro in presence of compliment Does not bind or inactivate lymphotoxin (TNFβ) | Rheumatoid arthritis Psoriatic arthritis Ankylosing spondylitis | Adult: 40 mg every other week; some patients with RA not receiving methotrexate may benefit from increasing frequency to 40 mg every week | Adult: 40 mg every other week; some patients with RA not receiving methotrexate may benefit from increasing frequency to 40 mg every week | Administered by subcutaneous injection. |
| Juvenile idiopathic arthritis | For patients 4-17 yr, based on weight: 15kg (33 lb) to <30 kg (66 lb): 20 mg every other week ≥30 kg (66 lb): 40 mg every other week | For patients 4-17 yr, based on weight: 15 kg (33 lb) to <30 kg (66 lb): 20 mg every other week | Administered by subcutaneous injection. | ||
| Crohn’s disease | Adult: 160 mg day 1 (given as four 40-mg injections in 1 day or as two 40-mg injections per day for 2 consecutive days) | Followed by 80 mg 2 weeks later (day 15); 2 weeks later (day 29) begin maintenance dose of 40 mg every other week | Administered by subcutaneous injection. | ||
| Plaque psoriasis | Adult: initial dose of 80 mg | 40 mg every other week starting 1 week after initial dose | Administered by subcutaneous injection. | ||
| Anakinra/Kineret | Blocks the biologic activity of IL-1 by competitively inhibiting IL-1 binding to IL-1 type 1 receptor, which is expressed in a wide variety of tissues and organs | Rheumatoid arthritis | 100 mg/day Physician should consider a dose of 100 mg every other day for RA patients who have severe renal insufficiency or end-stage renal disease | 100 mg/day Physician should consider a dose of 100 mg every other day for RA patients who have severe renal insufficiency or end-stage renal disease | Administered by subcutaneous injection. Administer dose at approximately same time every day. |
| Certolizumab pegol/Cimzia | Binds and inhibits human TNFα | Crohn’s disease | Adult: 400 mg initially | At weeks 2 and 4; if response occurs, follow with 400 mg every 4 weeks | Administered by subcutaneous injection. |
| Efalizumab/Raptiva | Binds to CD11a and inhibits binding of LFA-1 to ICAM-1, thereby inhibiting adhesion of leukocytes to other cell types | Plaque psoriasis | A single 0.7 mg/kg conditioning dose | Followed by weekly doses of 1 mg/kg (maximum single dose not to exceed total of 200 mg) | Administered by subcutaneous injection. |
| Etanercept/Enbrel | Binds specifically to TNF and blocks its interaction with cell-surface receptors | Rheumatoid arthritis Psoriatic arthritis Ankylosing spondylitis | Adult: 50 mg per week | Adult: 50 mg per week | Administered by subcutaneous injection. |
| Juvenile idiopathic arthritis | Pediatric (ages 2-17 yr): 0.8 mg/kg per week up to maximum of 50 mg per week | Pediatric (ages 2-17 yr): 0.8 mg/kg per week up to maximum of 50 mg per week | Administered by subcutaneous injection. 50-mg prefilled syringe or autoinjector may be used for pediatric patients weighing 63 kg or more. 25-mg prefilled syringe is not recommended for pediatric patients weighing less than 31 kg. | ||
| Plaque psoriasis | Adult: 50 mg given twice weekly (administered 3 or 4 days apart) for 3 months | Followed by a reduction to maintenance dose of 50 mg per week. | Administered by subcutaneous injection. | ||
| Natalizumab/Tysabri | Binds to alpha 4 subunit of α 4β 1– and α 4β 7-integrins expressed on surface of all leukocytes except neutrophils, and inhibits alpha 4-mediated adhesion of leukocytes to their counter-receptors | Relapsing form of multiple sclerosis | 300 mg every 4 weeks | 300 mg every 4 weeks | Administer intravenously over 1 hour. Only prescribers registered in MS Touch prescribing program may prescribe Tysabri. |
| Crohn’s disease | 300 mg every 4 weeks | 300 mg every 4 weeks | Administer intravenously over 1 hour. Only prescribers registered in CD Touch prescribing program may prescribe Tysabri. | ||
| Infliximab/Remicade | Neutralizes biological activities of TNFα by binding with high affinity to soluble and transmembrane forms of TNFα; inhibits binding of TNFα with its receptors Does not neutralize TNFβ, a related cytokine that uses same receptors as TNF | Rheumatoid arthritis | 3 mg/kg as first infusion | Followed by 3 mg/kg at 2 and 6 weeks after first infusion and then every 8 weeks thereafter For patients with incomplete response, adjust dose up to 10 mg/kg or treat as often as every 4 weeks | Should be given in combination with methotrexate. Administered as an intravenous infusion over 2 hours. |
| Ankylosing spondylitis | 5 mg/kg | Followed by additional doses of 5 mg/kg at 2 and 6 weeks after first infusion, then every 6 weeks | Administered as an intravenous infusion over 2 hours. | ||
| Psoriatic arthritis | 5 mg/kg | Followed by additional doses of 5 mg/kg at 2 and 6 weeks after first infusion, then every 8 weeks | |||
Crohn’s disease Fistulizing Crohn’s disease | Adult: 5 mg/kg induction regimen at 0, 2, and 6 weeks | Adult: followed by maintenance regimen of 5 mg/kg every 8 weeks thereafter For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg | Administered as an intravenous infusion over 2 hours. Patients who do not respond by week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue Remicade. | ||
| Pediatric Crohn’s disease | Pediatric: 5 mg/kg induction regimen at 0, 2, and 6 weeks | Followed by maintenance regimen of 5 mg/kg every 8 weeks thereafter | Administered as an intravenous infusion over 2 hours. | ||
| Ulcerative colitis | 5 mg/kg | Followed by additional doses of 5mg/kg at 2 and 6 weeks after first infusion, then every 8 weeks thereafter | Administered as an intravenous infusion over 2 hours. Can be given with or without methotrexate. | ||
| Plaque psoriasis | 5 mg/kg | Followed by additional doses of 5 mg/kg at 2 and 6 weeks after first infusion, then every 8 weeks thereafter | Administered as an intravenous infusion over 2 hours. | ||
| Rituximab/Rituxan | Binds to CD20 antigen on B lymphocytes and recruits immune effector function to mediate B-cell lysis | Rheumatoid arthritis Note: rituximab is approved for NHL but not included in discussion of autoimmune disorders | Two, 1000-mg infusions separated by 2 weeks | Given in combination with methotrexate. Premedicate before each infusion. First infusion: initiate infusion at rate of 50 mg/hr. In absence of infusion toxicity, increase infusion rate by 50 mg/hr increments every 30 min, to maximum of 400 mg/hr. Subsequent infusion: initiate infusion at rate of 100 mg/hr. In absence of infusion toxicity, increase rate by 100 mg/hr increments at 30-min intervals, to maximum of 400 mg/hr. Interrupt infusion or slow infusion rate for infusion reactions. Continue infusion at half previous rate upon improvement of symptoms. |
MONOCLONAL ANTIBODIES FOR AUTOIMMUNE DISORDERS
Monoclonal antibodies are produced using a technique developed by Kohler and Milstein in 1975 called hybridoma technique. This technique fuses an antibody-producing cell with a myeloma cell line, resulting in an “immortal hybrid” cell that produces a single antibody recognizing only a single antigen (Rieger, 2001). This technique made it possible to produce an unlimited batch of a pure monoclonal antibody that varied little and was highly specific for a single antigen (Rieger, 2001). The generic drug names of monoclonal antibodies end with the suffix “mab.” The infixes preceding the suffix stem identify the target disease state and the product source. Thus, looking at the drug name rituximab, the “tu” indicates that the drug is for “tumor”; “xi” indicates that the drug is a chimeric monoclonal antibody, meaning derived from two or more genetically distinct sources; and “mab” indicates that the drug is a monoclonal antibody (American Medical Association, 2008).
The monoclonal antibody targeting tumor necrosis factor-alpha (TNFα), which is a key component in the development of various autoimmune disorders, includes anti-TNFα agents, also known as TNF antagonists, TNF inhibitors, TNF neutralizers, or TNF blockers. In certain autoimmune disorders such as rheumatoid arthritis (RA), Crohn’s disease (CD), ulcerative colitis (UC), psoriasis, psoriatic arthritis, and ankylosing spondylitis, scientific evidence indicates that TNFα, a proinflammatory cytokine released by activated monocytes, macrophages, and T lymphocytes, is a central factor in the development of these diseases (Olsen and Stein, 2004). TNFα binds to its receptor cells, and the presence of TNFα in high concentration is responsible for inflammatory pathogenesis by mediating leukocyte recruitment and inflammation of the synovial membrane of the joint, intestinal mucosa, and skin (Feldmann and Maini, 2001 and Papadakis and Targan, 2000). The TNFα antagonists work by impairing TNF binding to its receptors and lysing cells that express TNFα on their surface (Olsen and Stein, 2004). TNFα has been implicated in a wide spectrum of diseases beyond those previously mentioned, including sepsis, diabetes, cancer, osteoporosis, and multiple sclerosis (Chen and Goeddel, 2002). Examples of these agents are infliximab (Remicade, Centocor), adalimumab (Humira, Abbott), and certolizumab pegol (Cimzia, UCB). For the recommended dosing and administration of each agent, refer to Table 16-1.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
